A New Low-Energy Proton Irradiation Facility to Unveil the Mechanistic Basis of the Proton-Boron Capture Therapy Approach
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiation Biophysics Beamline Setup and Experimental Procedures
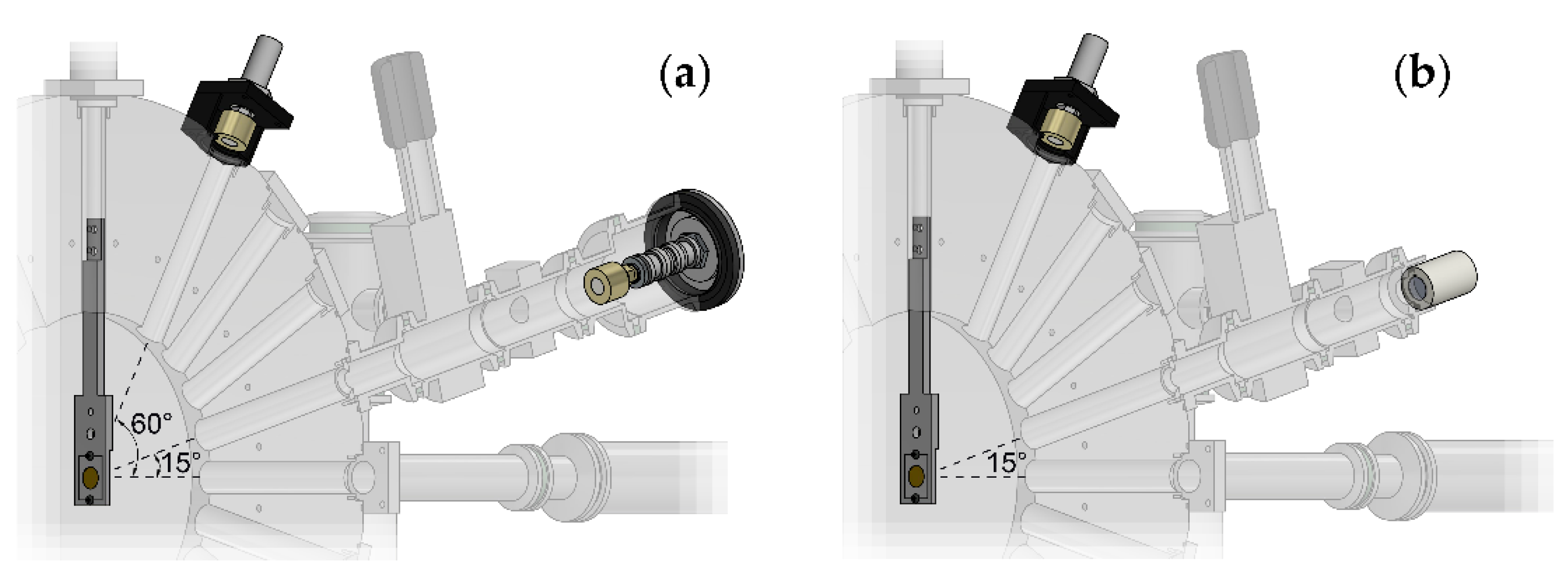
2.1.1. Scattering Chamber
- A disk-shaped scattering chamber, in whose centre it is possible to mount a specially designed aluminium target-holder, provided with two beam collimators (3 and 5 mm diameter) and a frame-holder on which there is a beam scattering Au foil (10 mm diameter, 1 μm thickness, Goodfellow Cambridge Ltd., London, UK). The target holder can be moved in the vertical direction to remove it from the beam path. From the centre of the chamber depart several radial channels, by 15° steps in the forward direction, and some backwards channels.
- An SSBD (Si BU-011-05-300, useful area 0.50 cm2, 300 μm of minimum depletion depth, Ametek Ortec, Oak Ridge, TN, USA) for the beam monitoring, placed at the end of the forward channel at 60°.
- A channel, placed at the end of the forward channel at 15°, equipped with a gate valve and ending with a window (13 mm diameter); next to the window, it is possible to mount, alternatively, a second SSBD, identical to the one described above, or a CR39 SSTD (13 mm diameter and 0.5 mm thickness, Mi.AM Srl, Piacenza, Italy) for beam monitoring and flux calibration, respectively. During the irradiation of the biological samples, the detectors were replaced by the sample holder assembly (see Section 2.1.2); hence, in what follows, we shall refer to this channel as cell channel. A knife-edge collimator (Figure 1) is mounted on the outlet of the scattering chamber, (8 mm internal diameter, 12 mm external diameter) to collimate the scattered beam on the solid angle intercepted by the cell sample or by the detectors placed at the bottom of the channel.
- A Faraday Cup (FC), placed at the end of the 0° forward channel, which allows to measure the current intensity of the beam.
2.1.2. Sample Holder Assembly
2.2. Irradiation System and Dosimetry
2.3. Biological Sample Preparation
2.3.1. Cell Culture
2.3.2. Boron Carrier
2.4. Measurement of pB-Mediated Enhancement of Proton Biological Effectiveness
3. Results
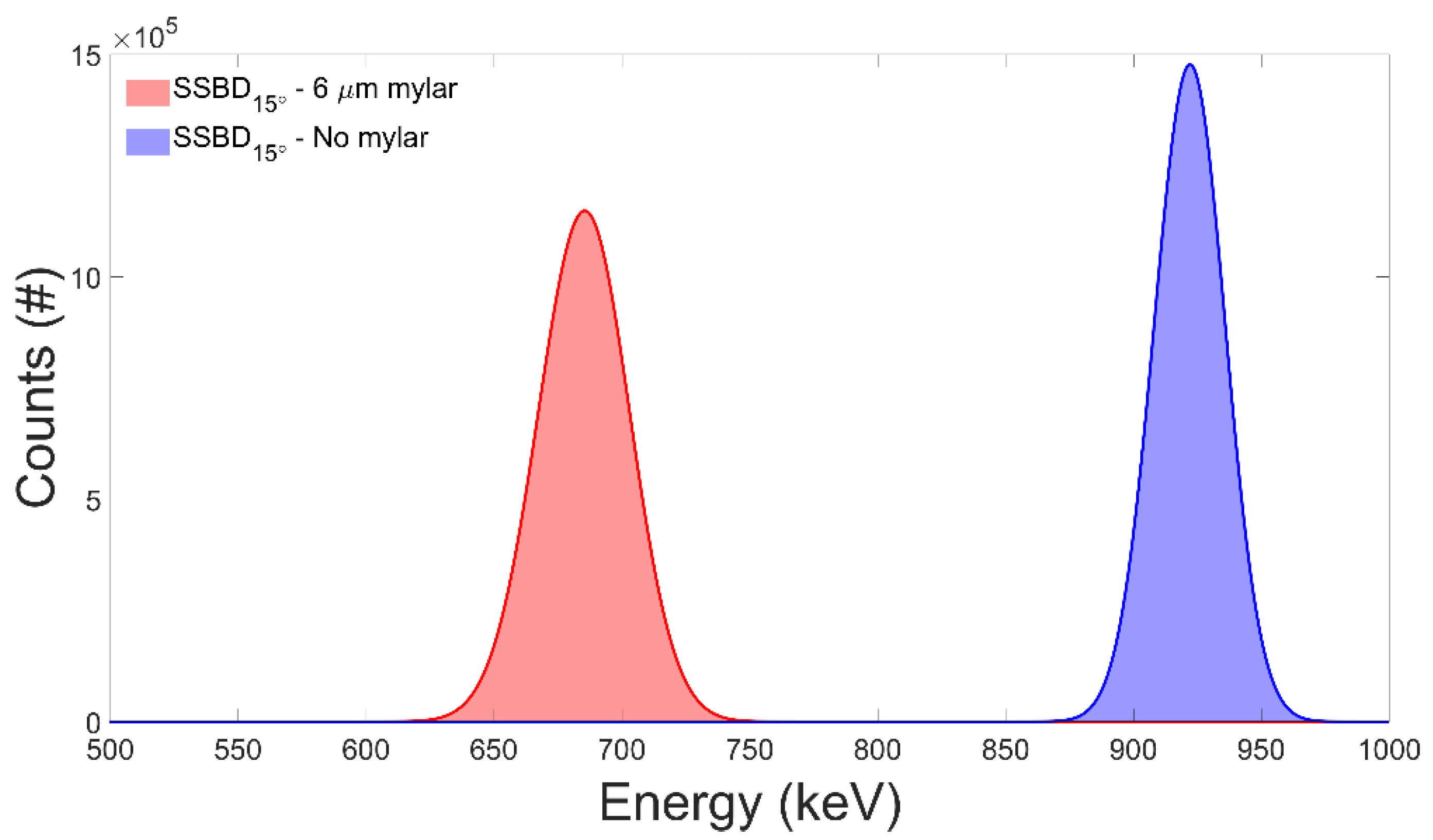
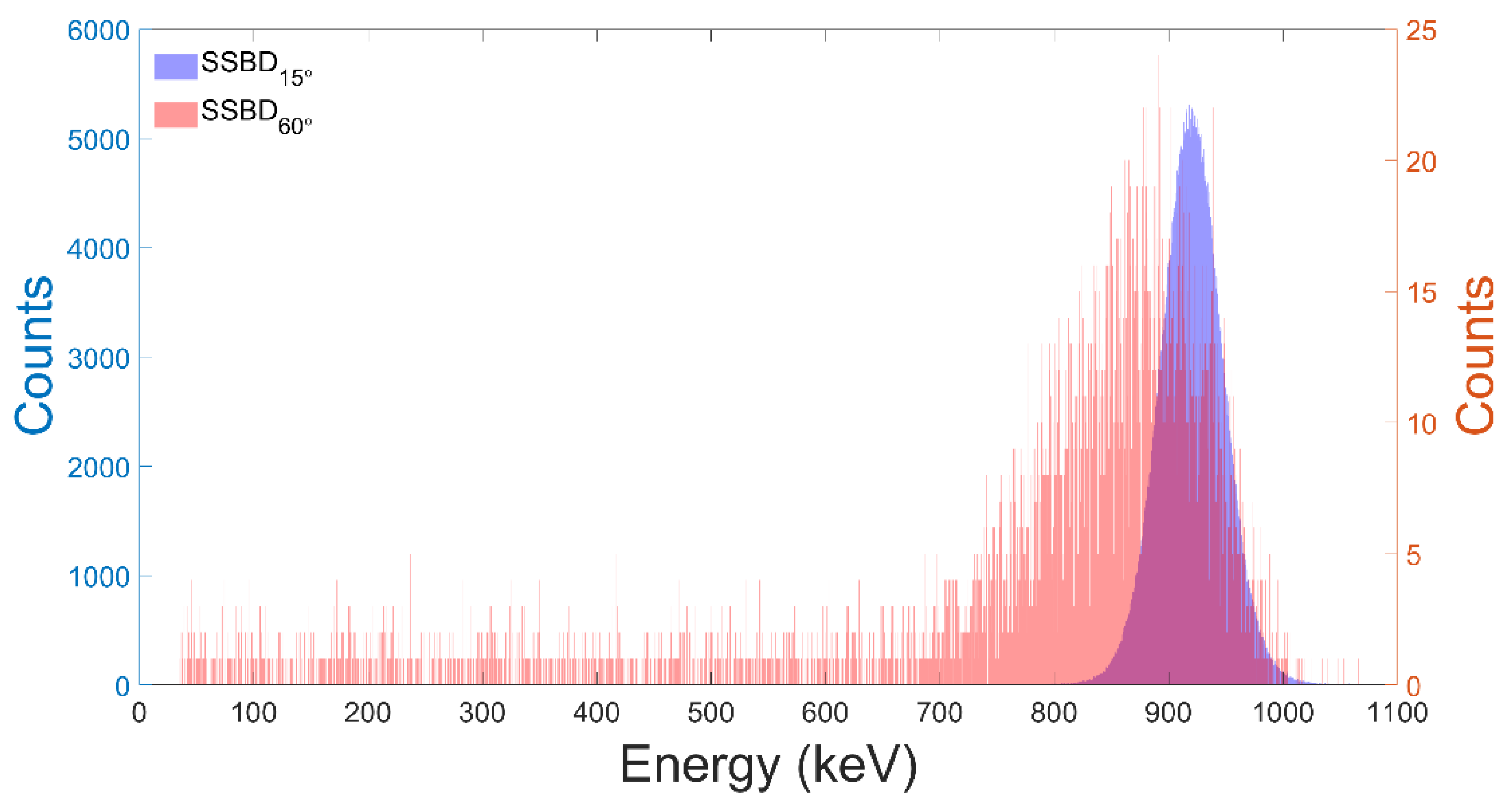
3.1. Test of the Facility Performance
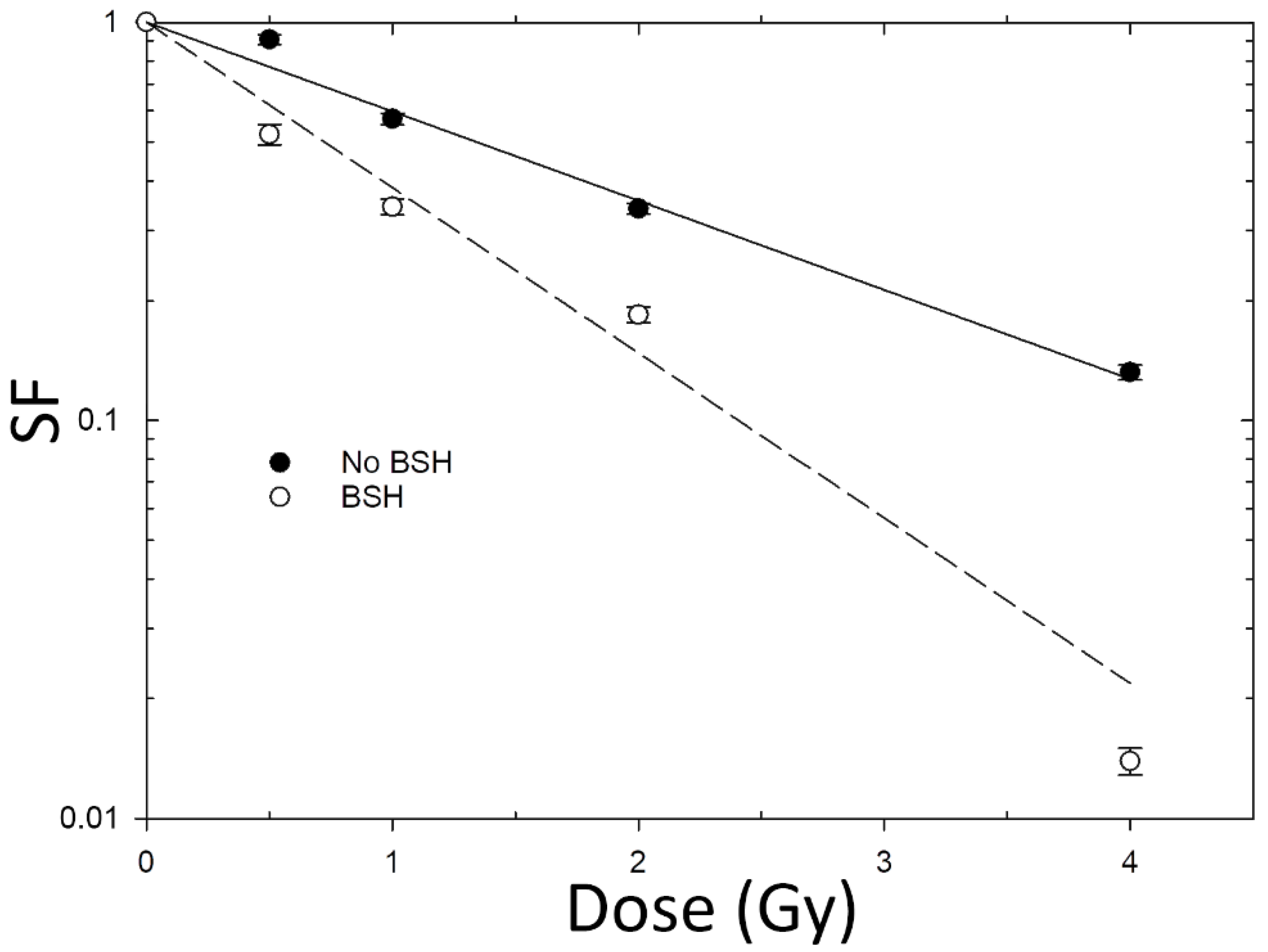
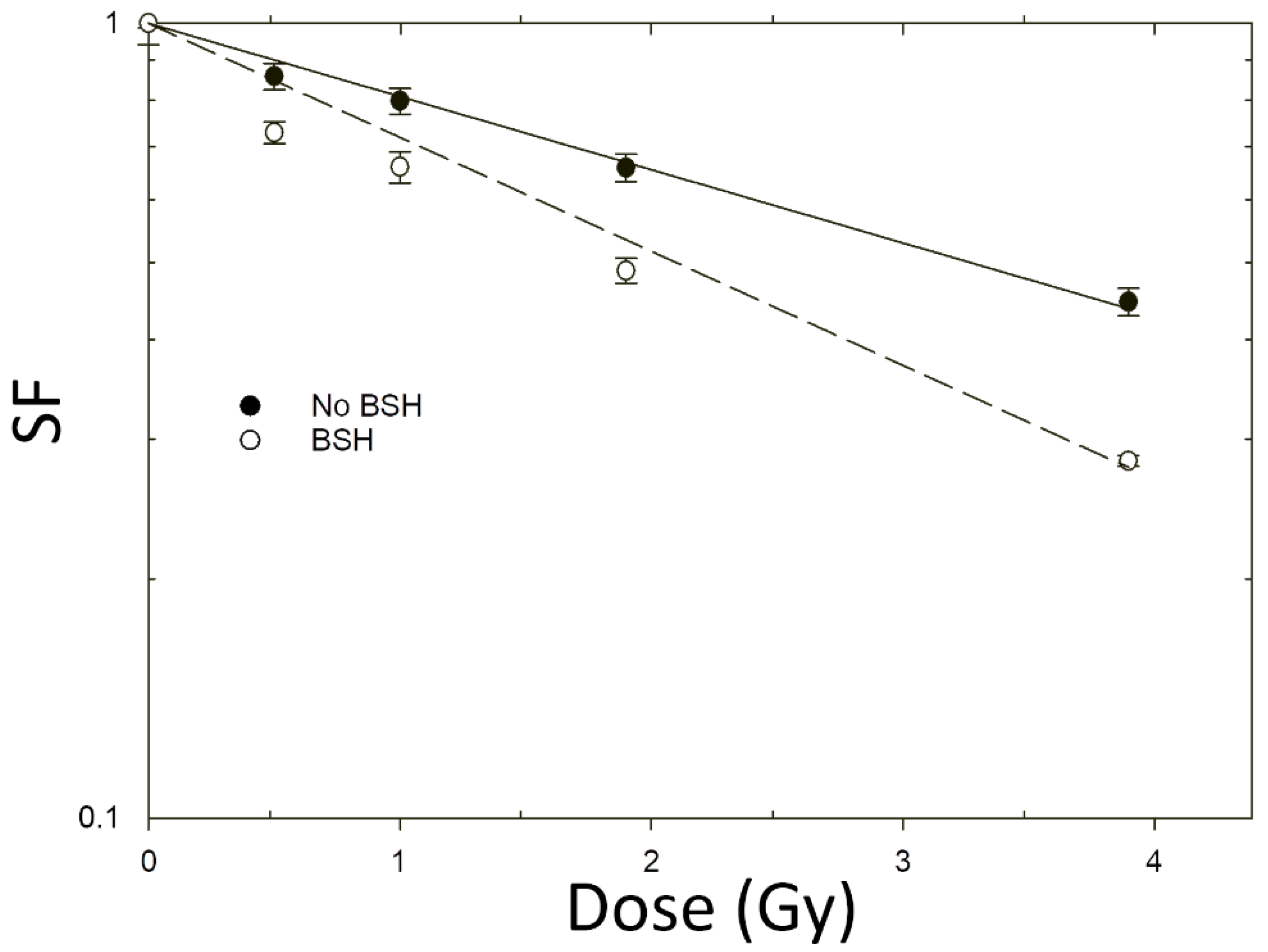
3.2. Increase in Cancer Cell Death Following Proton Irradiation in the Presence of BSH
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Particle Therapy Co-Operative Group (PTCOG). Available online: https://www.ptcog.ch/index.php/facilities-in-operation (accessed on 19 September 2021).
- Eaton, B.R.; MacDonald, S.M.; Yock, T.I.; Tarbell, N.J. Secondary Malignancy Risk Following Proton Radiation Therapy. Front. Oncol. 2015, 5, 261. [Google Scholar] [CrossRef]
- Mizumoto, M.; Murayama, S.; Akimoto, T.; Demizu, Y.; Fukushima, T.; Ishida, Y.; Oshiro, Y.; Numajiri, H.; Fuji, H.; Okumura, T.; et al. Long-term follow-up after proton beam therapy for pediatric tumors: A Japanese national survey. Cancer Sci. 2017, 108, 444–447. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers 2021, 13, 23. [Google Scholar] [CrossRef]
- Petringa, G.; Romano, F.; Manti, L.; Pandola, L.; Attili, A.; Cammarata, F.; Cuttone, G.; Forte, G.; Manganaro, L.; Pipek, J.; et al. Radiobiological quantities in proton-therapy: Estimation and validation using Geant4-based Monte Carlo simulations. Phys. Med. 2019, 58, 72–80. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Verstrepen, K.; Nuyts, S. Clinical Progress in Proton Radiotherapy: Biological Unknowns. Cancers 2021, 13, 604. [Google Scholar] [CrossRef]
- Mirjolet, C.; Nicol, A.; Limagne, E.; Mura, C.; Richard, C.; Morgand, V.; Rousseau, M.; Boidot, R.; Ghiringhelli, F.; Noel, G.; et al. Impact of proton therapy on antitumor immune response. Sci. Rep. 2021, 11, 13444. [Google Scholar] [CrossRef]
- Cunningham, C.; de Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization Effect of Gold Nanoparticles in Proton Therapy. Front. Public Health 2021, 9, 699822. [Google Scholar] [CrossRef]
- Yoon, D.-K.; Jung, J.-Y.; Suh, T.S. Application of proton boron fusion reaction to radiation therapy: A Monte Carlo simulation study. Appl. Phys. Lett. 2014, 105, 223507/1–223507/4. [Google Scholar] [CrossRef]
- Becker, H.W.; Rolfs, C.; Trautvetter, H.P. Low-energy cross sections for 11B(p,3alpha). Z. Phys. At. Nucl. 1987, 327, 341–355. [Google Scholar] [CrossRef]
- Hodgkins, P.S.; O’Neil, P.; Stevens, D.; Fairman, M.P. The severity of alpha-particle-induced DNA damage is revealed by exposure to cell-free extracts. Radiat. Res. 1996, 146, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sasai, K.; Evans, J.W.; Kovacs, M.S.; Brown, J.M. Prediction of human cell radiosensitivity: Comparison of clonogenic assay with chromosome aberrations scored using premature chromosome condensation with fluorescence in situ hybridization. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 1127–1132. [Google Scholar] [CrossRef]
- Manti, L.; Durante, M.; Grossi, G.; Lattuada, M.; Pugliese, M.; Sabini, M.G.; Scampoli, P.; Valastro, L.; Gialanella, G. Modelled microgravity does not modify the yield of chromosome aberrations induced by high-energy protons in human lymphocytes. Int. J. Radiat. Biol. 2005, 81, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cirrone, G.A.P.; Manti, L.; Margarone, D.; Petringa, G.; Giuffrida, L.; Minopoli, A.; Picciotto, A.; Russo, G.; Cammarata, F.; Pisciotta, P.; et al. First experimental proof of Proton Boron Capture Therapy (PBCT) to enhance protontherapy effectiveness. Sci. Rep. 2018, 8, 1141. [Google Scholar] [CrossRef]
- Bláha, P.; Feoli, C.; Agosteo, S.; Calvaruso, M.; Cammarata, F.P.; Catalano, R.; Ciocca, M.; Cirrone, G.A.P.; Conte, V.; Cuttone, G.; et al. The Proton-Boron Reaction Increases the Radiobiological Effectiveness of Clinical Low-and High-Energy Proton Beams: Novel Experimental Evidence and Perspectives. Front. Oncol. 2021, 11, 682647. [Google Scholar] [CrossRef] [PubMed]
- Manti, L.; Braselmann, H.; Calabrese, M.L.; Massa, R..; Pugliese, M.; Scampoli, P.; Sicignano, G.; Grossi, G. Effects of modulated microwave radiation at cellular–telephone frequency (1.95 GHz) on X ray-induced chromosome aberrations in human lymphocytes in vitro. Radiat. Res. 2008, 169, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pignalosa, D.; Bertucci, A.; Gialanella, G.; Grossi, G.; Manti, L.; Pugliese, M.; Scampoli, P.; Durante, M. Chromosome inter- and intrachanges detected by arm-specific DNA probes in the progeny of human lymphocytes exposed to energetic heavy ions. Radiat. Res. 2008, 170, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, N.; Wang, X.; Hosseinabadi, R.; Samiei, F. Is the proton–boron fusion therapy effective? J. Radiother. Pract. 2021, 20, 153–157. [Google Scholar] [CrossRef]
- Mazzucconi, D.; Bortot, D.; Pola, A.; Fazzi, A.; Cazzola, L.; Conte, V.; Cirrone, G.A.P.; Petringa, G.; Cuttone, G.; Manti, L.; et al. Experimental investigation at CATANA facility of n-10B and p-11B reactions for the enhancement of proton therapy. Phys. Med. 2021, 89, 226–231. [Google Scholar] [CrossRef] [PubMed]
- IonBeamCenters.eu. Available online: https://www.ionbeamcenters.eu/ion-beam-facilities (accessed on 19 September 2021).
- Belli, M.; Cherubini, R.; Galeazzi, G.; Mazzucato, S.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A. Proton irradiation facility for radiobiological studies at a 7 MV Van de Graaff accelerator. Nucl. Instr. Meth. Phys. Res. A 1987, 256, 576–580. [Google Scholar] [CrossRef]
- Gialanella, L.; Di Leva, A.; Terrasi, F. Nuclear Astrophysics at CIRCE. Nucl. Phys News 2018, 28, 20–24. [Google Scholar] [CrossRef]
- Terrasi, F.; Rogalla, D.; De Cesare, N.; D’Onofrio, A.; Lubritto, C.; Marzaioli, F.; Passariello, I.; Rubino, M.; Sabbarese, C.; Casa, G.; et al. A new AMS facility in Caserta/Italy. Nucl. Instr. Meth. Phys. Res. B 2007, 259, 14–17. [Google Scholar] [CrossRef]
- FAIR-INFN Sezione di Napoli. Available online: http://www.esalerno.com/gerardogiordano/products/fair/index_lllita.html (accessed on 24 September 2021).
- Durante, M.; Grossi, G.; Pugliese, M.; Manti, L.; Nappo, M.; Gialanella, G. Single-charged particle damage to living cells: A new method to detect traversals based on track-etch detectors. Nucl. Instr. Meth. Phys. Res. B 1994, 94, 251–258. [Google Scholar] [CrossRef]
- Coppola, F.; Durante, M.; Gialanella, G.; Grossi, G.; Manti, L.; Pugliese, M.; Scampoli, P. Development of an automated scanning system for the analysis of heavy ions’ fragmentation reactions by nuclear track detectors. Radiat. Meas. 2009, 44, 802–805. [Google Scholar] [CrossRef][Green Version]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter. Nucl. Instr. Meth. Phys. Res. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Manti, L.; Jamali, M.; Prise, K.M.; Michael, B.D.; Trott, K.R. Genomic instability in Chinese hamster cells after exposure to X-rays, neutrons or alpha-particles of different LET. Radiat. Res. 1997, 147, 22–28. [Google Scholar] [CrossRef]
- Puck, T.T.; Marcus, P.I. Action of X-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Manti, L.; Davies, H.E.; Venables, S.; Bowen, I.D.; Court, J.B. Correlation between the clonogenic initial slope and the response of polykaryon-forming units: The behavior of strains defective in XRCC5 and ATM and the heritability of small variations in radioresponse. Radiat. Res. 2000, 154, 650–658. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA, a simulation program for the analysis of NRA, RBS and ERDA. AIP Conf. Proc. 1999, 475, 541. [Google Scholar] [CrossRef]
- Asanuma, K.; Moriai, R.; Yajima, T.; Yagihashi, A.; Yamada, M.; Kobayashi, D.; Watanabe, N. Survivin as a radioresistance factor in pancreatic cancer. Jpn. J. Cancer Res. 2000, 91, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Frick, S.; Brunner, T.; Pilarsky, C.; Grützmann, R.; Sipos, B.; Klöppel, G.; McKenna, W.G.; Bernhard, E.J. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene 2007, 26, 6851–6862. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.P.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar] [CrossRef]
- Jolly, S.; Owen, H.; Schippers, M.; Welsch, C. Technical challenges for FLASH proton therapy. Phys. Med. 2020, 78, 71–82. [Google Scholar] [CrossRef]
- Schillaci, F.; Anzalone, A.; Cirrone, G.A.P.; Carpinelli, M.; Cuttone, G.; Cutroneo, M.; De Martinis, C.; Giove, D.; Korn, G.; Maggiore, M.; et al. ELIMED, MEDical and multidisciplinary applications at ELI-Beamlines. J. Phys. Conf. Ser. 2014, 508, 012010. [Google Scholar] [CrossRef]
- Manti, L.; Perozziello, F.M.; Borghesi, M.; Candiano, G.; Chaudhary, P.; Cirrone, G.A.P.; Doria, D.; Gwynne, D.; Leanza, R.; Prise, K.M.; et al. The radiobiology of laser-driven particle beams: Focus on sub-lethal responses of normal human cells. J. Instr. 2017, 12, C03084. [Google Scholar] [CrossRef][Green Version]


| CR-39 | Irradiation Time (s) | Counts | Fluence (cm−2) | Fluenceexpected (cm−2) | Dose (mGy) | Doseexpected (mGy) |
|---|---|---|---|---|---|---|
| #1 | 15 ± 1 | 36 ± 6 | (4.20 ± 0.11)·104 | (4.33 ± 0.09)·104 | 1.77 ± 0.05 | 1.82 ± 0.04 |
| #2 | 30 ± 1 | 71 ± 8 | (8.4 ± 0.2)·104 | (8.6 ± 0.2)·104 | 3.53 ± 0.08 | 3.62 ± 0.08 |
| Treatment | ||
|---|---|---|
| BSH | 0.52 ± 0.03 | - |
| No BSH | 0.96 ± 0.08 | 1.85 ± 0.19 |
| Treatment | ||
|---|---|---|
| BSH | 0.212 ± 0.006 | - |
| No BSH | 0.330 ± 0.012 | 1.56 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, V.; Bláha, P.; Buompane, R.; Crescente, G.; Cuttone, G.; Gialanella, L.; Michaličková, K.; Pacifico, S.; Porzio, G.; Manti, L. A New Low-Energy Proton Irradiation Facility to Unveil the Mechanistic Basis of the Proton-Boron Capture Therapy Approach. Appl. Sci. 2021, 11, 11986. https://doi.org/10.3390/app112411986
Ricciardi V, Bláha P, Buompane R, Crescente G, Cuttone G, Gialanella L, Michaličková K, Pacifico S, Porzio G, Manti L. A New Low-Energy Proton Irradiation Facility to Unveil the Mechanistic Basis of the Proton-Boron Capture Therapy Approach. Applied Sciences. 2021; 11(24):11986. https://doi.org/10.3390/app112411986
Chicago/Turabian StyleRicciardi, Valerio, Pavel Bláha, Raffaele Buompane, Giuseppina Crescente, Giacomo Cuttone, Lucio Gialanella, Katarina Michaličková, Severina Pacifico, Giuseppe Porzio, and Lorenzo Manti. 2021. "A New Low-Energy Proton Irradiation Facility to Unveil the Mechanistic Basis of the Proton-Boron Capture Therapy Approach" Applied Sciences 11, no. 24: 11986. https://doi.org/10.3390/app112411986
APA StyleRicciardi, V., Bláha, P., Buompane, R., Crescente, G., Cuttone, G., Gialanella, L., Michaličková, K., Pacifico, S., Porzio, G., & Manti, L. (2021). A New Low-Energy Proton Irradiation Facility to Unveil the Mechanistic Basis of the Proton-Boron Capture Therapy Approach. Applied Sciences, 11(24), 11986. https://doi.org/10.3390/app112411986









