Dark-Field Hyperspectral Microscopy for Carbon Nanotubes Bioimaging
Abstract
:1. Introduction
2. Dark-Field Microscopy
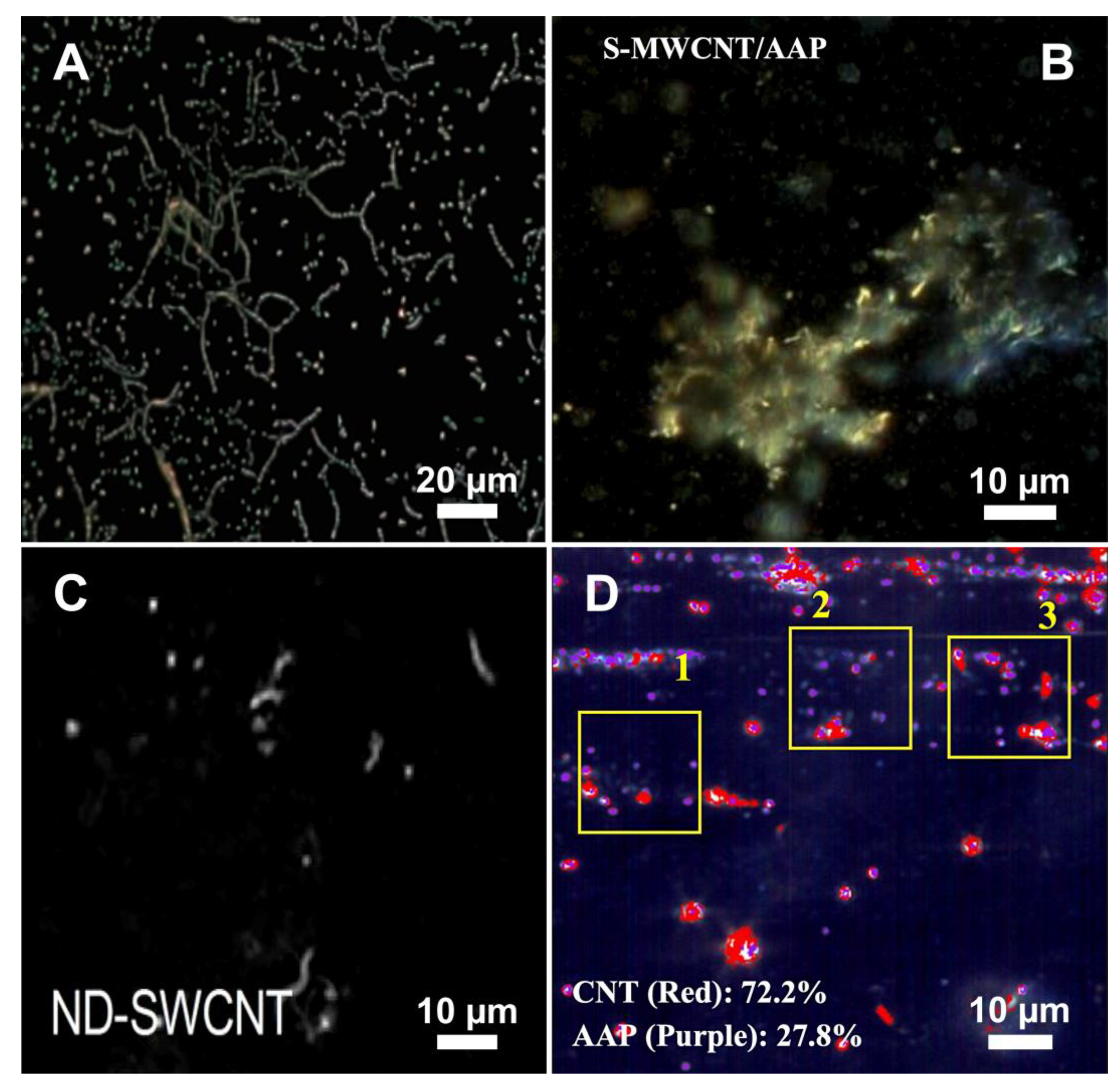
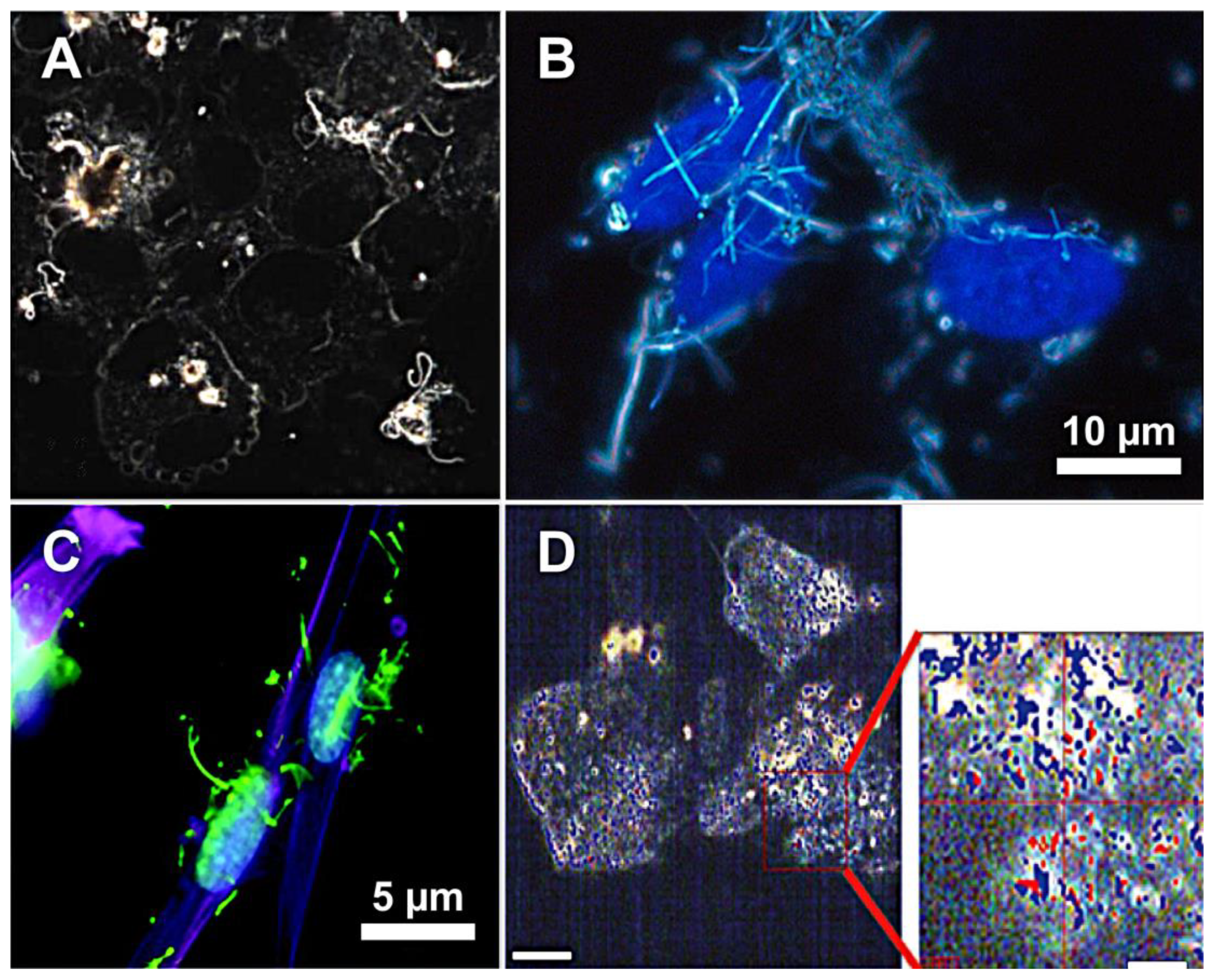
3. In Vitro Studies of CNTs
| System | Cell Culture | Type of CNTs | Size of Tested CNTs | Treatment Condition | Sample Preparation | Results | Reference |
|---|---|---|---|---|---|---|---|
| CytoViva enhanced dark-field microscopy setup | PANC-1 cell line | SWCNTs | L: 0.3–3.0 µm, D: 0.7–1.6 nm | 10 µg/mL for 1, 4, 8 and 24 h | Living cell samples | Imaging showed time-dependent cellular uptake/accumulation of SWCNTs started at 4 h of exposure | [62] |
| MWCNTs-PEG | Hydrodynamic diameter is 298–728 nm | 5, 10 and 50 µg/mL for 1 h | Not specified | EDFM demonstrated dose-dependent accumulation of CNTs in cells | [63] | ||
| RAW 264.7 cell line | MWCNTs | L: >2 µm, D: 8–15 nm | 0.2 µg/cm2 for 2, 4, 6, 24 and 48 h | Living cell samples | Imaging revealed time-dependent accumulation of CNTs on the surface and inside the cells | [64] | |
| BEAS-2B cell line | TUBALLTM and HiPco SWCNTs | TUBALLTM (L: >5 µm, D: 1.6 ± 0.4 nm), HiPco (L: 0.1–1.0 µm, D: 0.8–1.2 nm) | 2.5 µg/mL for 48 h | Living cell samples | Microscopy confirmed cell penetration of all types CNTs | [65] | |
| CytoViva dual mode fluorescence-enhanced dark-field microscopy setup | A549 cell line | MWCNTs | L: ≈5.6 µm, W: 60 nm | 5 and 10 µg/mL for 24 h | Fixed with PFA, stained with rhodamine-phalloidin and DAPI | Imaging confirmed both attachment to the cell membrane and partial internalisation of MWCNTs | [66] |
| Macrophages, A549 and MRC-5 cell lines | MWCNTs | L: 5.66 ± 4.7 µm, D: 60.1 ± 18.2 nm | 5 and 10 µg/mL for 24 and 96 h | Fixed with PFA, stained with rhodamine-phalloidin and DAPI | Microscopy demonstrated dose-dependent interaction of MWCNTs in all cell lines with increased association noted in THP-1 cells | [67] | |
| Organotypic model of human alveolar tissue MatTek EpiAlveolar, cocultured with (+) or without (-) MDMs | Mitsui-7 (long and thick) and Nanocyl-7000 (short and thin) MWCNTs | Not specified | ≈0.9 and ≈2 µg/cm2 of Mitsui-7 or ≈1 µg/cm2 of Nanocyl-7000 for 3 weeks of repeated exposure in liquid aerosol form | Fixed with PFA, stained with rhodamine-phalloidin and DAPI | Imaging confirmed the association of both types of CNTs with cells in EpiAlveolar tissue. Nanocyl-7000 could only be seen after the additional processing of samples | [69] | |
| A549, 16HBE14o-, MeT-5A and J774A.1 cell lines | Mitsui-7 and Nanocyl-7000 MWCNTs | Mitsui-7 (L: 5.6 ± 4.7 µm, D: 60 ± 19 nm), Nanocyl-7000 (L: 0.8 ± 0.5 µm, D: 11 ± 5 nm) | 10 µg/mL for 24 h | Fixed with PFA, stained with rhodamine-phalloidin and DAPI | Both types of MWCNTs were detectable within all cell types. Nanocyl-7000 were densely packed in cellular vesicles | [70] | |
| Rat aortic endothelial cells and RAW264.7 cell line | Uncoated and BSA-coated pristine (MWCNTs), carboxylated (F-MWCNTs), and base-washed carboxylated (BW-F-MWCNTs) nanotubes | MWCNTs (L: 1205 ± 360 nm, D: 34 ± 9 nm) ,F-MWCNTs (L: 767 ± 527 nm, D: 35 ± 11 nm), BW-F-MWCNTs (L: 737 ± 457 nm, D: 35 ± 7 nm) | 50 µg/mL for 24 h in serum-free medium | Fixed with PFA and stained with DAPI | Microscopy confirmed the lack of the influence of the coating on uptake of all types of MWCNTs by cells: F-MWCNTs and BW-F-MWCNTs are greater internalised in cells than pristine tubes | [71] | |
| CytoViva enhanced dark-field microscopy setup | SAECs | SWCNTs and MWCNTs | SWCNTs (L: 1.08 µm, W: 270 nm), MWCNTs (L: 5.1 µm, W: 78 nm) | 0.1 µg/mL for 24 and 48 h | Fixed with NBF and stained with toluidine blue | Imaging showed that both types of CNTs were co-localized in the cytoplasm of cells or puncturing the cellular and nuclear membranes | [72] |
| ‘As-prepared’ (pMWCNT), carboxylated- (MW-COOH), and aminated- (MW-NHx) CNTs | pMWCNT (L: 1.51 ± 0.001 µm, W: 26.0 ± 5.4 nm), MW-COOH (L: 1.86 ± 0.16 µm, W: 26.5 ± 1.0 nm), MW-NHx (D: 1.50 ± 0.0078 µm, W: 21.6 ± 0.6 nm) | 0.288 µg/mL for 24 h | Not specified | Imaging showed that all the MWCNT particles co-localized with either the cytoplasm or nucleus of the cells | [73] | ||
| MWCNTs | L: 8.1 ± 5 µm, D: 8.2 nm | 0.06 µg/cm2 for 24 h | Fixed with NBF and stained with toluidine blue | Microscopy analysis confirmed co-localization of CNTs with the plasma membrane, cytoplasm, and nucleus | [74] | ||
| CytoViva dual mode fluorescence-enhanced dark-field microscopy setup | Bone-marrow-derived macrophages | Uncoated and OVA-coated carboxylated MWCNT-2 and MWCNT-30 | MWCNT-2 (L: ≈500 nm, D: 26 ± 5 nm) MWCNT-30 (L: ≈500 nm, D: 18 ± 3 nm) | 25 µg/mL for 6 h in serum-free medium | Fixed with PFA and stained with DAPI | Imaging confirmed delivery of OVA into cells by both types of MWCNTs | [75] |
| BEAS-2B | MWCNTs, MWCNT-HT and MWCNT-ND | MWCNTs (L: 5 ± 4 µm, D: 49 ± 13 nm), MWCNT-HT (L: 5 ± 4 µm, D: 57 ± 24 nm in), MWCNT-ND (L: 2 ± 3 µm, D: 30 ± 23 nm) | 0.024, 0.24, 2.4, and 24 µg/mL for 24 h | Fixed with 100% ice cold methanol and stained with DAPI | Imaging showed a dose-dependent increase in all types of MWCNTs within the cell nucleus with higher partitioning of raw MWCNTs | [76] | |
| A549 cell line and human skin fibroblasts | SWCNTs | Hydrodynamic size is 7–214 nm | 0.1, 0.25, 0.5, and 1 mg/mL for 24 h with AuNP | Fixed with PFA and stained with DAPI | SWCNTs were seen as large aggregates on the cells in EDF images which was not observed in TEM analysis | [77] | |
| CytoViva dual mode fluorescence-enhanced dark-field microscopy coupled with hyperspectral imaging | SAECs | SWCNTs | Hydrodynamic radius is 106–243 nm | 50 µg/mL for 24 h followed by viral infection | Fixed with cold acetone and methanol solution | Mapping showed that SWCNTs in isolated exposure appeared as irregular extracellular aggregates on cells; the distribution pattern of both virus and CNTs have changed during co-exposure | [78] |
| A549 cell line | MWCNTs | L: 414.3 ± 79.3 nm | 100 µg/mL for 24 h | Living cell samples. Samples fixed with PFA and stained with DAPI | Microscopy coupled with spectral matching revealed co-localisation of CNTs on the cell membrane | [79] | |
| CRL-1490 cell line | SWCNTs and MWCNTs | SWCNTs (L: 1 µm, W: 0.27 µm, MWCNTs (L: 5.1 µm, W: 0.078 µm) | 0.02 µg/cm2 for 24 h | Fixed with formaldehyde, stained with phalloidin and DAPI | Fluorescence microscopy showed uptake of all types of CNTs, which was then confirmed by hyperspectral imaging | [80] | |
| CytoViva enhanced dark-field microscopy combined with SERS | BEAS-2B and HepG2 cell lines | LW-MWCNTs, LN-MWCNTs and SN-MWCNTs | LW-MWCNTs (L: 10–30 µm, D: 20–30 nm), LN-MWCNTs (L: 10–30 µm, D: 8–15 nm), SN-MWCNTs (L: 0.5–2 µm, D: 8–15 nm) | 1 µg/mL for 24 h | Living cell samples | Imaging showed the interaction of all types of MWCNTs with both cell lines. Subsequent SERS analysis confirmed internalisation only of SN-MWCNTs | [55] |
| ImageStreamX multispectral imaging flow cytometer | Adherent macrophages and HUVEC line | FITC-MWCNTs conjugates | L: 340 nm, D: 20–30 nm | 0, 10, 20, and 50 μg/mL for 20 h at 37 °C or 2 h at 4 °C. The conditioned medium was then incubated with recipient cells for 48 h | Trypsinised and fixed with formaldehyde | Flow cytometry imaging with dark-field, bright-field and fluorescent channels confirmed the dose-dependent increase in CNTs-labelled cells. The method also confirmed the possibility of CNT-labelling of recipient cells from conditioned medium | [81] |
4. In Vivo Studies of CNTs
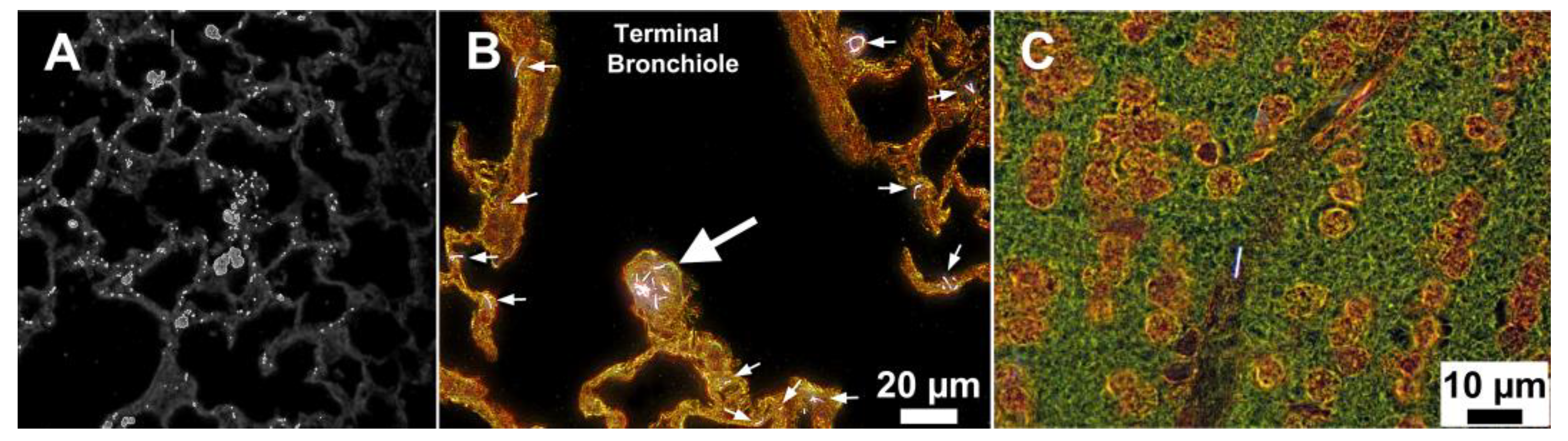
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Saliev, T. The Advances in Biomedical Applications of Carbon Nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Wang, Y.; Ren, Z.F. Physics and applications of aligned carbon nanotubes. Adv. Phys. 2011, 60, 553–678. [Google Scholar] [CrossRef]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Gupta, E.; Kanu, N.J. Plethora of Carbon Nanotubes Applications in Various Fields–A State-of-the-Art-Review. Smart Sci. 2021, 1–24. [Google Scholar] [CrossRef]
- Cai, L.; Wang, C. Carbon Nanotube Flexible and Stretchable Electronics. Nanoscale Res. Lett. 2015, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of Applications of Polymer/Carbon Nanotubes and Epoxy/CNT Composites. Polym. Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Zainal, Z.; Yusof, N. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Aqel, A.; El-Nour, K.M.M.A.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-de-Arellano, J.M.; Canales, M.; Magaña, L.F. Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems. Molecules 2021, 26, 5346. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 2908. [Google Scholar] [CrossRef]
- Yang, C.-T.; Ghosh, K.K.; Padmanabhan, P.; Langer, O.; Liu, J.; Eng, D.N.C.; Halldin, C.; Gulyás, B. PET-MR and SPECT-MR multimodality probes: Development and challenges. Theranostics 2018, 8, 6210–6232. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, A.G.; Garashchenko, B.L.; Ivanova, M.K.; Vinokurov, S.E.; Myasoedov, B.F. Carbon Nanomaterials for Sorption of 68Ga for Potential Using in Positron Emission Tomography. Nanomaterials 2020, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. In Surface-Modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Puente-Santiago, A.R., Rodríguez-Padrón, D., Eds.; Springer International Publishing: New York, NY, USA, 2020; pp. 177–217. ISBN 978-3-030-55501-6. [Google Scholar]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hong, H.; Cai, W. Imaging with Raman Spectroscopy. Curr. Pharm. Biotechnol. 2010, 11, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Godin, A.G.; Setaro, A.; Gandil, M.; Haag, R.; Adeli, M.; Reich, S.; Cognet, L. Photoswitchable single-walled carbon nanotubes for super-resolution microscopy in the near-infrared. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- Yudasaka, M.; Yomogida, Y.; Zhang, M.; Tanaka, T.; Nakahara, M.; Kobayashi, N.; Okamatsu-Ogura, Y.; Machida, K.; Ishihara, K.; Saeki, K.; et al. Near-Infrared Photoluminescent Carbon Nanotubes for Imaging of Brown Fat. Sci. Rep. 2017, 7, 44760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Lu, C.; Chen, M.; Xu, X.; Shu, G.; Du, Y.; Ji, J. Recent advances in near-infrared II imaging technology for biological detection. J. Nanobiotechnology 2021, 19, 132. [Google Scholar] [CrossRef]
- Lee, W.; Parpura, V. Carbon nanotubes as substrates/scaffolds for neural cell growth. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2009; pp. 110–125. ISBN 978-0-444-53431-6. [Google Scholar]
- Vicentini, N.; Gatti, T.; Salerno, M.; Hernandez Gomez, Y.S.; Bellon, M.; Gallio, S.; Marega, C.; Filippini, F.; Menna, E. Effect of different functionalized carbon nanostructures as fillers on the physical properties of biocompatible poly(l-lactic acid) composites. Mater. Chem. Phys. 2018, 214, 265–276. [Google Scholar] [CrossRef]
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 016018. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review and the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Boyles, M.S.P.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.W.; Proudfoot, L.; Windle, A.H.; et al. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. Vitr. 2015, 29, 1513–1528. [Google Scholar] [CrossRef]
- Manke, A.; Luanpitpong, S.; Dong, C.; Wang, L.; He, X.; Battelli, L.; Derk, R.; Stueckle, T.; Porter, D.; Sager, T.; et al. Effect of Fiber Length on Carbon Nanotube-Induced Fibrogenesis. Int. J. Mol. Sci. 2014, 15, 7444–7461. [Google Scholar] [CrossRef] [Green Version]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Methods in Molecular Biology (Clifton, N.J.); Humana Press: New York, NY, USA, 2019; pp. 1–29. ISBN 978-1-4939-8916-4. [Google Scholar]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Spaeth, P.; Kar, A.; Baaske, M.D.; Khatua, S.; Orrit, M. Photothermal Microscopy: Imaging the Optical Absorption of Single Nanoparticles and Single Molecules. ACS Nano 2020, 14, 16414–16445. [Google Scholar] [CrossRef] [PubMed]
- Ghamsari, M.S. Introductory Chapter: Nano-bioimaging—Past, Present, and Future. In State of the Art in Nano-Bioimaging; InTech: London, UK, 2018; ISBN 978-1-78923-294-3. [Google Scholar]
- Ostrowski, A.; Nordmeyer, D.; Boreham, A.; Holzhausen, C.; Mundhenk, L.; Graf, C.; Meinke, M.C.; Vogt, A.; Hadam, S.; Lademann, J.; et al. Overview about the localization of nanoparticles in tissue and cellular context by different imaging techniques. Beilstein J. Nanotechnol. 2015, 6, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Fakhrullin, R.; Nigamatzyanova, L.; Fakhrullina, G. Dark-field/hyperspectral microscopy for detecting nanoscale particles in environmental nanotoxicology research. Sci. Total Environ. 2021, 772, 145478. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Rubio, L.; Vila, L.; Xamena, N.; Velázquez, A.; Marcos, R.; Hernández, A. The Comet Assay as a Tool to Detect the Genotoxic Potential of Nanomaterials. Nanomaterials 2019, 9, 1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Fujioka, Y.; Kubono, A.; Akiyama, R. Electrically developed morphology of carbon nanoparticles in suspensions monitored by in situ optical observations under sinusoidal electric field. Colloid Polym. Sci. 2006, 284, 562–567. [Google Scholar] [CrossRef]
- Truong, P.L.; Ma, X.; Sim, S.J. Resonant Rayleigh light scattering of single Au nanoparticles with different sizes and shapes. Nanoscale 2014, 6, 2307. [Google Scholar] [CrossRef]
- Fong, K.E.; Yung, L.-Y.L. Localized surface plasmon resonance: A unique property of plasmonic nanoparticles for nucleic acid detection. Nanoscale 2013, 5, 12043. [Google Scholar] [CrossRef]
- Weigel, A.; Sebesta, A.; Kukura, P. Dark Field Microspectroscopy with Single Molecule Fluorescence Sensitivity. ACS Photonics 2014, 1, 848–856. [Google Scholar] [CrossRef]
- Gaillard, J.; Skove, M.; Rao, A.M. Mechanical properties of chemical vapor deposition-grown multiwalled carbon nanotubes. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Mishra, A.; Clayton, K.; Velasco, V.; Williams, S.J.; Wereley, S.T. Dynamic optoelectric trapping and deposition of multiwalled carbon nanotubes. Microsystems Nanoeng. 2016, 2, 16005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeevi, G.; Shlafman, M.; Tabachnik, T.; Rogachevsky, Z.; Rechnitz, S.; Goldshtein, I.; Shlafman, S.; Gordon, N.; Alchanati, G.; Itzhak, M.; et al. Automated circuit fabrication and direct characterization of carbon nanotube vibrations. Nat. Commun. 2016, 7, 12153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhang, Y.; Zhang, Q.; Xie, H.; Wang, H.; Nie, J.; Wen, Q.; Wei, F. Optical visualization of individual ultralong carbon nanotubes by chemical vapour deposition of titanium dioxide nanoparticles. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhen, S.J.; Sang, Y.; Li, J.Y.; Wang, Y.; Zhan, L.; Peng, L.; Wang, J.; Li, Y.F.; Huang, C.Z. Controllable preparation of metal nanoparticle/carbon nanotube hybrids as efficient dark field light scattering agents for cell imaging. Chem. Commun. 2010, 46, 4303–4305. [Google Scholar] [CrossRef]
- Zamora-Perez, P.; Tsoutsi, D.; Xu, R.; Rivera_Gil, P. Hyperspectral-Enhanced Dark Field Microscopy for Single and Collective Nanoparticle Characterization in Biological Environments. Materials 2018, 11, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, S. Enhanced Darkfield Optical Microscopy Opens New Nano-Scale Imaging Possibilities. Micros. Today 2021, 29, 50–55. [Google Scholar] [CrossRef]
- Sanderson, J. Condensers and Eyepieces. In Understanding Light Microscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 161–175. ISBN 9780470973752. [Google Scholar]
- Gorbachevskii, M.V.; Stavitskaya, A.V.; Novikov, A.A.; Fakhrullin, R.F.; Rozhina, E.V.; Naumenko, E.A.; Vinokurov, V.A. Fluorescent gold nanoclusters stabilized on halloysite nanotubes: In vitro study on cytotoxicity. Appl. Clay Sci. 2021, 207, 106106. [Google Scholar] [CrossRef]
- Austin, C.A.; Hinkley, G.K.; Mishra, A.R.; Zhang, Q.; Umbreit, T.H.; Betz, M.W.; Wildt, B.E.; Casey, B.J.; Francke-Carroll, S.; Hussain, S.M.; et al. Distribution and accumulation of 10 nm silver nanoparticles in maternal tissues and visceral yolk sac of pregnant mice, and a potential effect on embryo growth. Nanotoxicology 2016, 10, 654–661. [Google Scholar] [CrossRef]
- Roth, G.A.; Tahiliani, S.; Neu-Baker, N.M.; Brenner, S.A. Hyperspectral microscopy as an analytical tool for nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Yang, J.; Kim, S.; Joo, S.W.; Choi, J. Diameter size and aspect ratio as critical determinants of uptake, stress response, global metabolomics and epigenetic alterations in multi-wall carbon nanotubes. Carbon N. Y. 2016, 108, 529–540. [Google Scholar] [CrossRef]
- Huebschman, M.L.; Schultz, R.A.; Garner, H.R. Characteristics and capabilities of the hyperspectral imaging microscope. IEEE Eng. Med. Biol. Mag. 2002, 21, 104–117. [Google Scholar] [CrossRef]
- Wang, L.; Castranova, V.; Mishra, A.; Chen, B.; Mercer, R.R.; Schwegler-Berry, D.; Rojanasakul, Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part. Fibre Toxicol. 2010, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fu, W.; Shen, Y.; Badireddy, A.R.; Zhang, W.; Huang, H. Hyperspectral Imaging Microscopy of Acetaminophen Adsorbed on Multiwalled Carbon Nanotubes. Langmuir 2018, 34, 13210–13218. [Google Scholar] [CrossRef]
- Schwab, F.; Bucheli, T.D.; Camenzuli, L.; Magrez, A.; Knauer, K.; Sigg, L.; Nowack, B. Diuron Sorbed to Carbon Nanotubes Exhibits Enhanced Toxicity to Chlorella vulgaris. Environ. Sci. Technol. 2013, 47, 7012–7019. [Google Scholar] [CrossRef]
- Akhatova, F.; Danilushkina, A.; Kuku, G.; Saricam, M.; Culha, M.; Fakhrullin, R. Simultaneous Intracellular Detection of Plasmonic and Non-Plasmonic Nanoparticles Using Dark-Field Hyperspectral Microscopy. Bull. Chem. Soc. Jpn. 2018, 91, 1640–1645. [Google Scholar] [CrossRef]
- Zamora-Perez, P.; Pelaz, B.; Tsoutsi, D.; Soliman, M.G.; Parak, W.J.; Rivera-Gil, P. Hyperspectral-enhanced dark field analysis of individual and collective photo-responsive gold–copper sulfide nanoparticles. Nanoscale 2021, 13, 13256–13272. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.; Xu, Y.; Dantuluri, V.; Mustafa, T.; Zhang, Y.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A. Carbon nanotubes enhance the internalization of drugs by cancer cells and decrease their chemoresistance to cytostatics. Nanotechnology 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.T.; Cojocaru, I.; Ilie, I.; Tabaran, F.A.; Zaharie, F.; Iancu, C.; Bartos, D.; Mocan, L. Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J. Cancer 2014, 5, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Khaliullin, T.O.; Fatkhutdinova, L.M.; Zalyalov, R.R.; Kisin, E.R.; Murray, A.R.; Shvedova, A.A. In vitro toxic effects of different types of carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012021. [Google Scholar] [CrossRef]
- Timerbulatova, G.A.; Dimiev, A.M.; Khamidullin, T.L.; Boichuk, S.V.; Dunaev, P.D.; Fakhrullin, R.F.; Khaertdinov, N.N.; Porfiryeva, N.N.; Khaliullin, T.O.; Fatkhutdinova, L.M. Dispersion of Single-Walled Carbon Nanotubes in Biocompatible Environments. Nanotechnologies Russ. 2020, 15, 437–444. [Google Scholar] [CrossRef]
- Septiadi, D.; Abdussalam, W.; Rodriguez-Lorenzo, L.; Spuch-Calvar, M.; Bourquin, J.; Petri-Fink, A.; Rothen-Rutishauser, B. Revealing the Role of Epithelial Mechanics and Macrophage Clearance during Pulmonary Epithelial Injury Recovery in the Presence of Carbon Nanotubes. Adv. Mater. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chortarea, S.; Zerimariam, F.; Barosova, H.; Septiadi, D.; Clift, M.J.D.; Petri-Fink, A.; Rothen-Rutishauser, B. Profibrotic Activity of Multiwalled Carbon Nanotubes Upon Prolonged Exposures in Different Human Lung Cell Types. Appl. Vitr. Toxicol. 2019, 5, 47–61. [Google Scholar] [CrossRef]
- Saarimäki, L.A.; Kinaret, P.A.S.; Scala, G.; del Giudice, G.; Federico, A.; Serra, A.; Greco, D. Toxicogenomics analysis of dynamic dose-response in macrophages highlights molecular alterations relevant for multi-walled carbon nanotube-induced lung fibrosis. NanoImpact 2020, 20, 100274. [Google Scholar] [CrossRef]
- Barosova, H.; Maione, A.G.; Septiadi, D.; Sharma, M.; Haeni, L.; Balog, S.; O’Connell, O.; Jackson, G.R.; Brown, D.; Clippinger, A.J.; et al. Use of EpiAlveolar Lung Model to Predict Fibrotic Potential of Multiwalled Carbon Nanotubes. ACS Nano 2020, 14, 3941–3956. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, L.; Bourquin, J.; Barosova, H.; Haeni, L.; Caldwell, J.; Milosevic, A.; Geers, C.; Bonmarin, M.; Taladriz-Blanco, P.; Rothen-Rutishauser, B.; et al. Rapid and sensitive quantification of cell-associated multi-walled carbon nanotubes. Nanoscale 2020, 12, 17362–17372. [Google Scholar] [CrossRef]
- Bai, W.; Wu, Z.; Mitra, S.; Brown, J.M. Effects of Multiwalled Carbon Nanotube Surface Modification and Purification on Bovine Serum Albumin Binding and Biological Responses. J. Nanomater. 2016, 2016, 2159537. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Stueckle, T.A.; Mishra, A.; Derk, R.; Meighan, T.; Castranova, V.; Rojanasakul, Y. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology 2014, 8, 485–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stueckle, T.A.; Davidson, D.C.; Derk, R.; Wang, P.; Friend, S.; Schwegler-Berry, D.; Zheng, P.; Wu, N.; Castranova, V.; Rojanasakul, Y.; et al. Effect of surface functionalizations of multi-walled carbon nanotubes on neoplastic transformation potential in primary human lung epithelial cells. Nanotoxicology 2017, 11, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Stueckle, T.A.; Davidson, D.C.; Derk, R.; Kornberg, T.G.; Schwegler-Berry, D.; Pirela, S.V.; Deloid, G.; Demokritou, P.; Luanpitpong, S.; Rojanasakul, Y.; et al. Evaluation of tumorigenic potential of CeO2 and Fe2O3 engineered nanoparticles by a human cell in vitro screening model. NanoImpact 2017, 6, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Bai, W.; Raghavendra, A.; Podila, R.; Brown, J.M. Defect density in multiwalled carbon nanotubes influences ovalbumin adsorption and promotes macrophage activation and CD4+ T-cell proliferation. Int. J. Nanomed. 2016, 11, 4357–4371. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, K.J.; Reynolds, S.H.; Porter, D.W.; Mercer, R.R.; Bauer, A.K.; Lowry, D.; Cena, L.; Stueckle, T.A.; Kashon, M.L.; Wiley, J.; et al. Mitsui-7, heat-treated, and nitrogen-doped multi-walled carbon nanotubes elicit genotoxicity in human lung epithelial cells. Part. Fibre Toxicol. 2019, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuku, G.; Saricam, M.; Akhatova, F.; Danilushkina, A.; Fakhrullin, R.; Culha, M. Surface-Enhanced Raman Scattering to Evaluate Nanomaterial Cytotoxicity on Living Cells. Anal. Chem. 2016, 88, 9813–9820. [Google Scholar] [CrossRef]
- Sanpui, P.; Zheng, X.; Loeb, J.C.; Bisesi, J.H.; Khan, I.A.; Afrooz, N.R.M.N.; Liu, K.; Badireddy, R.R.; Wiesner, M.R.; Ferguson, P.L.; et al. Single-walled carbon nanotubes increase pandemic influenza A H1N1 virus infectivity of lung epithelial cells. Part. Fibre Toxicol. 2014, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhina, E.; Batasheva, S.; Miftakhova, R.; Yan, X.; Vikulina, A.; Volodkin, D.; Fakhrullin, R. Comparative cytotoxicity of kaolinite, halloysite, multiwalled carbon nanotubes and graphene oxide. Appl. Clay Sci. 2021, 205, 106041. [Google Scholar] [CrossRef]
- Mishra, A.; Stueckle, T.A.; Mercer, R.R.; Derk, R.; Rojanasaku, Y.; Castranova, V.; Wang, L. Identification of TGF-β receptor-1 as a key regulator of carbon nanotube-induced fibrogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L821–L833. [Google Scholar] [CrossRef] [Green Version]
- Marangon, I.; Boggetto, N.; Ménard-Moyon, C.; Venturelli, E.; Béoutis, M.L.; Péchoux, C.; Luciani, N.; Wilhelm, C.; Bianco, A.; Gazeau, F. Intercellular carbon nanotube translocation assessed by flow cytometry imaging. Nano Lett. 2012, 12, 4830–4837. [Google Scholar] [CrossRef] [PubMed]
- Sager, T.M.; Wolfarth, M.W.; Battelli, L.A.; Leonard, S.S.; Andrew, M.; Steinbach, T.; Endo, M.; Tsuruoka, S.; Porter, D.W.; Castranova, V. Investigation of the pulmonary bioactivity of double-walled carbon nanotubes. J. Toxicol. Environ. Health-Part A Curr. Issues 2013, 76, 922–936. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Berthing, T.; Jackson, P.; Poulsen, S.S.; Mortensen, A.; Jacobsen, N.R.; Skaug, V.; Szarek, J.; Hougaard, K.S.; Wolff, H.; et al. Physicochemical predictors of Multi-Walled Carbon Nanotube–induced pulmonary histopathology and toxicity one year after pulmonary deposition of 11 different Multi-Walled Carbon Nanotubes in mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.S.; Porter, D.W.; Orandle, M.S.; Green, B.J.; Barnes, M.A.; Croston, T.L.; Wolfarth, M.G.; Battelli, L.A.; Andrew, M.E.; Beezhold, D.H.; et al. Resolution of Pulmonary Inflammation Induced by Carbon Nanotubes and Fullerenes in Mice: Role of Macrophage Polarization. Front. Immunol. 2020, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khaliullin, T.O.; Shvedova, A.A.; Kisin, E.R.; Zalyalov, R.R.; Fatkhutdinova, L.M. Evaluation of Fibrogenic Potential of Industrial Multi-Walled Carbon Nanotubes in Acute Aspiration Experiment. Bull. Exp. Biol. Med. 2015, 158, 684–687. [Google Scholar] [CrossRef] [Green Version]
- Porter, D.W.; Hubbs, A.F.; Chen, B.T.; McKinney, W.; Mercer, R.R.; Wolfarth, M.G.; Battelli, L.; Wu, N.; Sriram, K.; Leonard, S.; et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 2013, 7, 1179–1194. [Google Scholar] [CrossRef] [Green Version]
- Sager, T.M.; Wolfarth, M.W.; Andrew, M.; Hubbs, A.; Friend, S.; Chen, T.; Porter, D.W.; Wu, N.; Yang, F.; Hamilton, R.F.; et al. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology 2014, 8, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Holian, A.; Hamilton, R.F.; Wu, Z.; Deb, S.; Trout, K.L.; Wang, Z.; Bhargava, R.; Mitra, S. Lung deposition patterns of MWCNT vary with degree of carboxylation. Nanotoxicology 2019, 13, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F.; Wu, Z.; Mitra, S.; Holian, A. The effects of varying degree of MWCNT carboxylation on bioactivity in various in vivo and in vitro exposure models. Int. J. Mol. Sci. 2018, 19, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Sung, J.H.; Song, K.S.; Lee, J.H.; Kim, S.M.; Lee, G.H.; Ahn, K.H.; Lee, J.S.; Shin, J.H.; Park, J.D.; et al. Persistent DNA Damage Measured by Comet Assay of Sprague Dawley Rat Lung Cells after Five Days of Inhalation Exposure and 1 Month Post-Exposure to Dispersed Multi-Wall Carbon Nanotubes (MWCNTs) Generated by New MWCNT Aerosol Generation System. Toxicol. Sci. 2012, 128, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Sung, J.H.; Choi, B.G.; Ryu, H.Y.; Song, K.S.; Shin, J.H.; Lee, J.S.; Hwang, J.H.; Lee, J.H.; Lee, G.H.; et al. In vivo genotoxicity evaluation of lung cells from Fischer 344 rats following 28 days of inhalation exposure to MWCNTs, plus 28 days and 90 days post-exposure. Inhal. Toxicol. 2014, 26, 222–234. [Google Scholar] [CrossRef]
- Smith, B.R.; Ghosn, E.E.B.; Rallapalli, H.; Prescher, J.A.; Larson, T.; Herzenberg, L.A.; Gambhir, S.S. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 2014, 9, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, R.R.; Hubbs, A.F.; Scabilloni, J.F.; Wang, L.; Battelli, L.A.; Friend, S.; Castranova, V.; Porter, D.W. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Battelli, L.A.; McKinney, W.; Friend, S.; Wolfarth, M.G.; Andrew, M.; Castranova, V.; Porter, D.W. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, P.A.; Minarchick, V.C.; Cumpston, A.M.; McKinney, W.; Chen, B.T.; Sager, T.M.; Frazer, D.G.; Mercer, R.R.; Scabilloni, J.; Andrew, M.E.; et al. Impairment of coronary arteriolar endothelium-dependent dilation after multi-walled carbon nanotube inhalation: A time-course study. Int. J. Mol. Sci. 2012, 13, 13781–13803. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Wang, L.; Battelli, L.A.; McKinney, W.; Castranova, V.; Porter, D.W. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, Y.P.; Tkach, A.V.; Yanamala, N.; Stanley, S.; Gao, S.; Shurin, M.R.; Kisin, E.R.; Kagan, V.E.; Shvedova, A. Dual acute proinflammatory and antifibrotic pulmonary effects of short palate, lung, and nasal epithelium clone-1 after exposure to carbon nanotubes. Am. J. Respir. Cell Mol. Biol. 2013, 49, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, V.E.; Kapralov, A.A.; St. Croix, C.M.; Watkins, S.C.; Kisin, E.R.; Kotchey, G.P.; Balasubramanian, K.; Vlasova, I.I.; Yu, J.; Kim, K.; et al. Lung macrophages Digest carbon nanotubes using a superoxide/peroxynitrite oxidative pathway. ACS Nano 2014, 8, 5610–5621. [Google Scholar] [CrossRef] [PubMed]

| System | Animal Model | Type of CNTs | Size of Tested CNTs | Treatment Condition | Sample Preparation | Results | Reference |
|---|---|---|---|---|---|---|---|
| CytoViva enhanced dark-field microscopy | C57BL/6J male mouse | DWCNTs | L: <5 µm, D: 1–2 nm | 0, 1, 10, and 40 μg/mouse by pharyngeal aspiration 1-, 7- and 56-days post-exposure | Lungs were fixed with NBF, embedded, sectioned, and stained with Sirius Red | Microscopy revealed that CNTs generally observed within the interstitial tissue but also in condensed areas of alveolar macrophages | [82] |
| C57BL/6N female mouse | NM-401 and NRCWE-006 MWCNTs | NM-401 (L: 4.0 ± 0.37 µm, D: 67 ± 24 nm), NRCWE-006 (L: 5.7 ± 0.49 µm, D: 29–173 nm) | 54 µg/mouse by intratracheal instillation 1-year post-exposure | Lung and liver were fixed with formalin, embedded, sectioned, stained with haematoxylin and eosin | Imaging demonstrated the presence of single fibres both in lung and liver | [83] | |
| B6C3F1 male mice | MWCNTs | L: 4.46 µm, W: 58.5 nm | 40 µg/mouse by pharyngeal aspiration 1-, 7-, and 28-days post-exposure | Lungs were fixed, embedded, sectioned, and stained with Picrosirius red and haematoxylin | Microscopy confirmed the appearance of CNTs in sections of mouse lung at 28 days post-exposure | [84] | |
| C57BL/6J male mouse | MWCNTs | L: 2–15 µm, D: 8–15 nm | 20, 40 and 80 µg/mouse by pharyngeal aspiration 1-, 7-, 28-, and 56-days post-exposure | Precipitated cells from bronchoalveolar lavage fluid were stained with Romanovsky–Giemsa | Microscopy confirmed inclusions of CNTs in the cells after 56 days of exposure | [85] | |
| MWCNTs | The aerodynamic diameter is 1.3 µm | 10 mg/m3 for 2, 4, 8, and 12 days (5 h/day) by inhalation 1-day post-exposure | Lungs were fixed in 10% NBF, sectioned and stained with haematoxylin and eosin. The cells were isolated from whole lung lavage fluid | Imaging showed cell nucleus and pleural penetration by CNTs | [86] | ||
| Bare (B) and carboxylated (F) MWCNTs | BMWCNTs (D: 42 nm), FMWCNTs (D: 44 nm). The length was not measured | 40 µg/mouse by pharyngeal aspiration 56 days post-exposure | Lungs were fixed with NBF, embedded, sectioned, and stained with Sirius Red | Imaging demonstrated a greater amount of BMWCNTs within lungs in comparison with FMWCNTs | [87] | ||
| CytoViva enhanced dark-field microscopy coupled with hyperspectral imaging | BALB/c male mouse | Raw, minimally, and maximally carboxylated (f-) MWCNTs | MWCNTs (L: 10–30 µm), f-MWCNTs (L: 2.2 or 3.4 µm) | 50 µg/25 g mouse by pharyngeal aspiration 7- and 28-days post-exposure | Lungs were fixed with PFA, embedded, sectioned, and stained with haematoxylin and eosin | Microscopy coupled with hyperspectral imaging analysis confirmed that the degree of carboxylation affected the lung burden | [88] |
| Raw and carboxylated (f-) MWCNTs with different microwave radiation times (5–120 min) | MWCNTs (L: 10–30 µm, D: 20–30 nm). The hydrodynamic size of all MWCNT variants was in the range of 42–396 nm | 1 mg/mL by intratracheal instillation 3 days post-exposure | Not specified | Microscopy coupled with hyperspectral image showed that degree of functionalisation is critical to the distribution and the number of deposited CNTs on the epithelial cells | [89] | ||
| SD male rat | MWCNTs | L: 0.5–20 µm, D: 10–15 nm | 0.16 ± 0.01, 0.34 ± 0.02 and 0.94 ± 0.02 mg/m3 for 5 days by inhalation (6 h/day) 0- and 30-days post-exposure | Lungs fixed with NBF, embedded, sectioned, and stained with haematoxylin and eosin | EDFM-HSI showed that CNTs were deposited in the alveolar epithelium and the alveolar macrophages and persisted after 30 days of post-exposure | [90] | |
| Fischer 344 rat, male and female | MWCNTs | L: 0.5–20 µm, D: 10–15 nm | 0.2, 0.5 and 1.0 mg/m3 for 28 days by inhalation (6 h/day) 0-, 28- and 90-days post-exposure | Lungs fixed with NBF, embedded, sectioned, and stained with haematoxylin and eosin | Imaging coupled with spectral matching revealed persistence of CNTs even at 90 days of post-exposure; aggregated CNTs were observed at 28 days of post-exposure but resolved at 90 days | [91] | |
| SCID male mouse with tumour inoculation | Peptide- and dye-conjugated SWCNTs | L: 100–300, D: 0.8–1.2 nm | 0.068 mg/mL (180 µL) by intravenous injection 2- and 6-h post-exposure | CNTs-laden living cells were isolated from blood using FACS | Microscopy with hyperspectral image analysis showed uptake of CNTs by circulating cells and subcellular distribution of nanotubes | [92] | |
| CytoViva enhanced dark-field microscopy | C57BL/6J male mouse | MWCNTs | L: 3.86 µm, D: 49 ± 13.4 nm | 10, 20, 40 and 80 µg/mouse by pharyngeal aspiration 1-, 7-, 28- and 56-days post-exposure | Lungs were fixed with NBF, embedded, sectioned, and stained with Sirius Red and haematoxylin | Imaging demonstrated that CNTs readily penetrate all cell membranes/boundaries of the lungs; the majority of the MWCNTs were found within or penetrating alveolar macrophages but rarely observed in the airways by the 7th day after postexposure | [93] |
| MWCNTs | L: 4.3 µm | 5 mg/m3 for 12 days (5 h/day) by inhalation 1, 14, 28-, 84-, 168- and 336-days post-exposure | Tissue blocks (lung) were fixed, embedded, sectioned, and stained with Sirius Red and Mayer’s haematoxylin | Microscopy analysis confirmed a decrease in MWCNTs lung burden from 28 to 18 µg during 336 days of post-exposure; the presence of singular nanotubes was unchanged over 168 days post-exposure, while the concentration of aggregated particles decreased | [94] | ||
| SD male rat | MWCNTs | L: 3.9 µm, W: 49 nm | 5 mg/m3 for 1, 3 and 4 days (5 h/day) by inhalation 24 h post-exposure | Tissue blocks (lung, heart, kidney, and liver) were fixed, embedded, sectioned, and stained with Sirius Red and Mayer’s haematoxylin | Imaging confirmed small translocation of CNTs from the lung to the extrapulmonary organs | [95] | |
| C57BL/6J male mouse | MWCNTs | L: 4.3 µm | 5 mg/m3 for 12 days (5 h/day) by inhalation 1- and 336-days post-exposure | Tissue blocks (lung, tracheobronchial lymph nodes, diaphragm, heart, kidney, liver, and brain) were fixed, embedded, sectioned, and stained with Sirius Red and Mayer’s haematoxylin | Microscopy confirmed that inhaled MWCNTs are translocated from the lung to the extrapulmonary organs and accumulated with time | [96] | |
| WT and Scgb1a1-hSPLUNC1 TG mice | Chemically cut SWCNTs | L: ≈200 nm | 80 µg/mouse by pharyngeal aspiration 7 days post-exposure | Not specified | Microscopy revealed a higher concentration of CNTs in WT mice with the predominance in the alveolar tissue region | [97] | |
| NADPH-oxidase-deficient and C57BL/6 mice | Oxidised SWCNTs | L: 0.4–2.4 µm | 40 µg/mouse by pharyngeal aspiration 7- and 28-days post-exposure | Lungs were fixed, embedded, sectioned, stained with haematoxylin and eosin. | Microscopy confirmed the significant decrease in CNTs-laden macrophages by 28th-day post-exposure; the clearance of CNTs in NADPH-oxidase-deficient mouse was less effective compared to the control group | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishmukhametov, I.; Fakhrullin, R. Dark-Field Hyperspectral Microscopy for Carbon Nanotubes Bioimaging. Appl. Sci. 2021, 11, 12132. https://doi.org/10.3390/app112412132
Ishmukhametov I, Fakhrullin R. Dark-Field Hyperspectral Microscopy for Carbon Nanotubes Bioimaging. Applied Sciences. 2021; 11(24):12132. https://doi.org/10.3390/app112412132
Chicago/Turabian StyleIshmukhametov, Ilnur, and Rawil Fakhrullin. 2021. "Dark-Field Hyperspectral Microscopy for Carbon Nanotubes Bioimaging" Applied Sciences 11, no. 24: 12132. https://doi.org/10.3390/app112412132






