Placental mRNA and Protein Expression of VDR, CYP27B1 and CYP2R1 Genes Related to Vitamin D Metabolism in Preeclamptic Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. RNA Extraction and cDNA Synthesis
2.3. Real-Time PCR
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
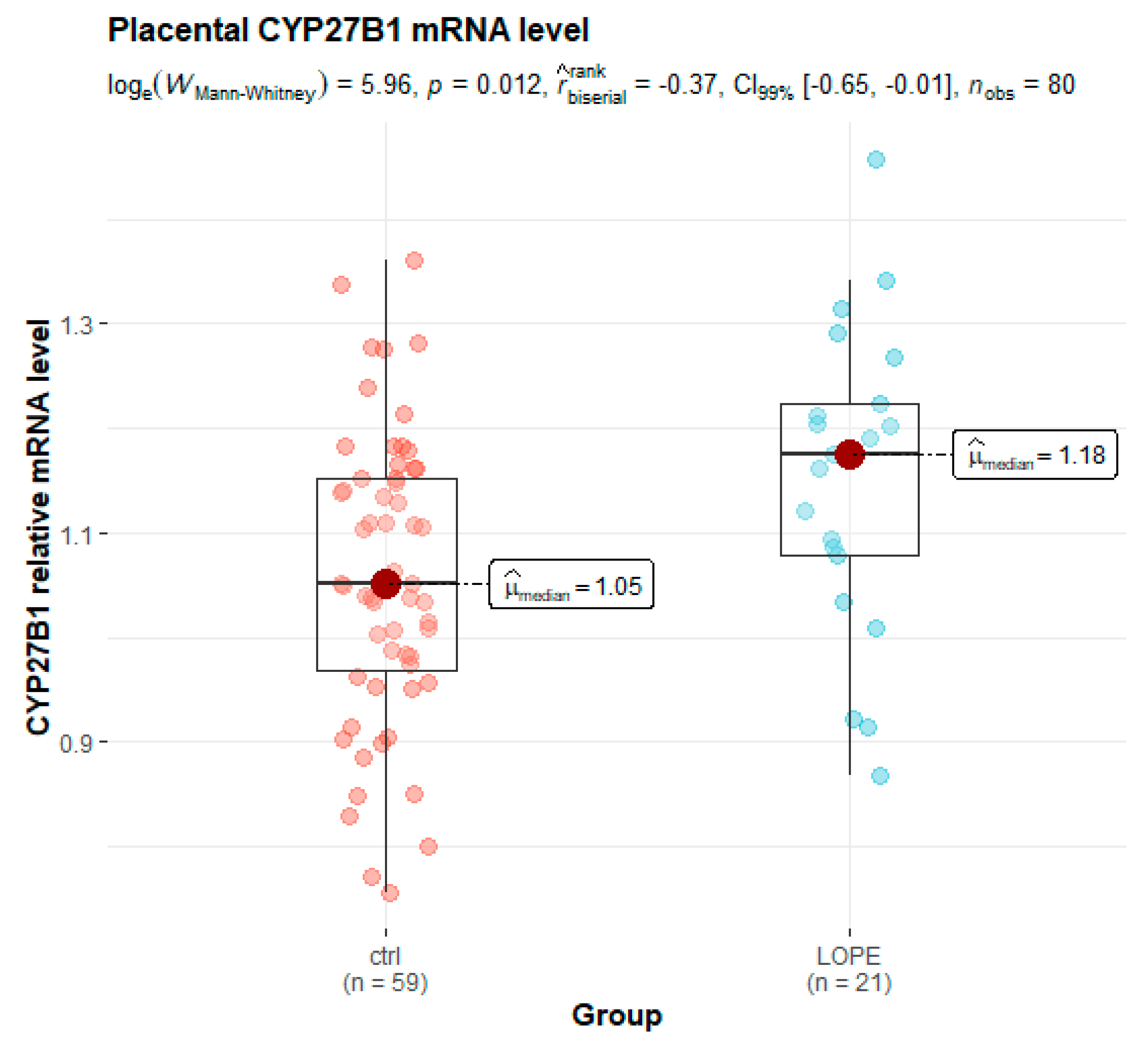
3.2. Placental Gene Expression
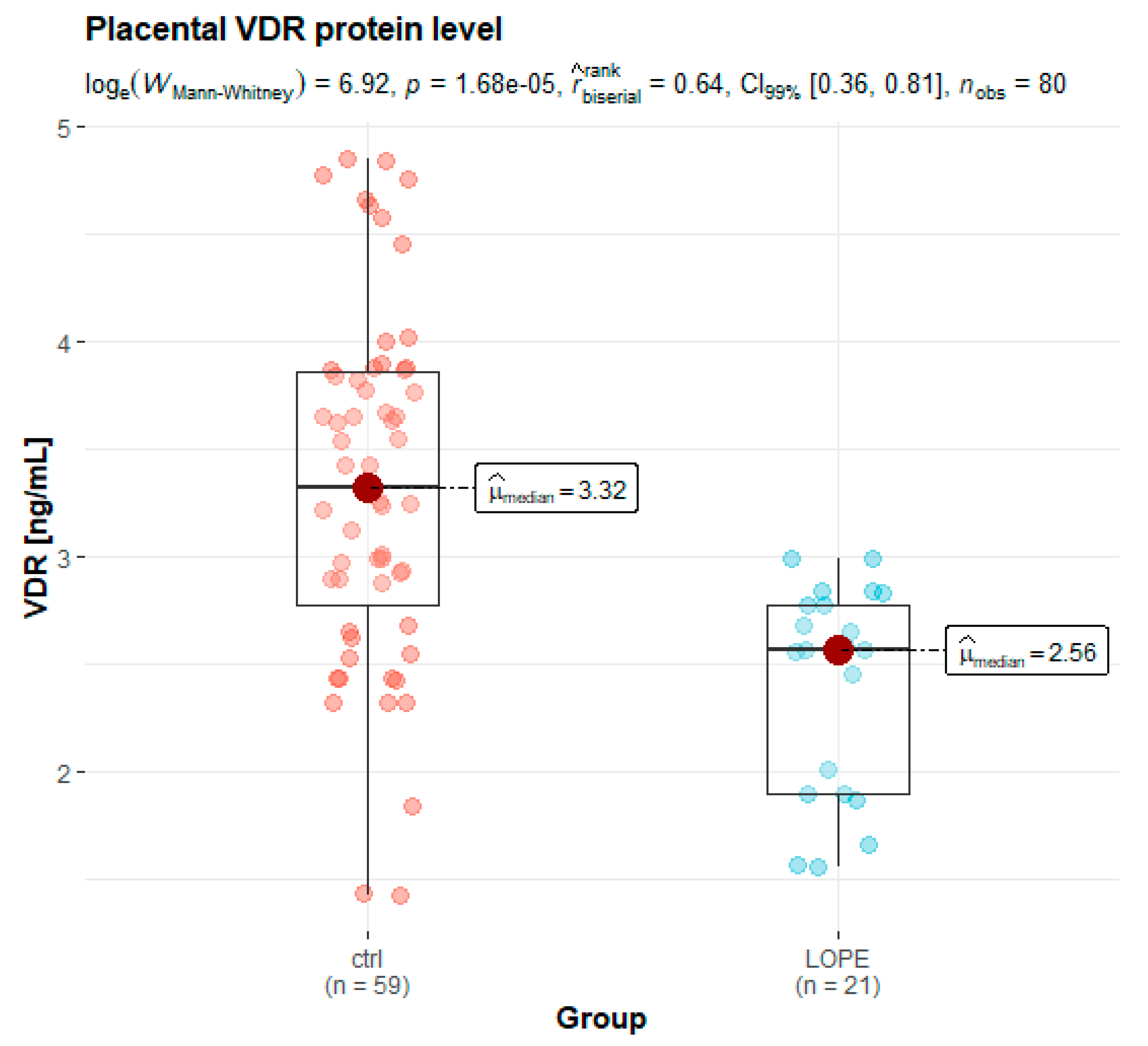
3.3. Placental Protein Expression
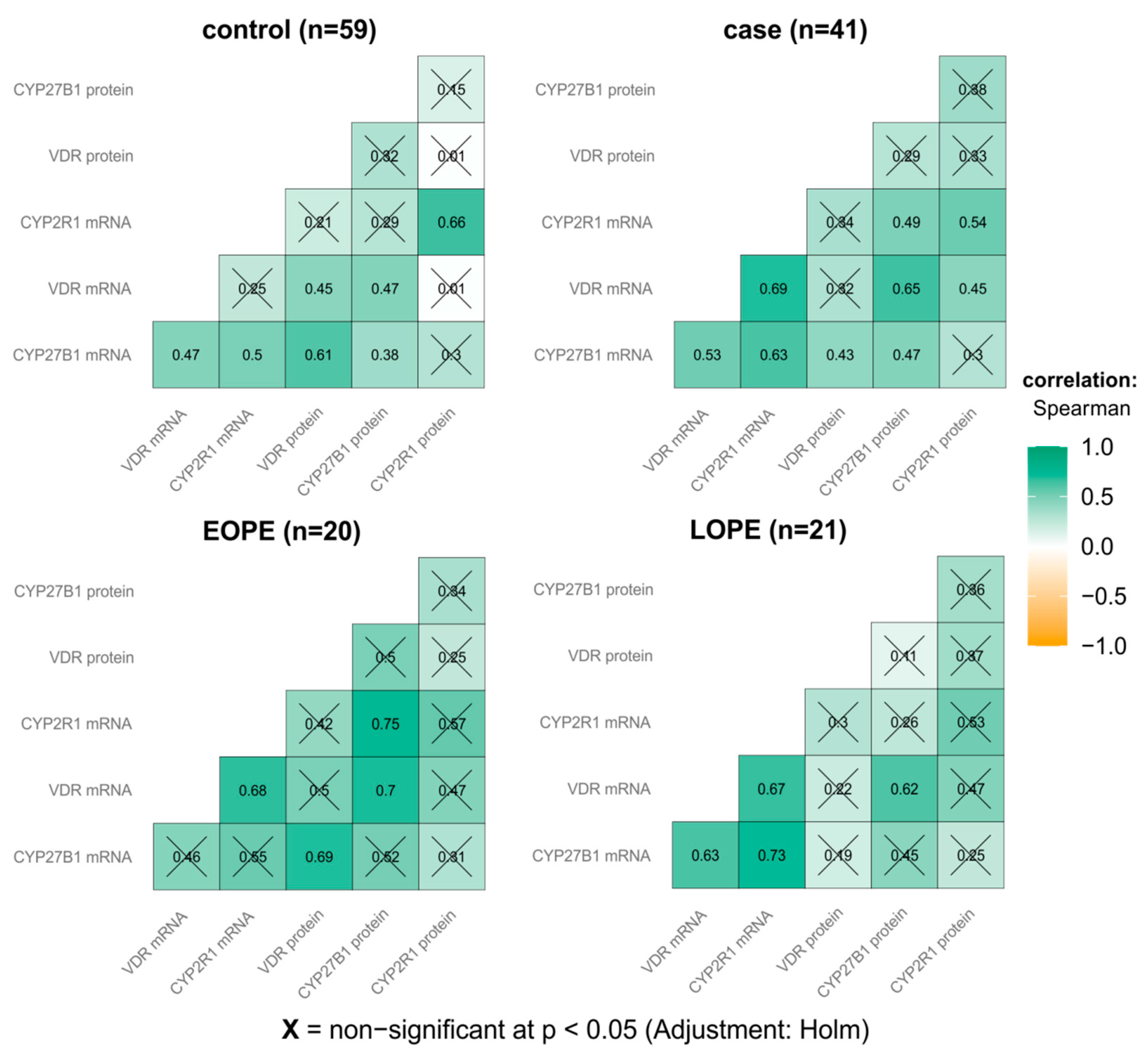
3.4. Correlations Analysis between Placental Gene/Protein Expression and Clinical Parameters
4. Discussion
4.1. VDR mRNA Expression
4.2. VDR Protein Level in Placentas
4.3. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Basso, O.; Rasmussen, S.; Weinberg, C.R.; Wilcox, A.J.; Irgens, L.M.; Skjaerven, R. Trends in fetal and infant survival following preeclampsia. JAMA 2006, 296, 1357–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odegård, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.A.; Austgulen, R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000, 96, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Metin-Gulmezoglu, A.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [Green Version]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]
- Akbari, S.; Khodadadi, B.; Ahmadi, S.A.Y.; Abbaszadeh, S.; Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis. Taiwan J. Obstet. Gynecol. 2018, 57, 241–247. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, 7, CD008873. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Amouzegar, A.; Azizi, F.; Ashrafivand, S.; Ahi, Z.; Saleh, M.; Mohaghegh, S.; Gargari, S.S. Prevalence of calcium and vitamin D deficiency and their association with feto-maternal outcomes in a sample of Iranian pregnant women. Hum. Antib. 2020, 28, 305–312. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynecologists. Vitamin D in Pregnancy; Scientific Impact Paper; Royal College of Obstetricians and Gynecologists: London, UK, 2014; pp. 1–11. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 495: Vitamin D: Screening and Supplementation during Pregnancy. Obstet. Gynecol. 2011, 118, 197–198. [Google Scholar] [CrossRef]
- Weisman, Y. Maternal, fetal and neonatal vitamin D and calcium metabolism during pregnancy and lactation. Vitam. Rickets. 2003, 6, 34–49. [Google Scholar] [CrossRef]
- Díaz, L.; Sánchez, I.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J. Clin. Endocrinol. Metab. 2000, 85, 2543–2549. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.Y.; Susarla, R.; Canovas, D.; Vasilopoulou, E.; Ohizua, O.; McCabe, C.J.; Hewison, M.; Kilby, M.D. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015, 36, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Barrera, D.; Avila, E.; Hernández, G.; Méndez, I.; González, L.; Halhali, A.; Larrea, F.; Morale, S.A.; Díaz, L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod. Biol. Endocrinol. 2008, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Stephanou, A.; Ross, R.; Handwerger, S. Regulation of human placental lactogen expression by 1,25-dihydroxyvitamin D3. Endocrinology 1994, 135, 2651–2656. [Google Scholar] [CrossRef]
- Barrera, D.; Avila, E.; Hernández, G.; Halhali, A.; Biruete, B.; Larrea, F.; Diaz, L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J. Steroid. Biochem. Mol. Biol. 2007, 103, 529–532. [Google Scholar] [CrossRef]
- Baker, A.R.; McDonnell, D.P.; Hughes, M.; Crisp, T.M.; Mangelsdorf, D.J.; Haussler, M.R.; Pike, J.W.; Shine, J.; O’Malley, B.W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 3294–3298. [Google Scholar] [CrossRef] [Green Version]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Norman, A.W. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal. 2009, 2, re4. [Google Scholar] [CrossRef]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women-Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Gynecol. Scand. 2021, 100, 480–488. [Google Scholar] [CrossRef]
- Yue, C.Y.; Gao, J.P.; Zhang, C.Y.; Ying, C.M. Is serum vitamin D deficiency before gestational 20 weeks a risk factor for preeclampsia? Clin. Nutr. 2021, 40, 4430–4435. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Magielda-Stola, J.; Drews, K.; Wolski, H.; Seremak-Mrozikiewicz, A. Vitamin D3 and its receptor in selected obstetrical complications. Ginekol. Pol. 2021, 92, 460–465. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. Early prediction of preeclampsia. Obstet. Gynecol. Int. 2014, 2014, 297397. [Google Scholar] [CrossRef] [Green Version]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef]
- McCurdy, R.D.; McGrath, J.J.; Mackay-Sim, A. Validation of the comparative quantification method of real-time PCR analysis and a cautionary tale of housekeeping gene selection. Gene Ther. Mol. Biol. 2008, 12, 15–24. [Google Scholar]
- Van der Meijden, K.; van Essen, H.W.; Bloemers, F.W.; Schulten, E.A.; Lips, P.; Bravenboer, N. Regulation of CYP27B1 mRNA expression in primary human osteoblasts. Calcif. Tissue Int. 2016, 99, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Meng, H.; Hou, J. Activity of 25-hydroxylase in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e52053. [Google Scholar] [CrossRef]
- Amatoril, S.; Persico, G.; Fanelli, M. Real-time quantitative PCR array to study drug-induced changes of gene expression in tumor cell lines. J. Cancer Metastasis Treat. 2017, 3, 90–99. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Res. Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, C.; Elisabetta, S.; Luigi, B.; Volha, Z.V.; Silvia, S.; Chiara, M.; Massimo, S.; Gianluca, A.; Annamaria, C.; Paolo, M. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Llewellyn, D.J.; Beauchet, O. Low serum vitamin D concentrations in Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2013, 33, 659–674. [Google Scholar] [CrossRef] [Green Version]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef] [Green Version]
- Knabl, J.; Vattai, A.; Ye, Y.; Jueckstock, J.; Hutter, S.; Kainer, F.; Mahner, S.; Jeschke, U. Role of placental VDR expression and function in common late pregnancy disorders. Int. J. Mol. Sci. 2017, 18, 2340. [Google Scholar] [CrossRef] [Green Version]
- Tamblyn, J.A.; Susarla, R.; Jenkinson, C.; Jeffery, L.E.; Ohizua, O.; Chun, R.F.; Chan, S.Y.; Kilby, M.D.; Hewison, M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017, 50, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Pospechova, K.; Rozehnal, V.; Stejskalova, L.; Vrzal, R.; Pospisilova, N.; Jamborova, G.; May, K.; Siegmund, W.; Dvorak, Z.; Nachtigal, P.; et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol. Cell Endocrinol. 2009, 299, 178–187. [Google Scholar] [CrossRef]
- Rebsamen, M.C.; Sun, J.; Norman, A.W.; Liao, J.K. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ. Res. 2002, 91, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.M.; Ralph, J.L.; Johnson, L.; Scheett, A.; Wright, M.L.; Taylor, J.Y.; Ohm, J.E.; Uthus, E. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci. 2015, 129, 10–15. [Google Scholar] [CrossRef]
- Xiao, J.P.; Lv, J.X.; Yin, Y.X.; Jiang, L.; Li, W.S.; Tao, T.; Liao, X.P.; Xu, Z.C. Lower maternal and fetal vitamin D status and higher placental and umbilical vitamin D receptor expression in preeclamptic pregnancies. Int. J. Clin. Exp. Pathol. 2017, 10, 10841–10851. [Google Scholar]
- Nguyen, T.P.; Yong, H.E.; Chollangi, T.; Borg, A.J.; Brennecke, S.P.; Murthi, P. Placental vitamin D receptor expression is decreased in human idiopathic fetal growth restriction. J. Mol. Med. 2015, 93, 795–805. [Google Scholar] [CrossRef]
- Nguyen, T.P.H.; Yong, H.E.J.; Chollangi, T.; Brennecke, S.P.; Fisher, S.J.; Wallace, E.M.; Ebeling, P.R.; Murthi, P. Altered downstream target gene expression of the placental Vitamin D receptor in human idiopathic fetal growth restriction. Cell Cycle 2018, 17, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Hutabarat, M.; Wibowo, N.; Obermayer-Pietsch, B.; Huppertz, B. Impact of vitamin D and vitamin D receptor on the trophoblast survival capacity in preeclampsia. PLoS ONE 2018, 13, e0206725. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, M.; Surma, S.; Więcek, A. Vitamin D and Arterial Hypertension: Facts and Myths. Curr. Hypertens. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef]
- Tamez, H.; Kalim, S.; Thadhani, R.I. Does vitamin D modulate blood pressure? Curr. Opin. Nephrol. Hypertens. 2013, 22, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Nema, J.; Sundrani, D.; Joshi, S. Prenatal vitamin D supplementation reduces blood pressure and improves placental angiogenesis in an animal model of preeclampsia. Food Funct. 2020, 11, 10413–10422. [Google Scholar] [CrossRef]
- Shahid, S.; Ladak, A.; Fatima, S.S.; Zaidi, F.A.; Farhat, S. Association of vitamin D levels with preeclampsia. J. Pak. Med. Assoc. 2020, 70, 2390–2393. [Google Scholar] [CrossRef]
- Zeng, S.; Cheng, X.; Chen, R.; Wu, J.; Zhou, J. Low level of vitamin D is a risk factor for the occurrence of early and late onset pre-eclampsia in pregnant women. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Forde, H.; Crowley, R.K.; McKenna, M.J.; Kilbane, M.T.; Conway, M.; McDonnell, C.M.; Twomey, P.J.; McAuliffe, F.M. No effect of calcium and vitamin D intake on maternal blood pressure in a healthy pregnant population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 8–14. [Google Scholar] [CrossRef]
- Budhwar, S.; Verma, P.; Verma, R.; Gupta, S.; Rai, S.; Rajender, S.; Singh, K. Altered cord serum 25-hydroxyvitamin D signaling and placental inflammation is associated with pre-term birth. Am. J. Reprod. Immunol. 2020, 83, e13201. [Google Scholar] [CrossRef]
- Dutra, L.V.; Affonso-Kaufman, F.A.; Cafeo, F.R.; Kassai, M.S.; Barbosa, C.P.; Santos Figueiredo, F.W.; Suano-Souza, F.I.; Bianco, B. Association between vitamin D plasma concentrations and VDR gene variants and the risk of premature birth. BMC Pregnancy Childbirth 2019, 20, 3. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Zmuda, J.M.; Cooper, M.E.; Parrott, M.S.; Roberts, J.M.; Marazita, M.L.; Simhan, H.N. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J. Nutr. 2010, 140, 999–1006. [Google Scholar] [CrossRef] [Green Version]
- Swamy, G.K.; Garrett, M.E.; Miranda, M.L.; Ashley-Koch, A.E. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am. J. Med. Genet. Part A 2011, 155A, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, Z.; Shakiba, M.; Rezavand, N.; Rahimi, Z.; Vaisi-Raygani, A.; Rahimi, Z.; Shakiba, E. Gene variants and haplotypes of Vitamin D biosynthesis, transport, and function in preeclampsia. Hypertens. Pregnancy 2021, 40, 1–8. [Google Scholar] [CrossRef]
- Rezavand, N.; Tabarok, S.; Rahimi, Z.; Vaisi-Raygani, A.; Mohammadi, E.; Rahimi, Z. The effect of VDR gene polymorphisms and vitamin D level on blood pressure, risk of preeclampsia, gestational age, and body mass index. J. Cell. Biochem. 2019, 120, 6441–6448. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse | Reference |
|---|---|---|---|
| VDR | GCCCACCATAAGACCTACGA | AGATTGGAGAAGCTGGACGA | [29] |
| CYP27B1 | TGGCCCAGATCCTAACACATTT | GTCCGGGTCTTGGGTCTAACT | [30] |
| CYP2R1 | TTGGAGGCATATCAACTGTGGT | CTCGGCCATATCTGGAATTGAG | [31] |
| GAPDH | GCAAATTCCATGGCACCGT | TCGCCCCACTTGATTTTGG | [32] |
| Characteristics | Preeclampsia n = 41 | Controls n = 59 | p |
|---|---|---|---|
| Maternal age (years) | 30.73 ± 5.00 | 30.98 ± 4.54 | 0.7988 |
| Gestational age (weeks) | 33.78 ± 3.24 | 39.02 ± 1.05 | <0.001 |
| Systolic blood pressure (mmHg) | 164.15 ± 18.39 | 107.50 ± 9.51 | <0.001 |
| Diastolic blood pressure (mmHg) | 105.49 ± 11.21 | 66.85 ± 7,27 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 25.16 ± 5.68 | 21.59 ± 3.52 | <0.001 |
| Post-pregnancy BMI (kg/m2) | 30.12 ± 5.08 | 26.87 ± 3.90 | <0.001 |
| Caesarean section n (%) | 41 (100.00) | 40 (67.80) | <0.001 * |
| Primipara n (%) | 24 (58.54) | 26 (44.07) | 0.2223 * |
| Infant birthweight (g) | 1948.61 ± 877.50 | 3476.55 ± 417.89 | < 0.001 |
| 1 min Apgar score | 9 (7–10) | 10 (10–10) | <0.001 # |
| 5 min Apgar score | 9 (8–10) | 10 (10–10) | <0.001 # |
| Placenta weight (g) | 385.31 ± 163.07 | 565.96 ± 103.94 | <0.001 |
| Urea (mg/dL) | 32.33 ± 12.25 | — | — |
| Uremic acid (mg/dL) | 16.67 ± 8.68 | — | — |
| Creatinine (mg/dL) | 0.77 ± 0.31 | — | — |
| Total protein (g/dL) | 5.42 ± 0.51 | — | — |
| Proteinuria (mg/dL) | 256.36 ± 206.14 | — | — |
| Proteinuria (g/24 h) | 4.21 ± 3.50 | — | — |
| ALT (IU/L) | 36.69 ± 46.84 | — | — |
| AST (IU/L) | 43.95 ± 54.98 | — | — |
| Early-onset PE n (%) | 20 (48.78) | — | — |
| Late-onset PE n (%) | 21 (51.22) | — | — |
| Gene | Preeclampsia n = 41 | Controls n = 59 | p |
|---|---|---|---|
| VDR/GAPDH | 1.15 (1.04–1.25) | 1.20 (1.15–1.28) | 0.030 * |
| CYP27B1/GAPDH | 1.17 (1.07–1.23) | 1.05 (0.97–1.15) | 0.006 * |
| CYP2R1/GAPDH | 2.01 (1.83–2.12) | 1.89 (1.65–2.02) | 0.039 * |
| Protein (ng/mL) | Preeclampsia n = 41 | Controls n = 59 | p |
|---|---|---|---|
| VDR | 2.56 (1.90–2.77) | 3.32 (2.78–3.86) | <0.001 * |
| CYP27B1 | 5.32 (4.68–5. 68) | 5.23 (4.67–5.81) | 0.530 * |
| CYP2R1 | 1.32 (0.98–1.45) | 1.43 (1.22–1.65) | 0.019 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magiełda-Stola, J.; Kurzawińska, G.; Ożarowski, M.; Bogacz, A.; Wolski, H.; Drews, K.; Karpiński, T.M.; Wolek, M.; Seremak-Mrozikiewicz, A. Placental mRNA and Protein Expression of VDR, CYP27B1 and CYP2R1 Genes Related to Vitamin D Metabolism in Preeclamptic Women. Appl. Sci. 2021, 11, 11880. https://doi.org/10.3390/app112411880
Magiełda-Stola J, Kurzawińska G, Ożarowski M, Bogacz A, Wolski H, Drews K, Karpiński TM, Wolek M, Seremak-Mrozikiewicz A. Placental mRNA and Protein Expression of VDR, CYP27B1 and CYP2R1 Genes Related to Vitamin D Metabolism in Preeclamptic Women. Applied Sciences. 2021; 11(24):11880. https://doi.org/10.3390/app112411880
Chicago/Turabian StyleMagiełda-Stola, Justyna, Grażyna Kurzawińska, Marcin Ożarowski, Anna Bogacz, Hubert Wolski, Krzysztof Drews, Tomasz M. Karpiński, Marlena Wolek, and Agnieszka Seremak-Mrozikiewicz. 2021. "Placental mRNA and Protein Expression of VDR, CYP27B1 and CYP2R1 Genes Related to Vitamin D Metabolism in Preeclamptic Women" Applied Sciences 11, no. 24: 11880. https://doi.org/10.3390/app112411880







