Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Gelatin Sponge
2.2. Thermogravimetric and Differential Thermal Analysis
2.3. Compression Test
2.4. Scanning Electron Microscopy, X-ray Diffraction, and Fourier Transform Infrared Spectroscopy Analysis
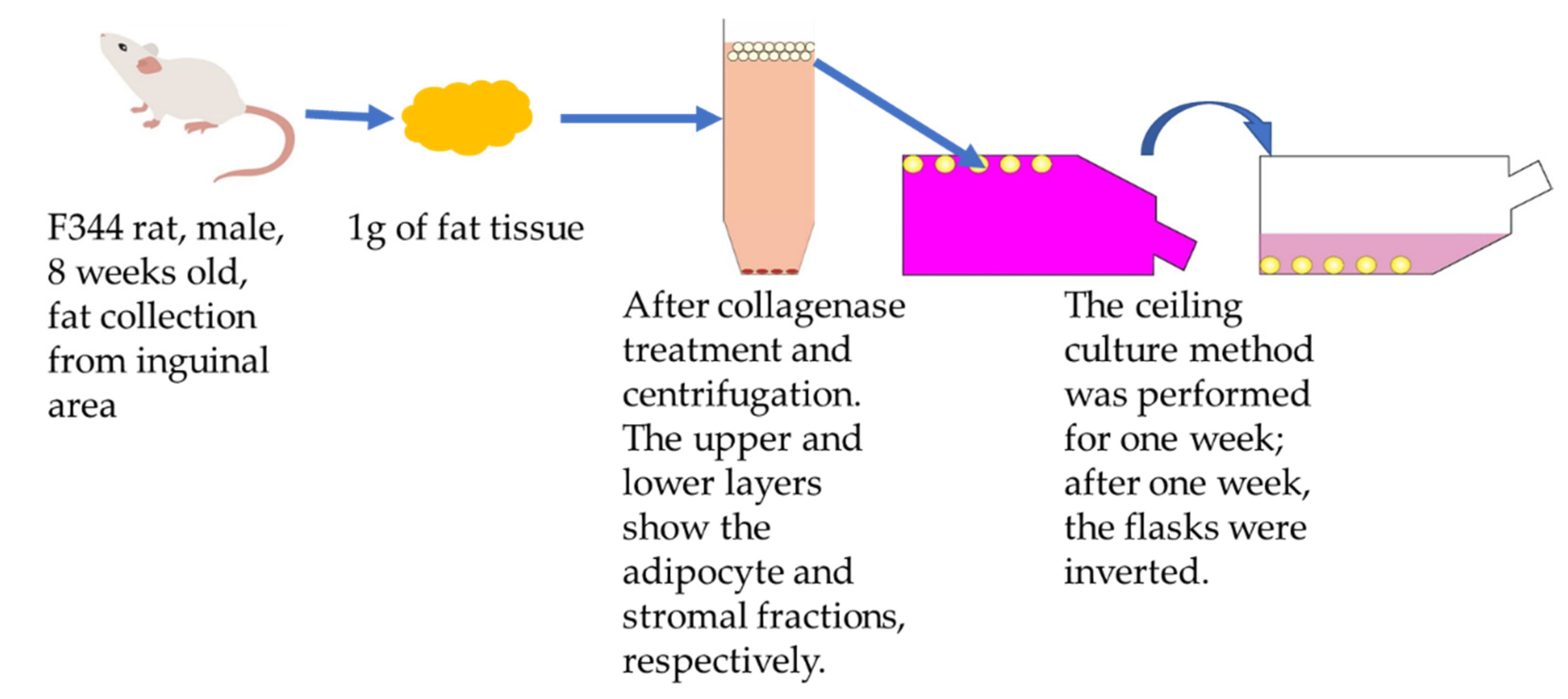
2.5. Fatty Tissue Collection
2.6. Isolation and Culture of Rat DFATs
2.7. Cell Seeding into the Scaffold and Scanning Electron Microscopy Analysis and DAPI Staining
2.8. Surgery
2.9. Bone Morphometric Analysis
2.10. Histological Assessment
2.11. Statistical Analysis
3. Results
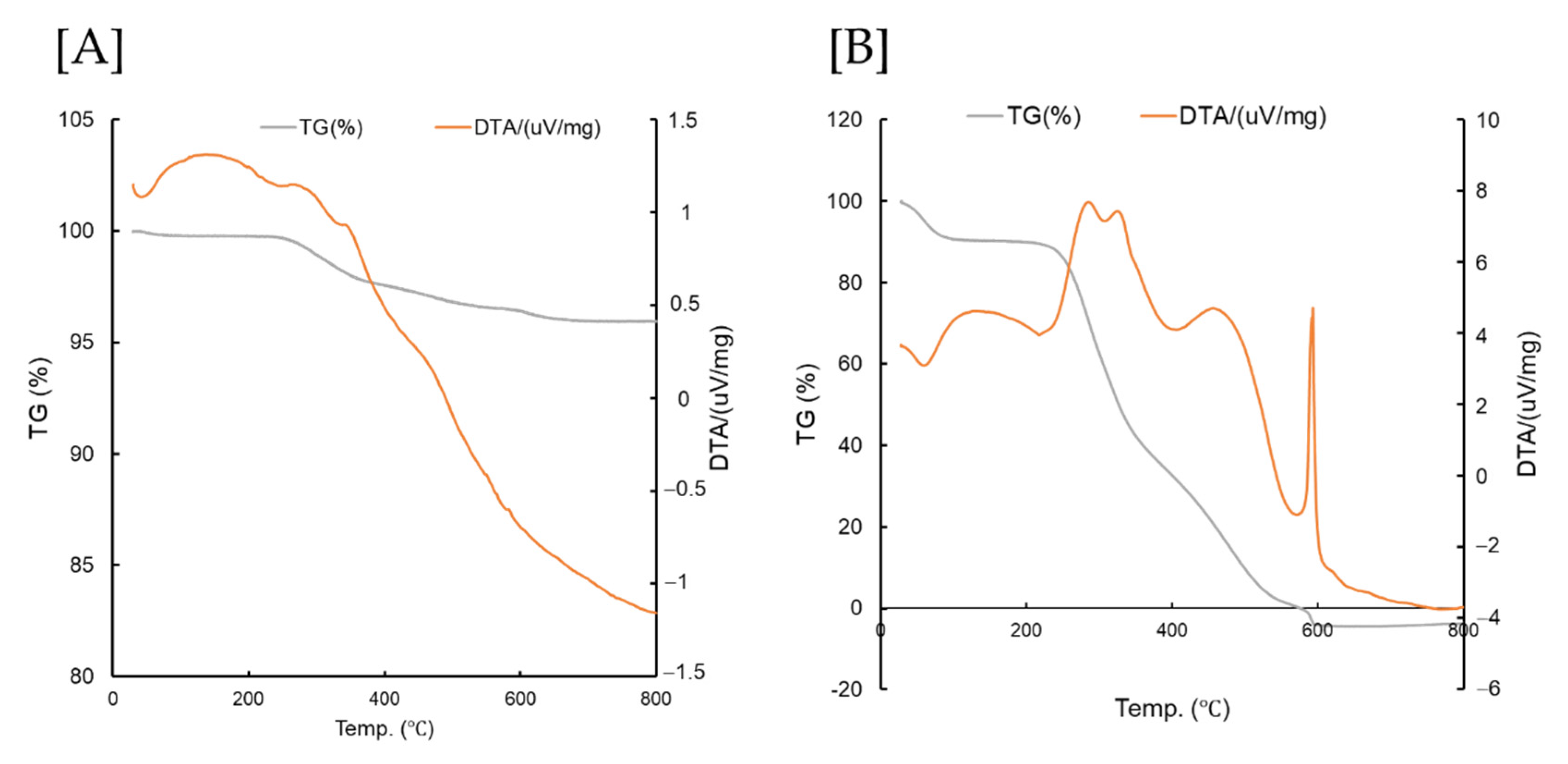
3.1. TG/DTA
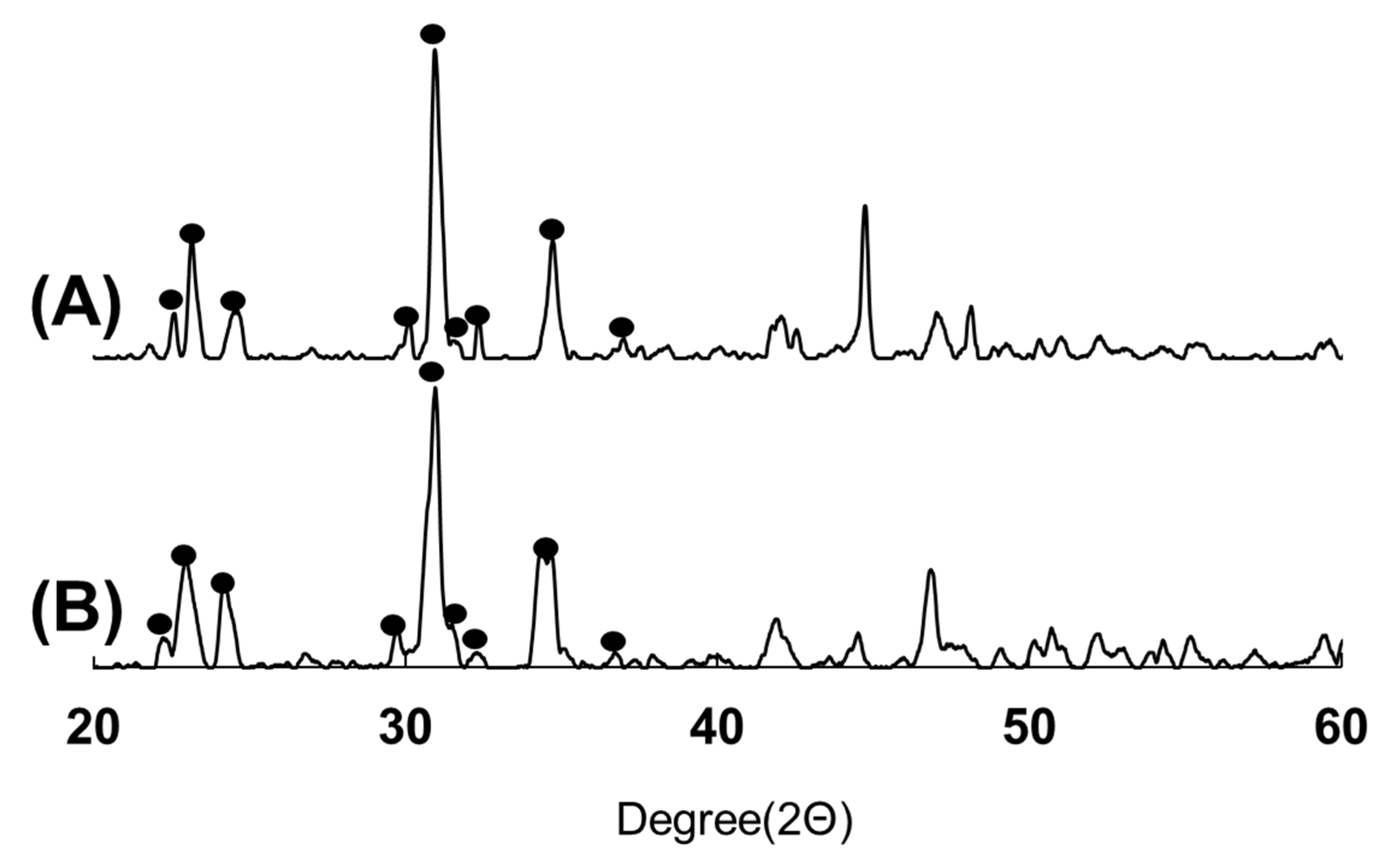
3.2. XRD
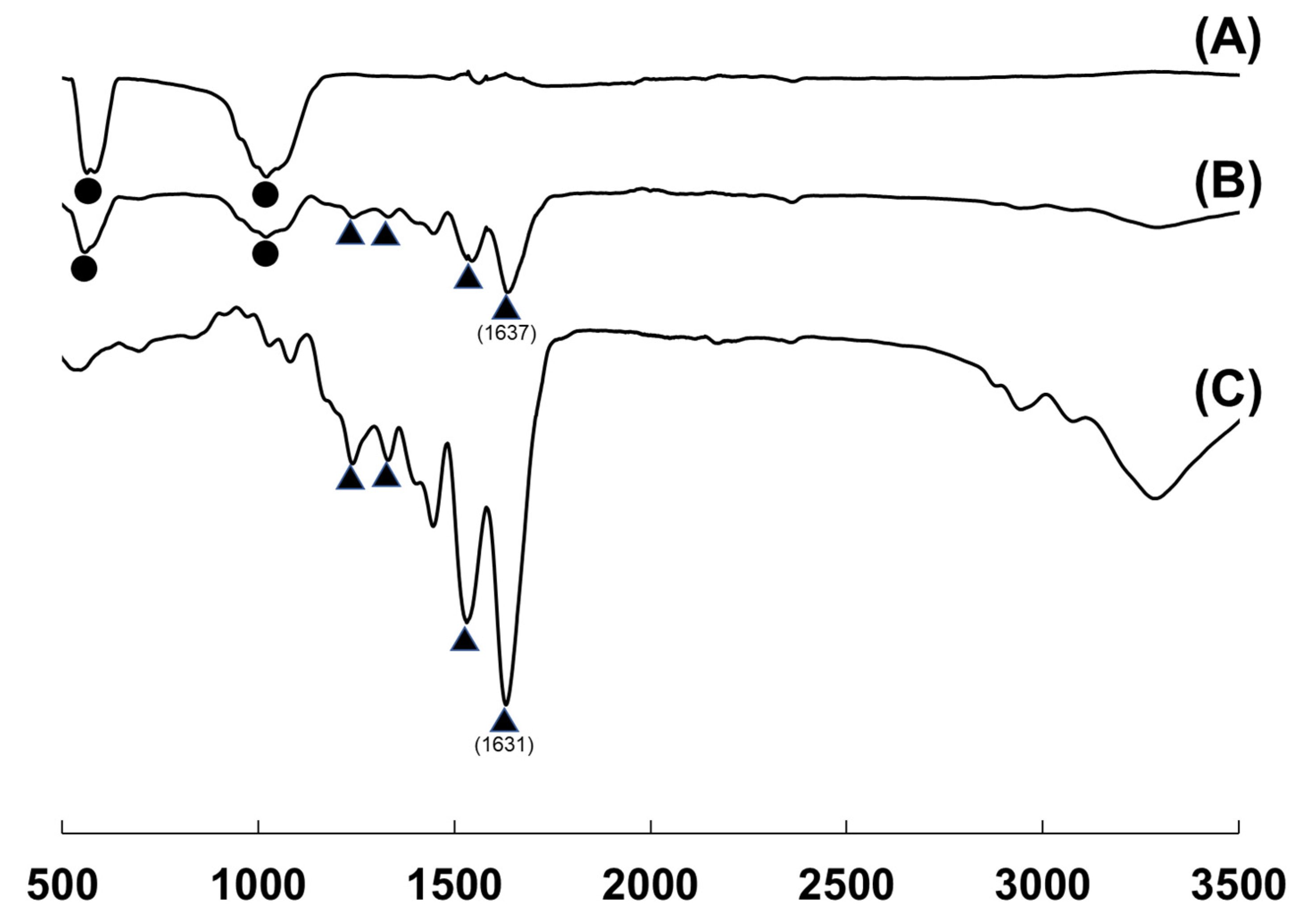
3.3. FTIR
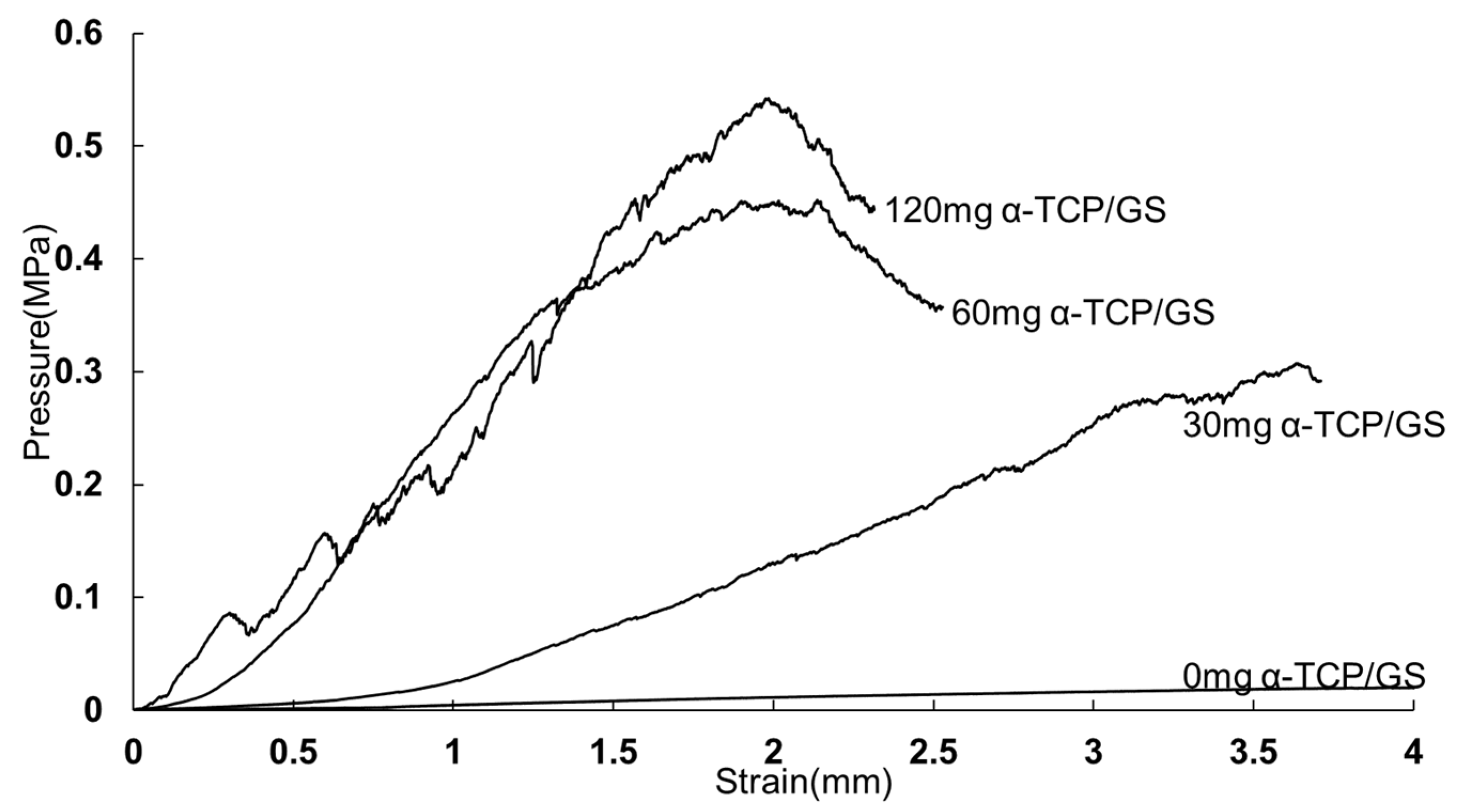
3.4. Compression Test
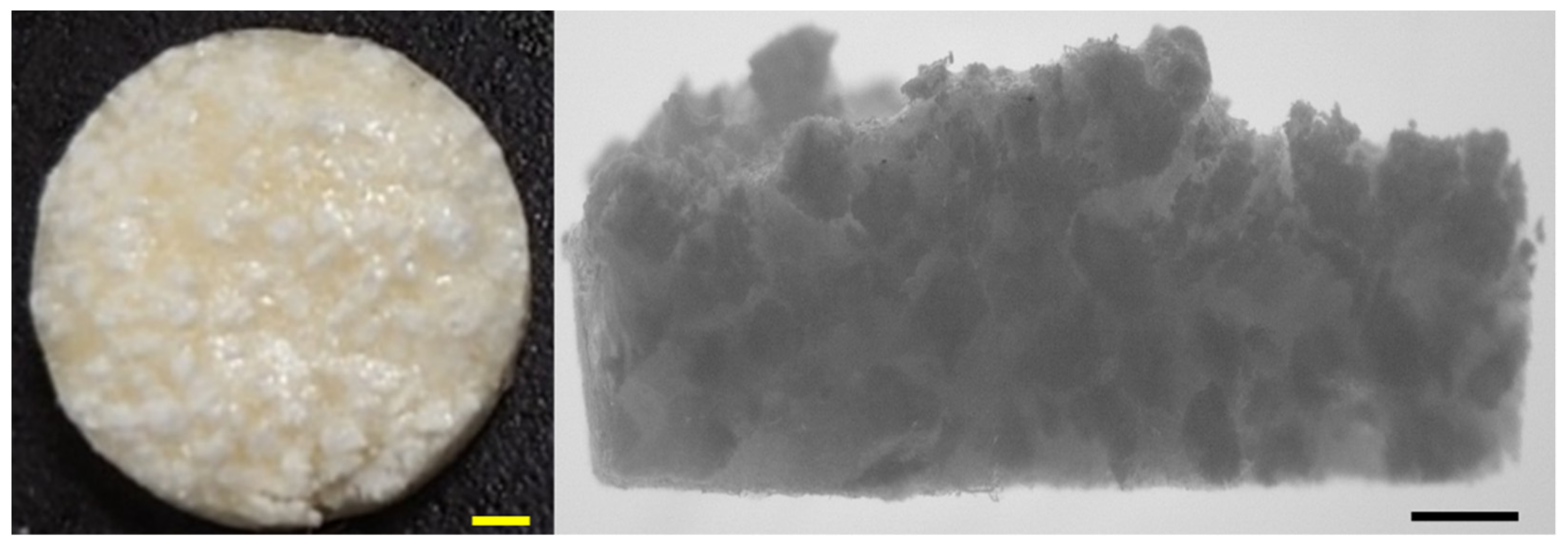
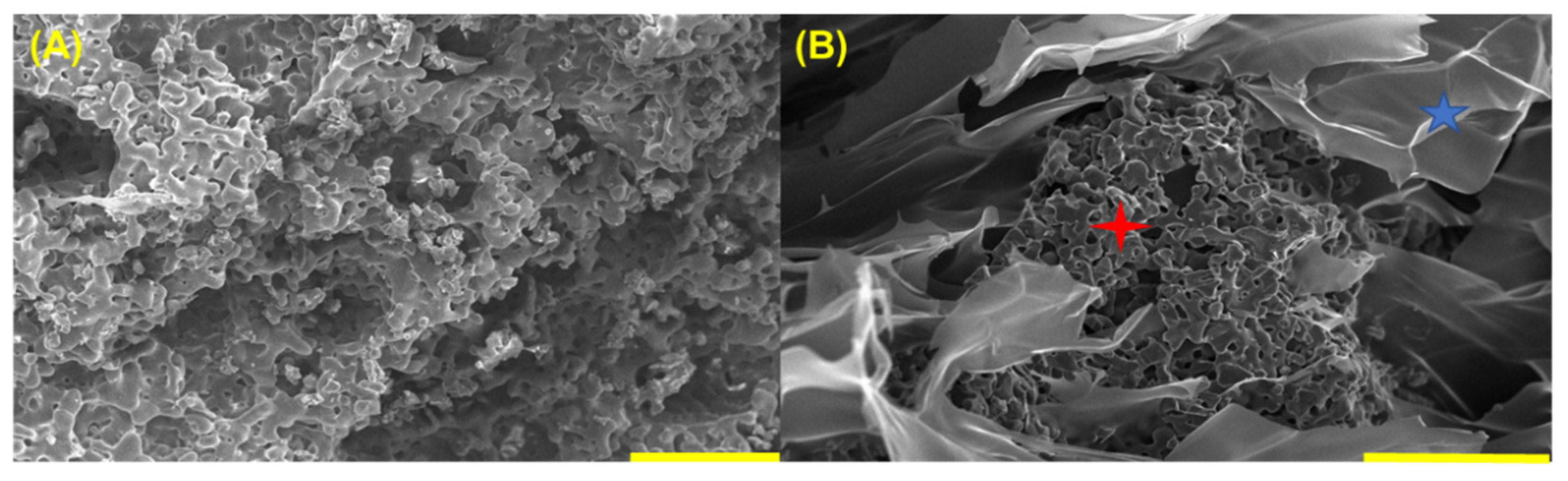
3.5. SEM of Synthesized Material
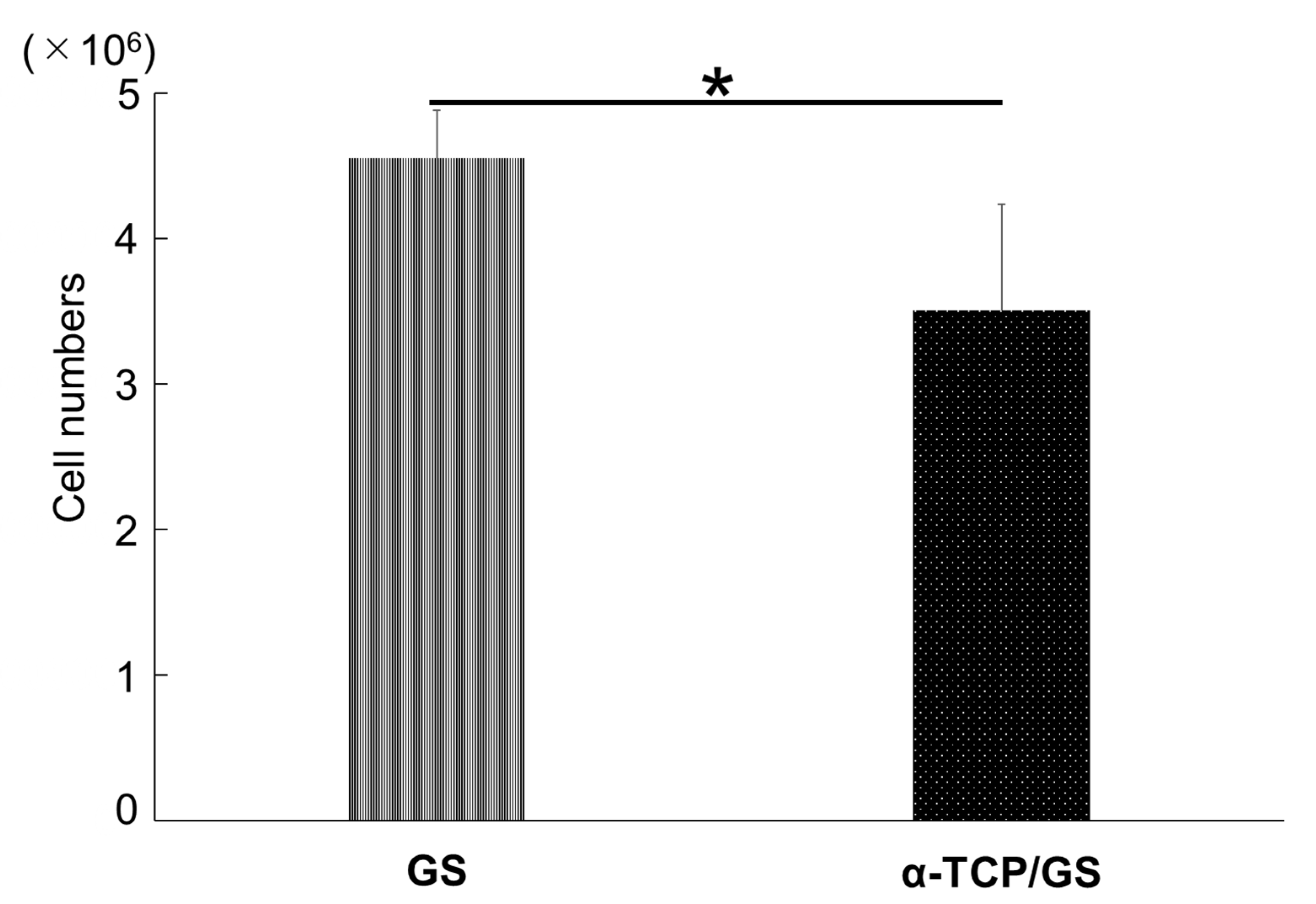
3.6. The Cell Numbers of GS and α-TCP/GS 24 h after DFAT Seeding
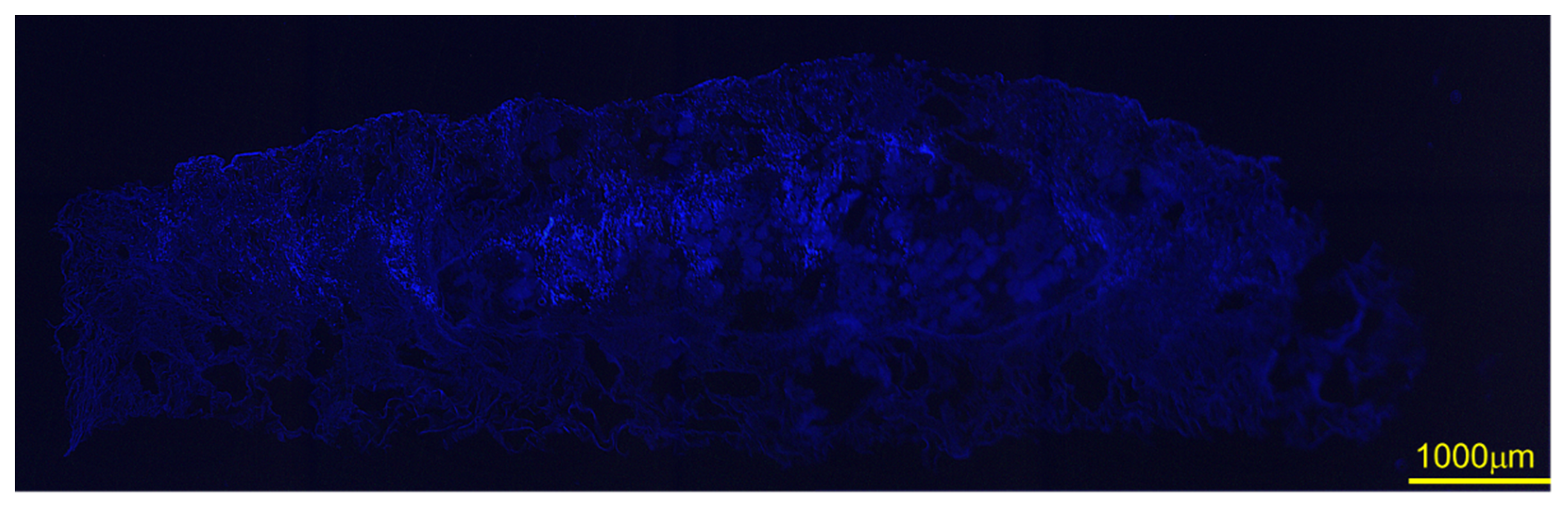
3.7. SEM after DFAT Seeding and DAPI Staining
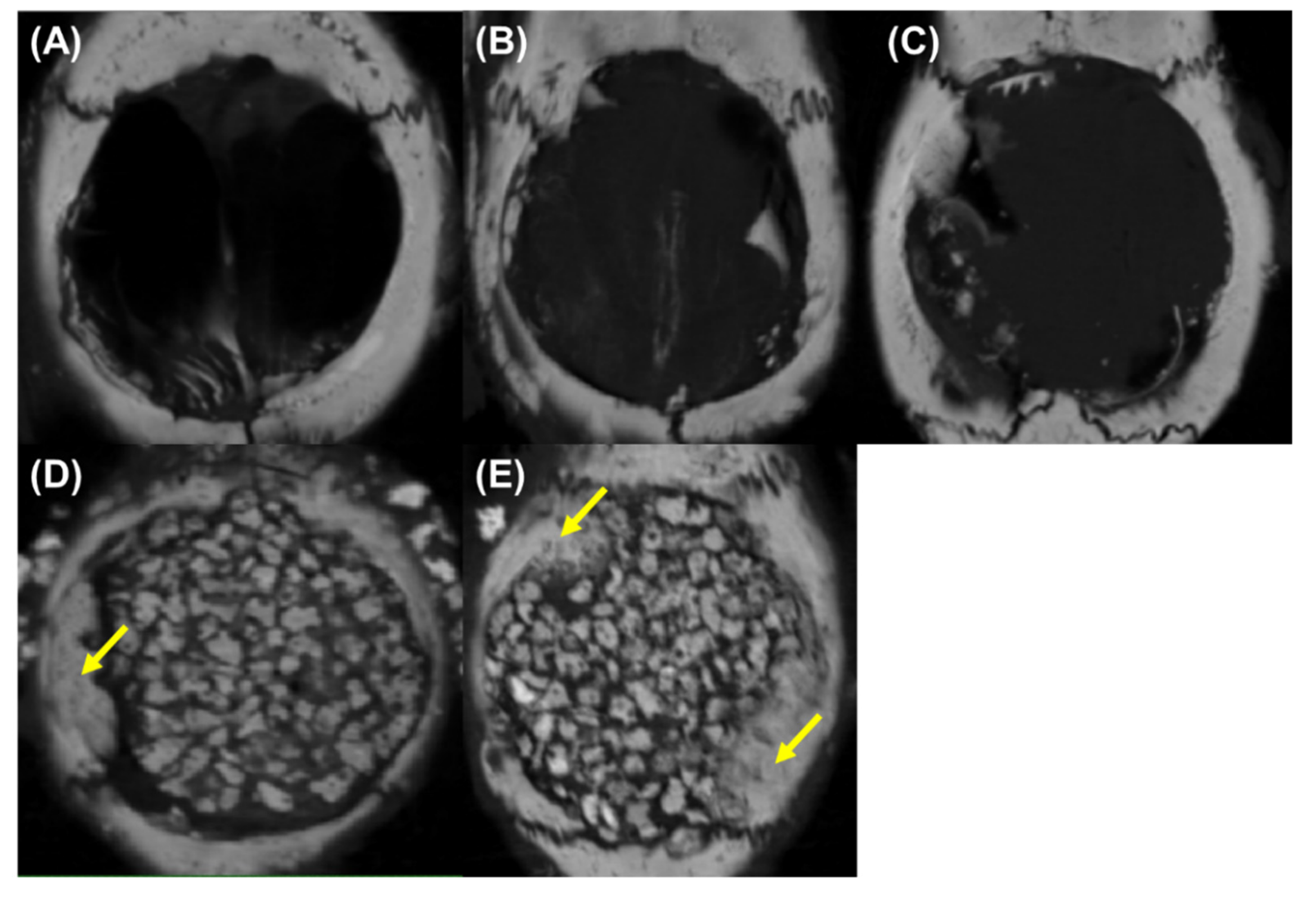
3.8. Bone Morphometric Analysis
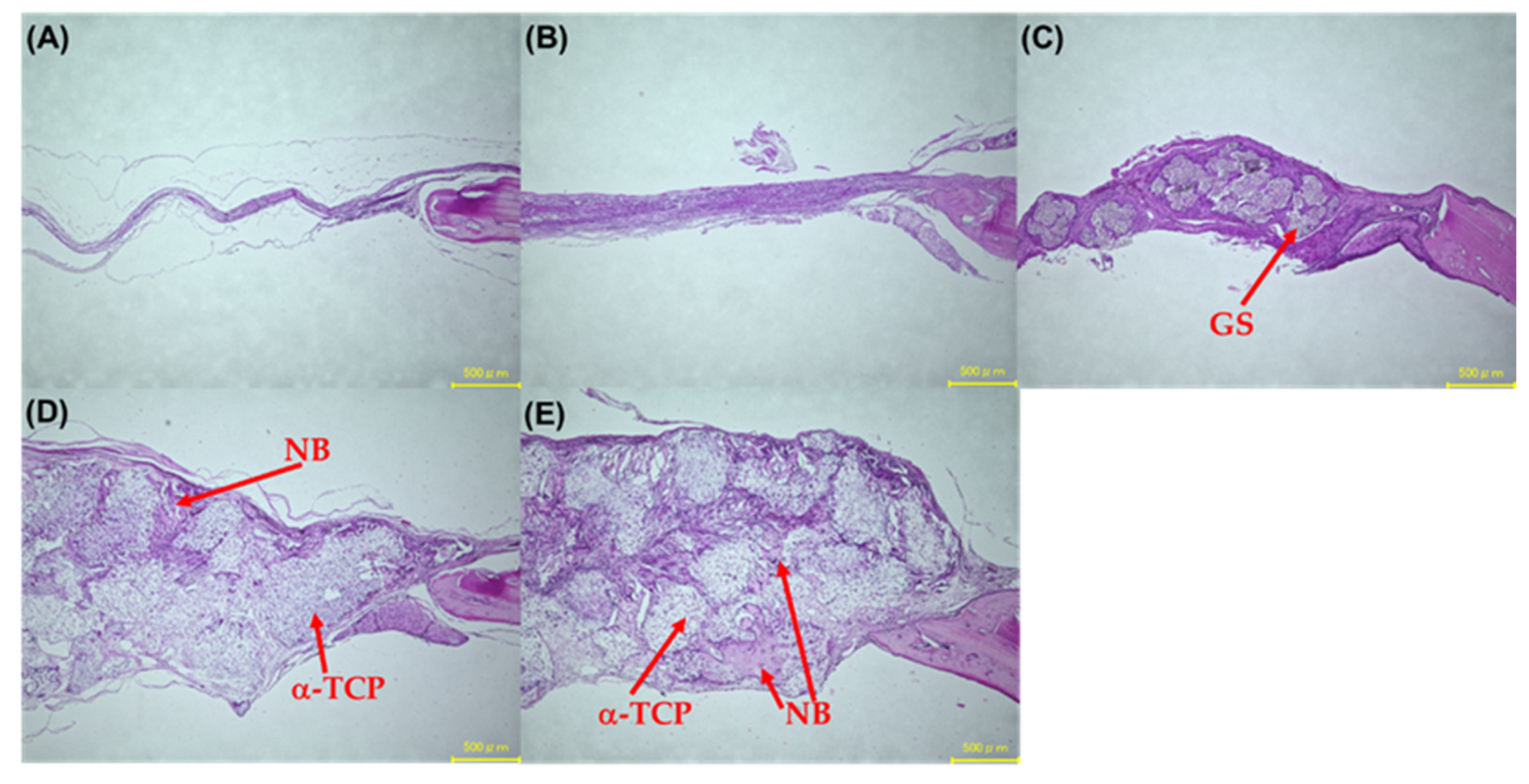
3.9. Hematoxylin and Eosin Stain
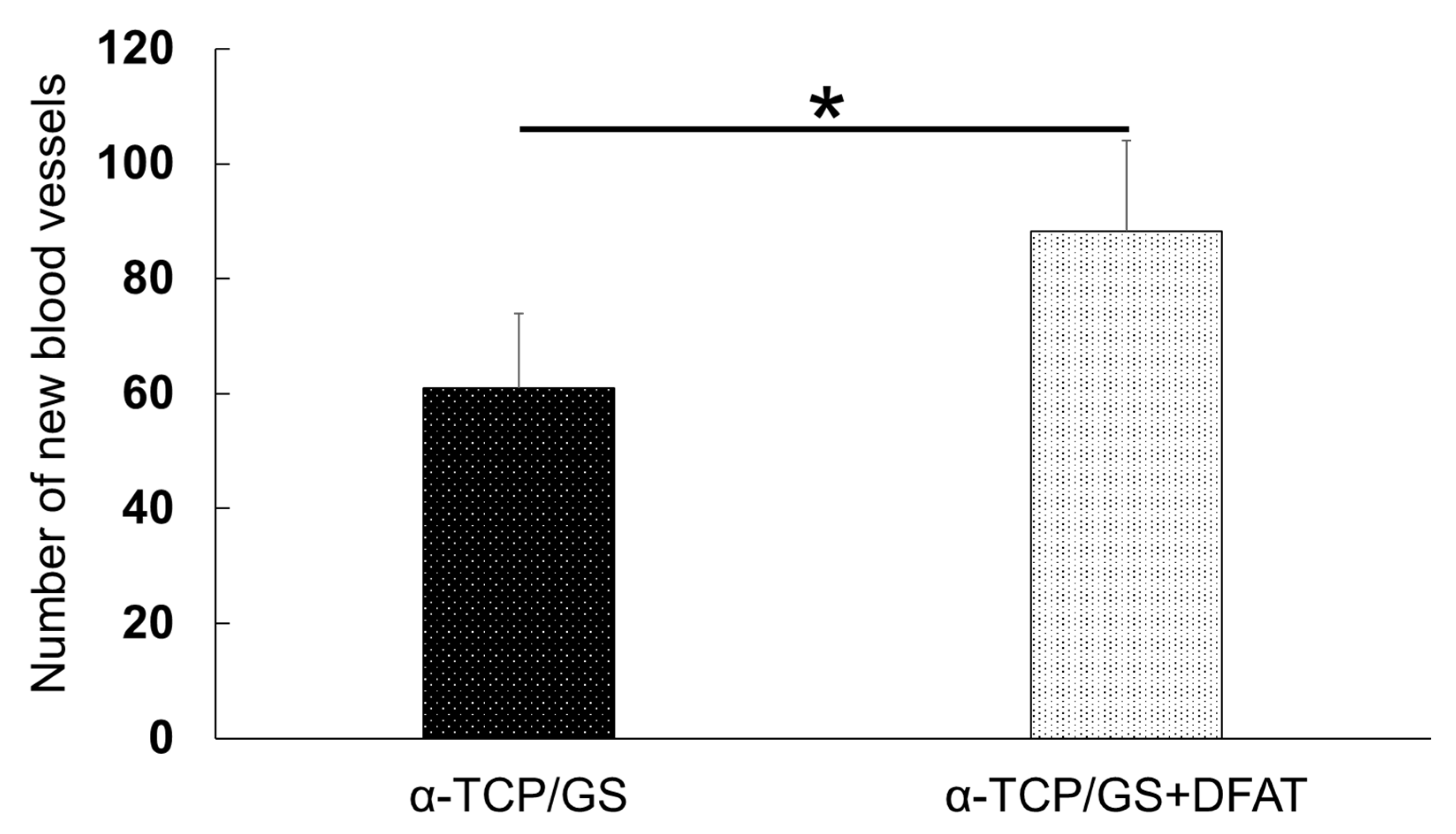
3.10. Immunohistochemical Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, M.; Polley, J.W.; Figueroa, A.A. Secondary (intermediate) alveolar bone grafting. Clin. Plast. Surg. 1993, 20, 691–705. [Google Scholar] [CrossRef]
- Ito, T.; Hashimoto, Y.; Baba, S.; Iseki, T.; Morita, S. Bone regeneration with a collagen model polypeptides/α-tricalcium phosphate sponge in a canine tibia defect model. Implant Dent. 2015, 24, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Inoue, T.; Hashimoto, Y.; Kimura, D.; Ueda, M.; Sakai, K.; Matsumoto, N.; Hiwa, C.; Adachi, T.; Hojo, M. Effectiveness of scaffolds with pre-seeded mesenchymal stem cells in bone regeneration—assessment of osteogenic ability of scaffolds implanted under the periosteum of the cranial bone of rats. Dent. Mater. J. 2010, 29, 673–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, T.; Nakamura, T.; Kuremoto, K.I.; Notazawa, S.; Nakahara, T.; Hashimoto, Y.; Satoh, T.; Shimizu, Y. Development of β-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent. Mater. J. 2006, 25, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Hashimoto, Y.; Baba, S.; Nishiura, A.; Matsumoto, N. Effects on bone regeneration when collagen model polypeptides are combined with various sizes of alpha-tricalcium phosphate particles. Dent. Mater. J. 2011, 30, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Kroschwitz, J.I. Encyclopedia of Polymer Science and Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Liu, X.; Smith, L.A.; Hu, J.; Ma, P.X. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 2009, 30, 2252–2258. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Adachi, S.; Matsuno, T.; Omata, K.; Yoshitaka, Y.; Ozeki, Y.; Umezu, Y.; Satoh, T.; Nakamura, M. Effect of an injectable 3D scaffold for osteoblast differentiation depends on bead size. Biomed. Mater. Eng. 2009, 19, 391–400. [Google Scholar] [CrossRef]
- Matsuno, T.; Hashimoto, Y.; Adachi, S.; Ozeki, Y.; Umezu, Y.; Tabata, Y.; Nakamura, M.; Yamaguchi, Y.; Satoh, T. Preparation of injectable 3D-formed β-tricalcium phosphate beadalginate composite for bone tissue engineering. Dent. Mater. J. 2008, 26, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Handa, T.; Anada, T.; Honda, Y.; Yamazaki, H.; Kobayashi, K.; Kanda, N.; Kamakura, S.; Echigo, S.; Suzuki, O. The effect of an octacalcium phosphate co-precipitated gelatin composite on the repair of critical-sized rat calvarial defects. Acta Biomater. 2012, 8, 1190–1200. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaida, K.; Uemura, N.; Kimura, D.; Matsuse, K.; Ueda, M.; Hirose, M.; Toda, I.; Komuro, A.; Kawaue, Y.; et al. Bone Regeneration with a Collagen Model Polypeptideporous Alpha-tricalcium Phosphate Sponge. J. Oral Tissue Engin 2016, 14, 41–50. [Google Scholar]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Dhar, S.; Chun, D.E.; Gharibjanian, N.A.; Evans, G.R. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007, 13, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Kondo, D.; Okazaki, Y.; Kano, K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem. Biophys. Res. Commun. 2004, 321, 967–974. [Google Scholar] [CrossRef]
- Kishimoto, N.; Momota, Y.; Hashimoto, Y.; Tatsumi, S.; Ando, K.; Omasa, A.; Kotani, J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin. Oral Investig. 2014, 18, 1893–1901. [Google Scholar] [CrossRef] [Green Version]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Kubo, H.; Nakajima, M.; Honda, Y.; Hashimoto, Y. Bone Regeneration Using Rat-Derived Dedifferentiated Fat Cells Combined with Activated Platelet-Rich Plasma. Materials 2020, 13, 5097. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Salih, V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef]
- Sharifi, E.; Ebrahimi-Barough, S.; Panahi, M.; Azami, M.; Ai, A.; Barabadi, Z.; Kajbafzadeh, A.M.; Ai, J. In vitro evaluation of human endometrial stem cell-derived osteoblast-like cells’ behavior on gelatin/collagen/bioglass nanofibers’ scaffolds. J. Biomed. Mater Res. A 2016, 104, 2210–2219. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, A.S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, P.; öztürk Er, E.; Bakırdere, S.; ülgen, K.; özbek, B. Application of supercritical gel drying method on fabrication of mechanically improved and biologically safe three-component scaffold composed of graphene oxide/chitosan/hydroxyapatite and characterization studies. J. Mater. Res. Technol. 2019, 8, 5201–5216. [Google Scholar] [CrossRef]
- Kitamura, M.; Ohtsuki, C.; Ogata, S.-I.; Kamitakahara, M.; Tanihara, M.; Miyazaki, T. Mesoporous Calcium Phosphate Via Post-Treatment of alpha-TCP. J. Am. Ceram. Soc. 2005, 88, 822–826. [Google Scholar] [CrossRef]
- Jeong, J.E.; Park, S.Y.; Shin, J.Y.; Seok, J.M.; Byun, J.H.; Oh, S.H.; Kim, W.D.; Lee, J.H.; Park, W.H.; Park, S.A. 3D Printing of Bone-Mimetic Scaffold Composed of Gelatin/beta-Tri-Calcium Phosphate for Bone Tissue Engineering. Macromol. Biosci. 2020, 20, e2000256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Yin, Y.; Yao, F.; Yao, K. Modulation of nano-hydroxyapatite size via formation on chitosan-gelatin network film in situ. Biomaterials 2007, 28, 781–790. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Tognana, E.; Penick, K.; Baskaran, H.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. A Rapid Seeding Technique for the Assembly of Large CellScaffold Composite Constructs. Tissue Eng. 2006, 12, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Asou, Y.; Sekiya, I.; Sotome, S.; Orii, H.; Shinomiya, K. Enhancement of tissue engineered bone formation by a low pressure system improving cell seeding and medium perfusion into a porous scaffold. Biomaterials 2006, 27, 2738–2746. [Google Scholar] [CrossRef]
- Yanagi, T.; Kajiya, H.; Fujisaki, S.; Maeshiba, M.; Yanagi, A.; Yamamoto, N.; Kakura, K.; Kido, H.; Ohno, J. Three-dimensional spheroids of dedifferentiated fat cells enhance bone regeneration. Regen. Ther. 2021, 18, 472–479. [Google Scholar] [CrossRef]
- Watanabe, H.; Goto, S.; Kato, R.; Komiyama, S.; Nagaoka, Y.; Kazama, T.; Yamamoto, C.; Li, Y.; Konuma, N.; Hagikura, K.; et al. The neovascularization effect of dedifferentiated fat cells. Sci. Rep. 2020, 10, 9211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.G.; Lee, S.W.; Oh, B.J.; Kim, J.H.; Kim, J.A.; Lee, G.; Jang, J.D.; Joe, Y.A. A Subset of Paracrine Factors as Efficient Biomarkers for Predicting Vascular Regenerative Efficacy of Mesenchymal Stromal/Stem Cells. Stem Cells 2019, 37, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsumano, N.; Kubo, H.; Imataki, R.; Honda, Y.; Hashimoto, Y.; Nakajima, M. Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model. Appl. Sci. 2021, 11, 11941. https://doi.org/10.3390/app112411941
Tsumano N, Kubo H, Imataki R, Honda Y, Hashimoto Y, Nakajima M. Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model. Applied Sciences. 2021; 11(24):11941. https://doi.org/10.3390/app112411941
Chicago/Turabian StyleTsumano, Nobuhito, Hirohito Kubo, Rie Imataki, Yoshitomo Honda, Yoshiya Hashimoto, and Masahiro Nakajima. 2021. "Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model" Applied Sciences 11, no. 24: 11941. https://doi.org/10.3390/app112411941
APA StyleTsumano, N., Kubo, H., Imataki, R., Honda, Y., Hashimoto, Y., & Nakajima, M. (2021). Bone Regeneration by Dedifferentiated Fat Cells Using Composite Sponge of Alfa-Tricalcium Phosphate and Gelatin in a Rat Calvarial Defect Model. Applied Sciences, 11(24), 11941. https://doi.org/10.3390/app112411941





