Anti-Inflammatory and Anti-Oxidant Effects of Epilobium amurense subsp. cephalostigma via Activation of Nrf2/HO-1 and Inhibition of NF-κB/p38 MAPK Signaling in LPS-Stimulated Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of EACEE
2.3. Cell Culture
2.4. MTT Assay
2.5. NO Assay
2.6. Western Blot Analysis
2.7. Intracellular ROS Assay
2.8. Statistical Analysis
3. Results
3.1. EACEE Inhibited NO Production and iNOS Protein Expression in LPS-Stimulated RAW 264.7 Macrophages
3.2. EACEE Regulated NF-κB Pathway in LPS-Stimulated RAW 264.7 Macrophages
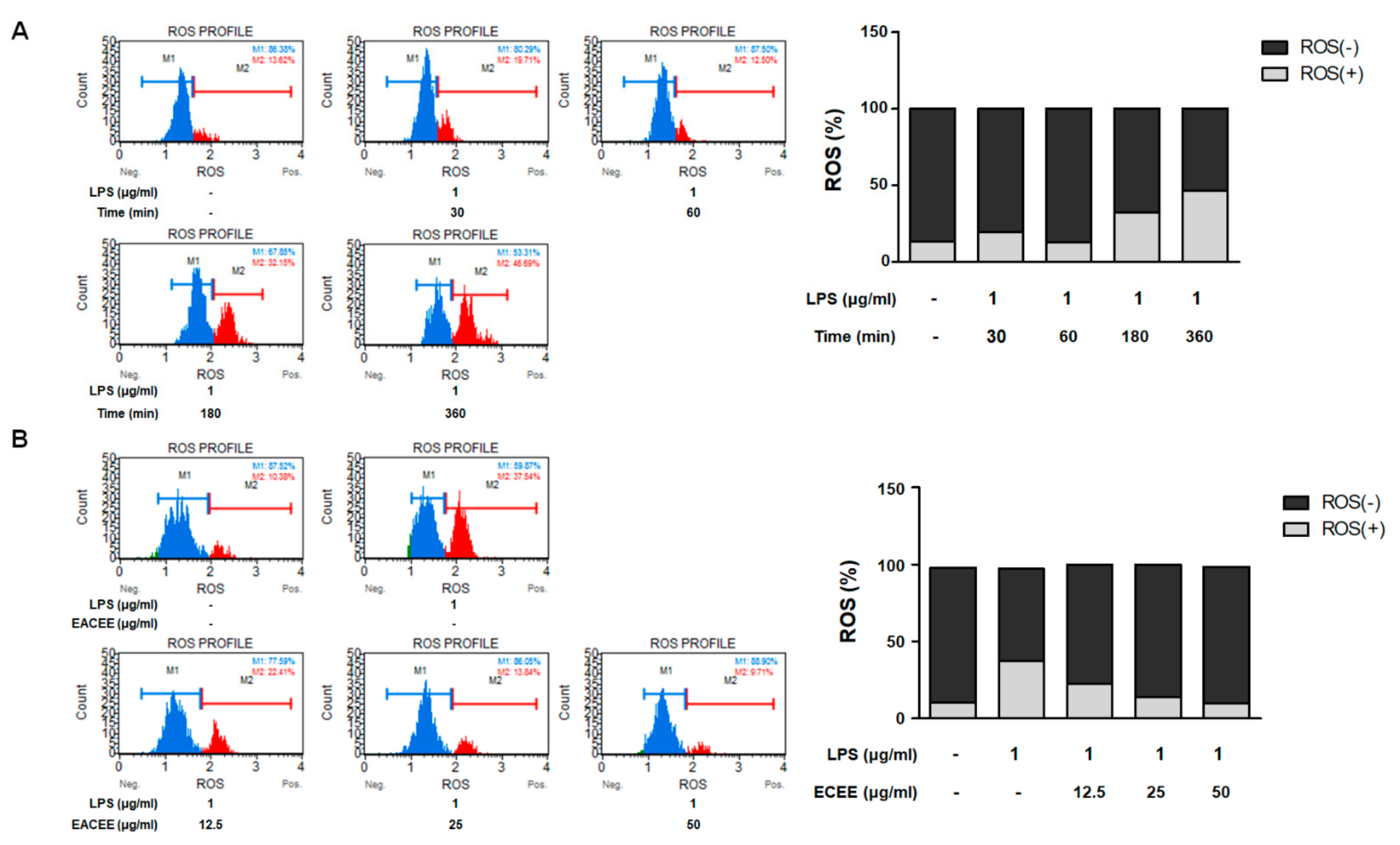
3.3. EACEE Inhibited Excess ROS Production in LPS-Stimulated RAW 264.7 Macrophages
3.4. EACEE Enhanced Nrf2 and HO-1 Protein Expression and Inhibited Overexpression of Nox4 in LPS-Stimulated RAW 264.7 Macrophages
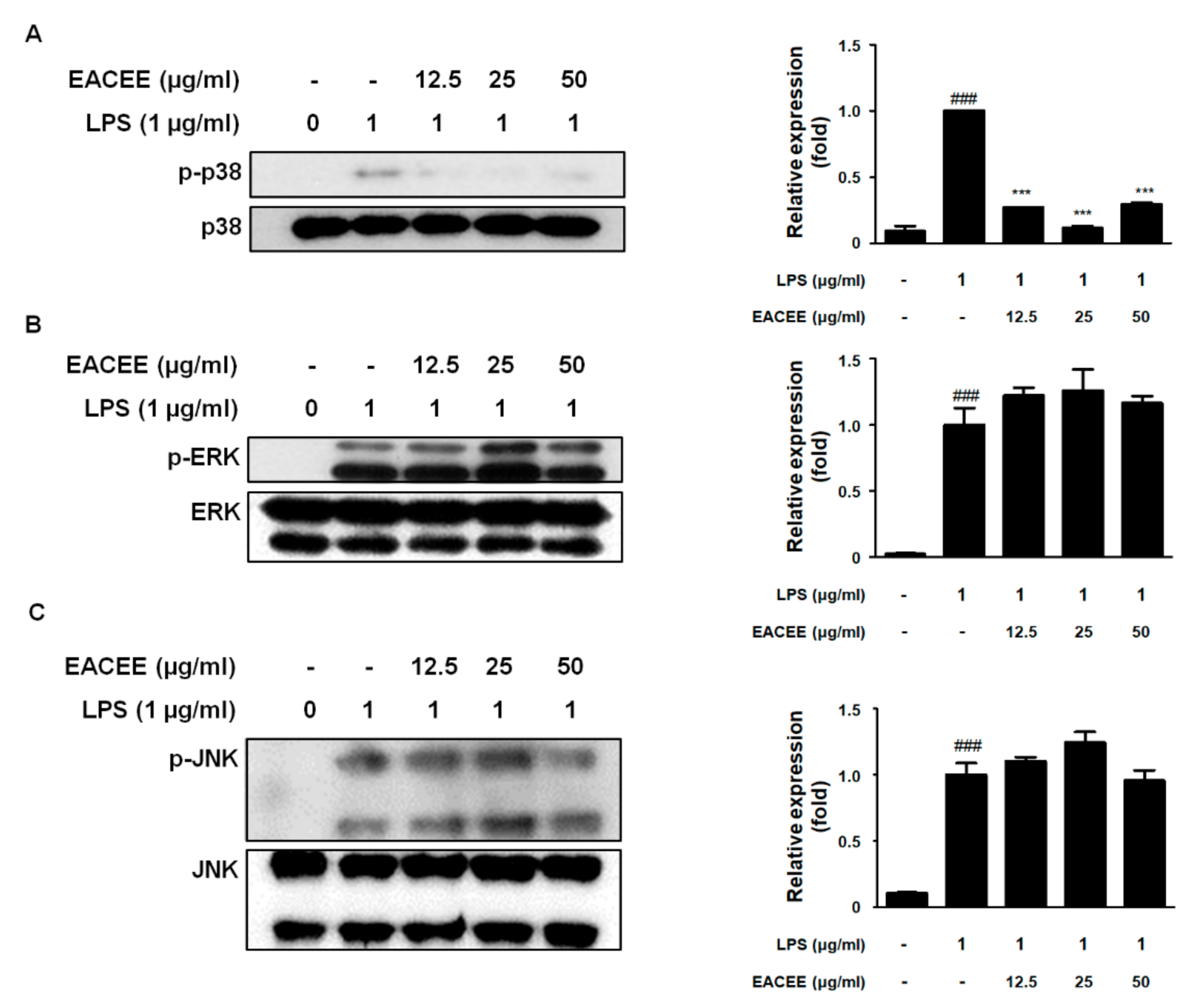
3.5. EACEE Suppressed p-p38 MAPK Protein Expression in LPS-Stimulated RAW 264.7 Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Granica, S.; Piwowarski, J.; Czerwińska, M.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Kungnip Sumogwŏn (Korea). Hanbando Chasaeng Singmul Yŏngŏirum Mongnokchip [English Names for Korean Native Plants]; Kungnip Sumogwŏn: Pocheon-si, Korea, 2015; 760p, Available online: https://www.nlb.gov.sg/biblio/203966604 (accessed on 1 December 2021).
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhang, H.; Wang, Z.; Ding, J.; Wang, S.; Huang, B.; Ke, S.; Gao, C. Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mater. Chem. B 2019, 7, 5019–5037. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.S.M.; Sheikh, S.I.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Druhan, L.J.; Zweier, J.L. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch. Biochem. Biophys. 2010, 494, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Rosanna, D.P.; Salvatore, C. Reactive oxygen species, inflammation, and lung diseases. Curr. Pharm. Des. 2012, 18, 3889–3900. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Schenten, D.; Medzhitov, R. The Control of Adaptive Immune Responses by the Innate Immune System. Adv. Immunol. 2011, 109, 87–124. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, Z.; Xiong, Y.; Zhang, Y.; Li, X. Cytotoxic and anti-inflammatory constituents from Momordica cochinchinensis seeds. Fitoterapia 2019, 139, 104360. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Cha, H.-J.; Choi, E.O.; Leem, S.-H.; Kim, G.-Y.; Moon, S.-K.; Chang, Y.-C.; Yun, S.-J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweet, M.; Hume, D. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 1996, 60, 8–26. [Google Scholar] [CrossRef]
- Rodriguez-Serrano, M.; Barany, I.; Prem, D.; Coronado, M.-J.; Risueno, M.C.; Testillano, P.S. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J. Exp. Bot. 2011, 63, 2007–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, P.C.; Abe, K.Y.; Schroeder, R.A. Oxidative Stress Increases Hepatocyte iNOS Gene Transcription and Promoter Activity. Biochem. Biophys. Res. Commun. 1997, 234, 289–292. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Huang, Q.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (-)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Innamorato, N.G.; Rojo, A.I.; García-Yagüe, Á.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.-W.; Yi, Y.-J.; Lee, M.-S.; Yun, B.-S.; Lee, S.-M. Differential Modulation of Lipopolysaccharide-Induced Inflammatory Cytokine Production by and Antioxidant Activity of Fomentariol in RAW264.7 Cells. Mycobiology 2015, 43, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef]
- Bist, G.; Pun, N.T.; Magar, T.B.T.; Shrestha, A.; Oh, H.J.; Khakurel, A.; Park, P.-H.; Lee, E.-S. Inhibition of LPS-stimulated ROS production by fluorinated and hydroxylated chalcones in RAW 264.7 macrophages with structure-activity relationship study. Bioorganic Med. Chem. Lett. 2017, 27, 1205–1209. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-L.; Pan, L.-L.; Long, F.; Wu, W.-J.; Yan, D.; Xu, P.; Liu, S.-Y.; Qin, M.; Jia, W.-W.; Liu, X.-H.; et al. Endogenous Hydrogen Sulfide Ameliorates NOX4 Induced Oxidative Stress in LPS-Stimulated Macrophages and Mice. Cell. Physiol. Biochem. 2018, 47, 458–474. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Shih, R.H.; Lin, C.C.; Chen, J.T.; Yang, C.M. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun. Signal. 2012, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Keum, Y.-S.; Yu, S.; Chang, P.P.-J.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.-N.T. Mechanism of Action of Sulforaphane: Inhibition of p38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element–Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006, 66, 8804–8813. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, S.-Y.; Kang, H.-A.; Jin, B.-R.; Kim, H.-J.; Park, Y.-J.; An, R.-B.; Kim, S.-Y.; An, H.-J. Anti-Inflammatory and Anti-Oxidant Effects of Epilobium amurense subsp. cephalostigma via Activation of Nrf2/HO-1 and Inhibition of NF-κB/p38 MAPK Signaling in LPS-Stimulated Macrophages. Appl. Sci. 2021, 11, 11715. https://doi.org/10.3390/app112411715
Cheon S-Y, Kang H-A, Jin B-R, Kim H-J, Park Y-J, An R-B, Kim S-Y, An H-J. Anti-Inflammatory and Anti-Oxidant Effects of Epilobium amurense subsp. cephalostigma via Activation of Nrf2/HO-1 and Inhibition of NF-κB/p38 MAPK Signaling in LPS-Stimulated Macrophages. Applied Sciences. 2021; 11(24):11715. https://doi.org/10.3390/app112411715
Chicago/Turabian StyleCheon, Se-Yun, Hyun-Ae Kang, Bo-Ram Jin, Hyo-Jung Kim, Yea-Jin Park, Ren-Bo An, Soo-Yong Kim, and Hyo-Jin An. 2021. "Anti-Inflammatory and Anti-Oxidant Effects of Epilobium amurense subsp. cephalostigma via Activation of Nrf2/HO-1 and Inhibition of NF-κB/p38 MAPK Signaling in LPS-Stimulated Macrophages" Applied Sciences 11, no. 24: 11715. https://doi.org/10.3390/app112411715
APA StyleCheon, S.-Y., Kang, H.-A., Jin, B.-R., Kim, H.-J., Park, Y.-J., An, R.-B., Kim, S.-Y., & An, H.-J. (2021). Anti-Inflammatory and Anti-Oxidant Effects of Epilobium amurense subsp. cephalostigma via Activation of Nrf2/HO-1 and Inhibition of NF-κB/p38 MAPK Signaling in LPS-Stimulated Macrophages. Applied Sciences, 11(24), 11715. https://doi.org/10.3390/app112411715






