From Feather to Adsorbent: Keratin Extraction, Chemical Modification, and Fe(III) Removal from Aqueous Solution
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
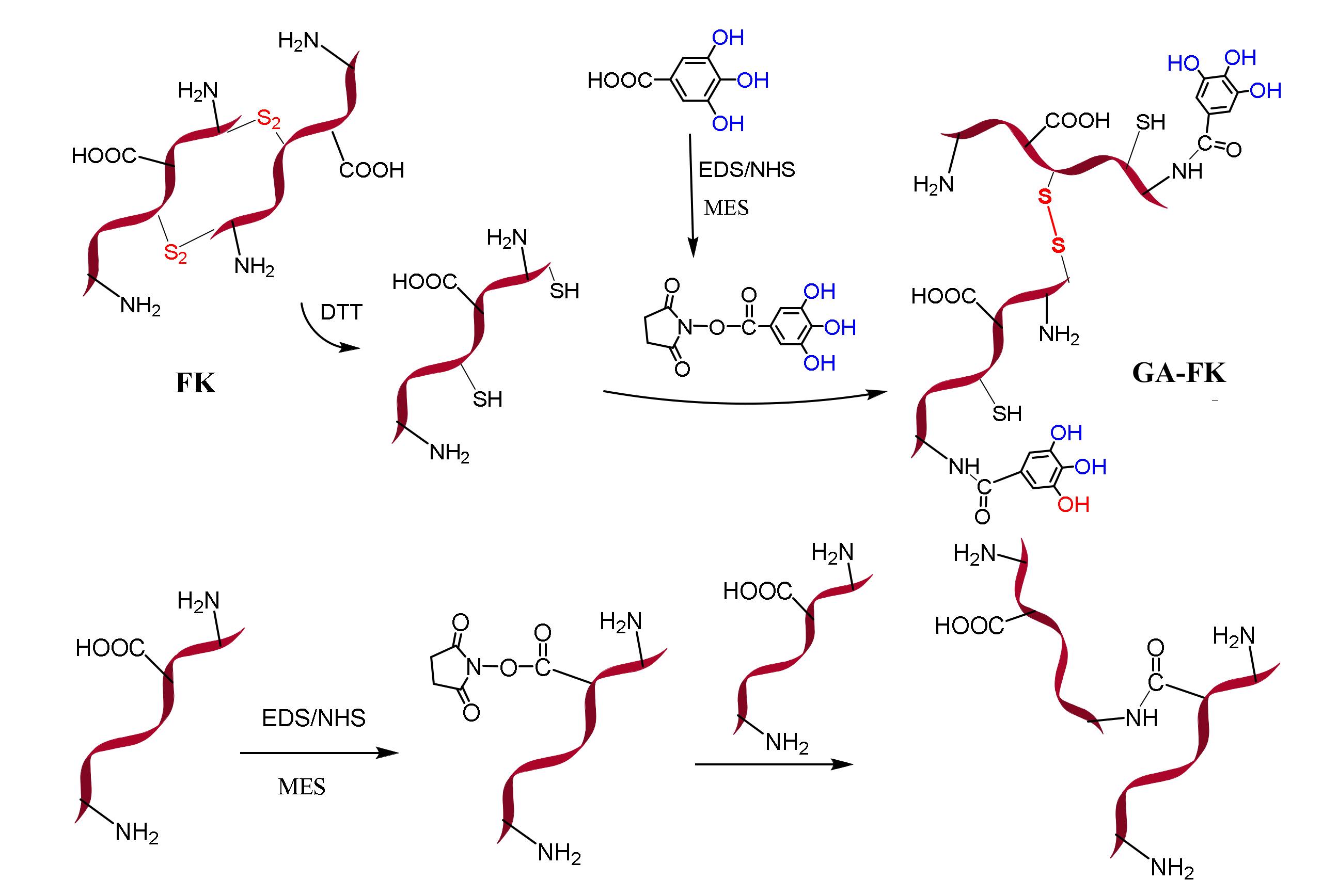
2.2. Preparation of GA-FK Gel
2.3. Characterization of FK
2.4. Characterization of GA-FK Gel
2.5. Adsorption Tests of GA-FK Gel to Fe(Ⅲ) in Aqueous Solution
2.6. Adsorption Kinetics and Isotherms
3. Results
3.1. Preparation Mechanism of GA-FK Gel
3.2. Characterization of FK
3.2.1. Molecular Weight of FK
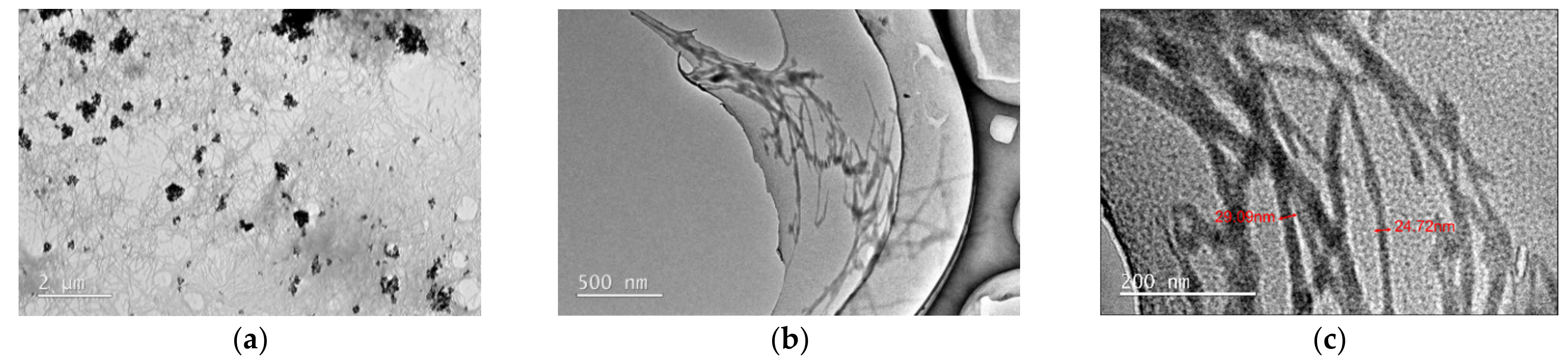
3.2.2. Aggregation Morphology of FK in an Aqueous Environment
3.3. Characterization of GA-FK Gel
3.3.1. IR Analysis
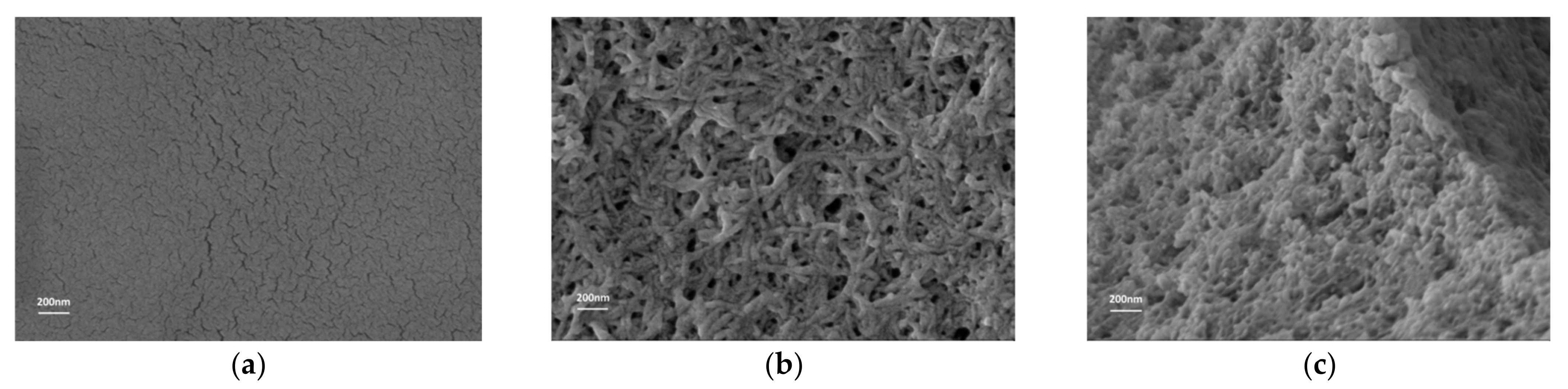
3.3.2. Micro-Morphology of GA-FK Gel
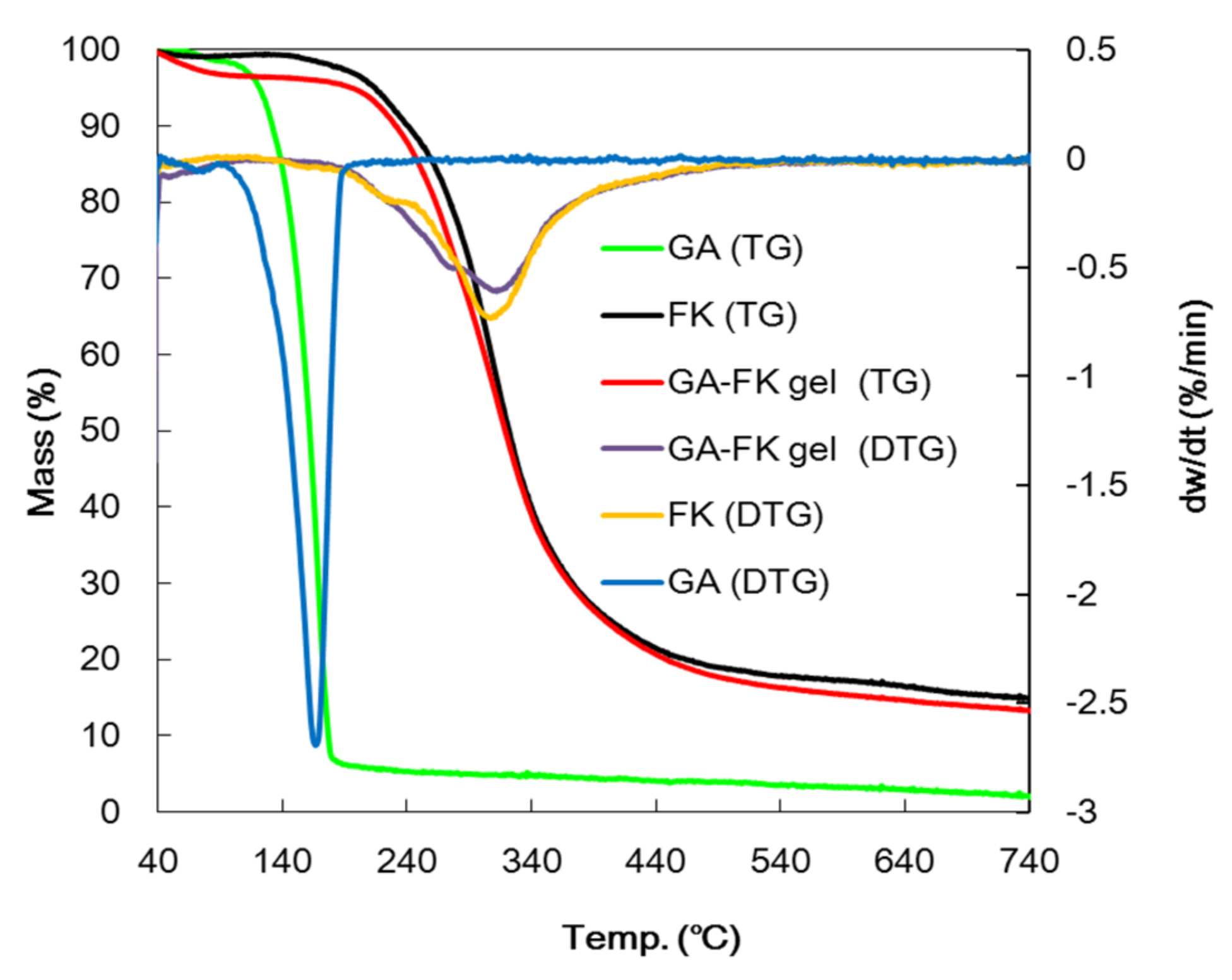
3.3.3. TG Analysis
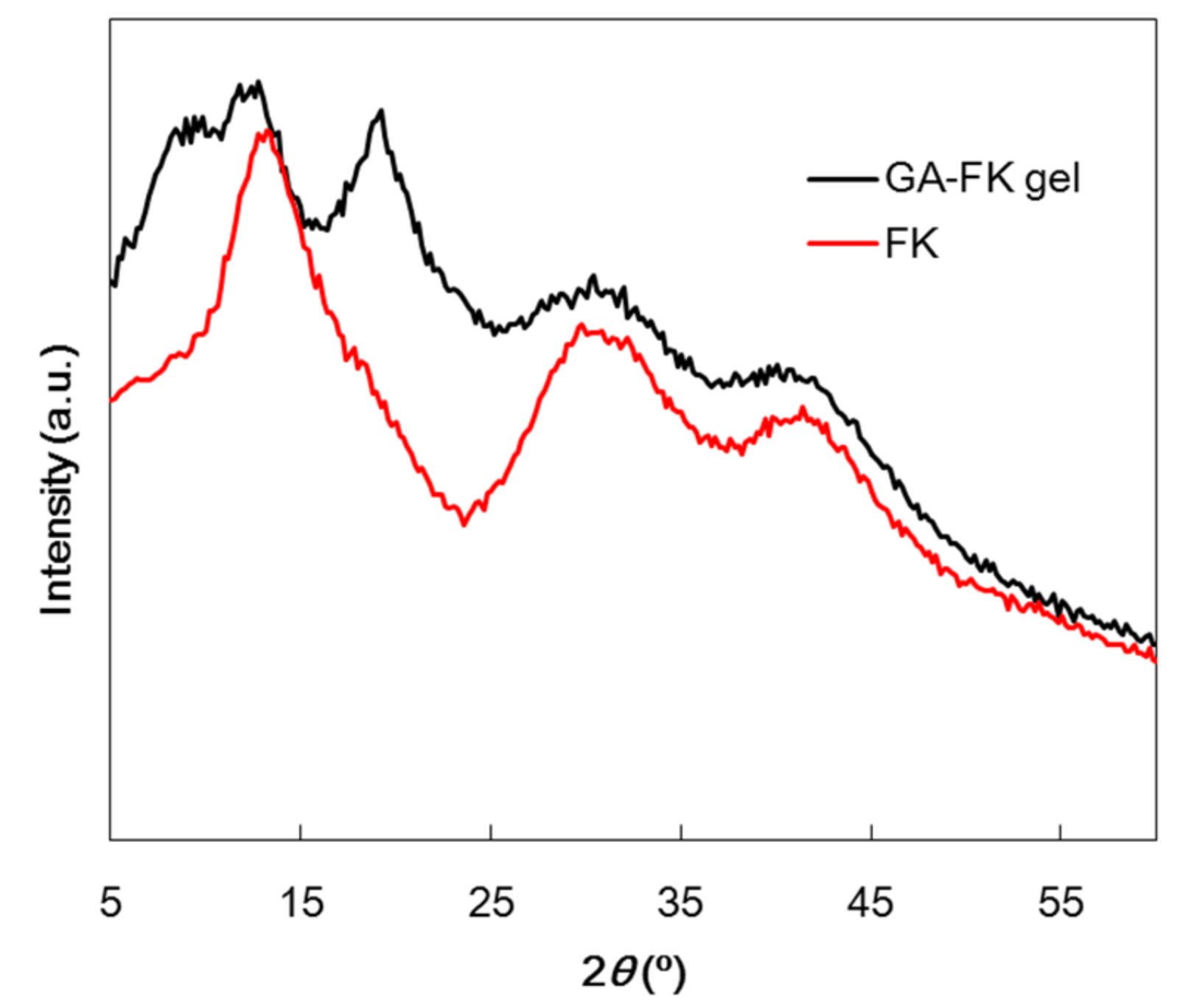
3.3.4. XRD Analysis
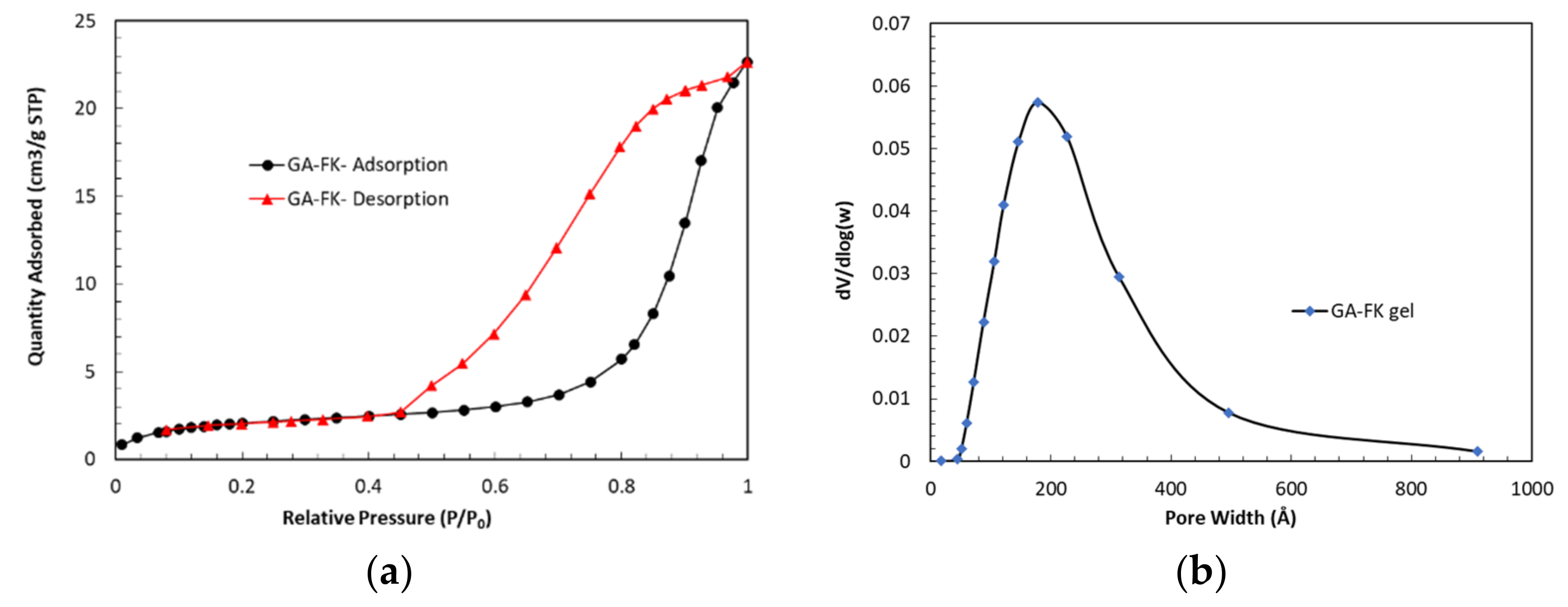
3.3.5. Nitrogen Adsorption Isotherms and Pore Size Distribution
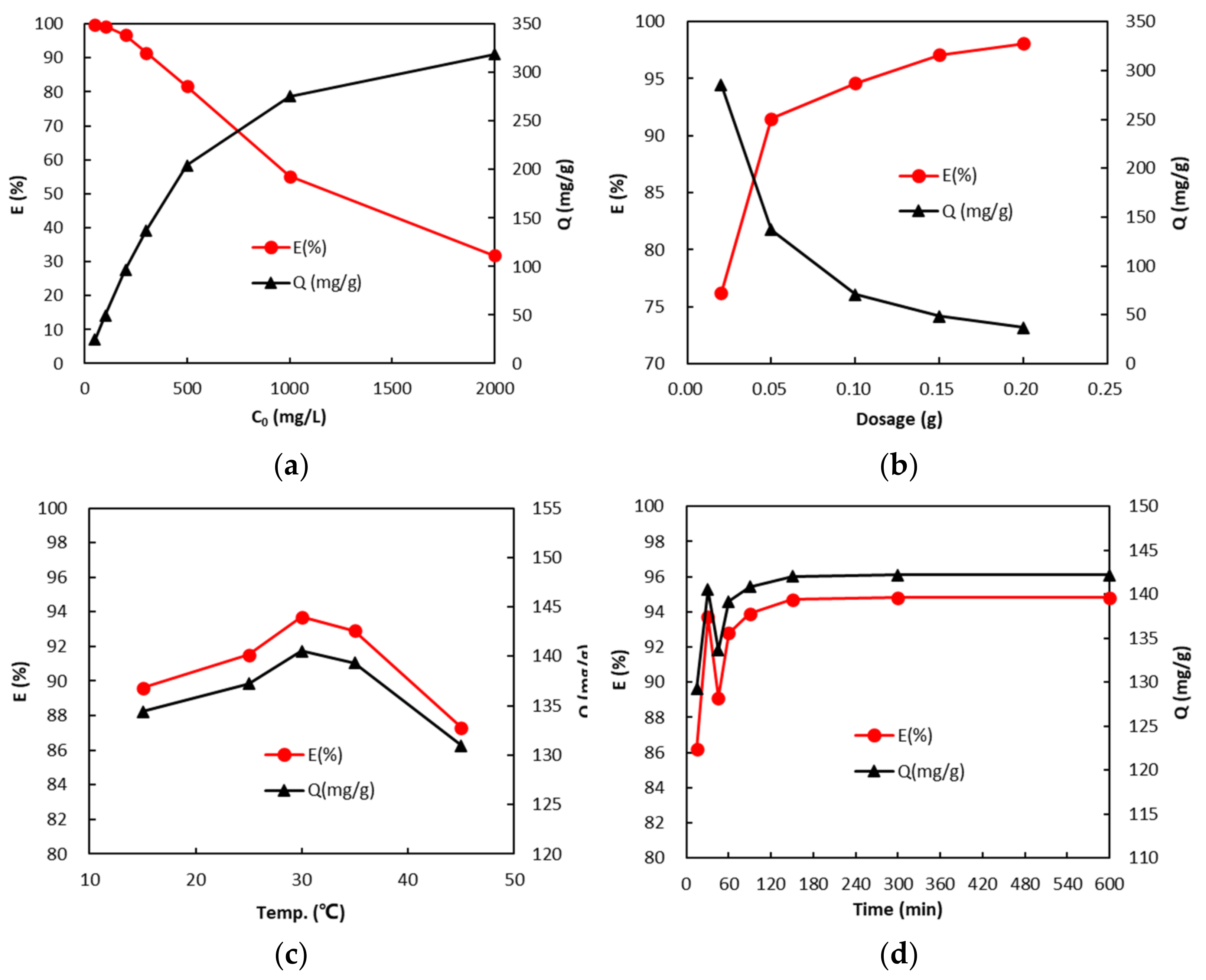
3.4. Adsorption Properties of GA-FK Gel
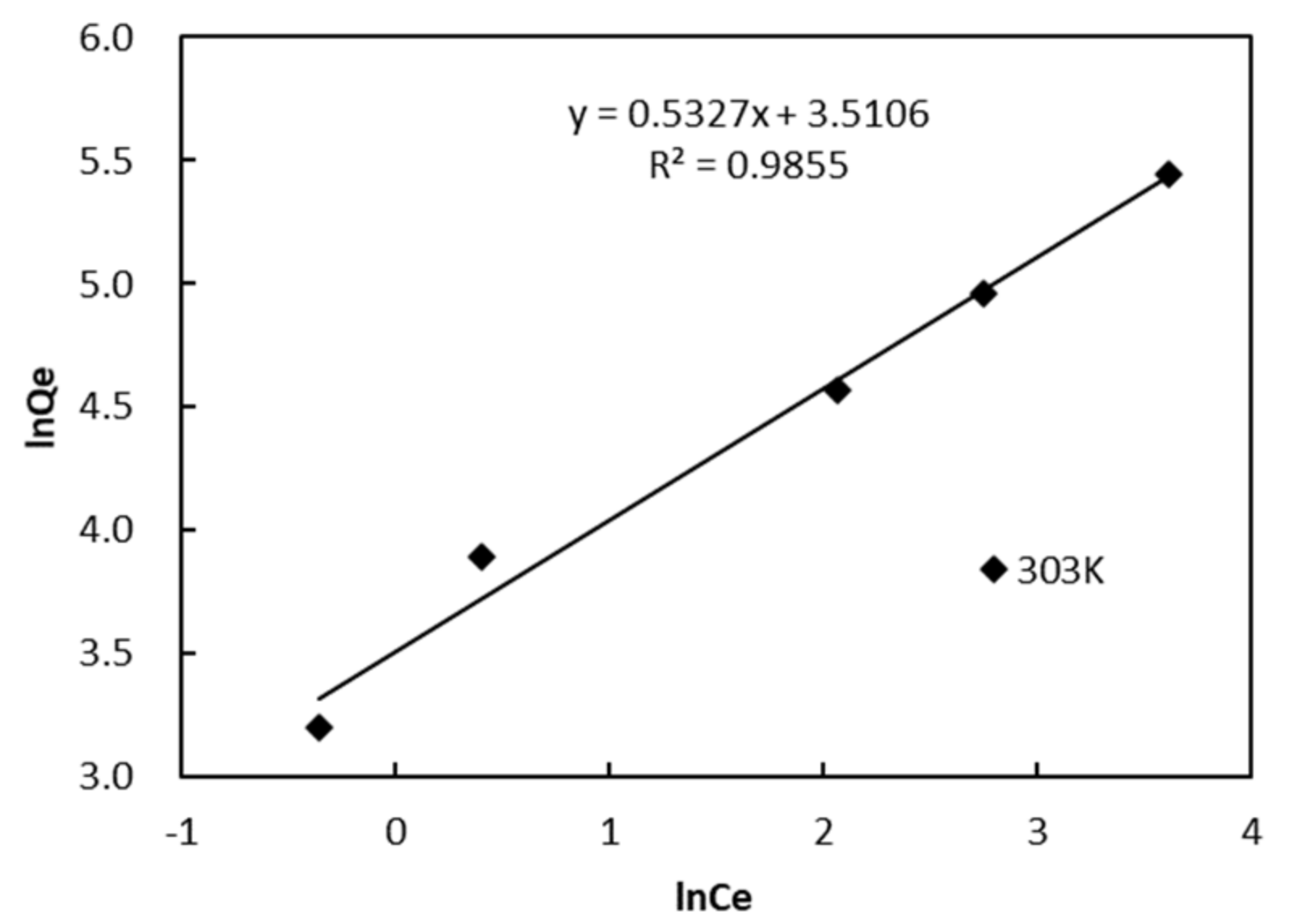
3.5. Adsorption Isotherm
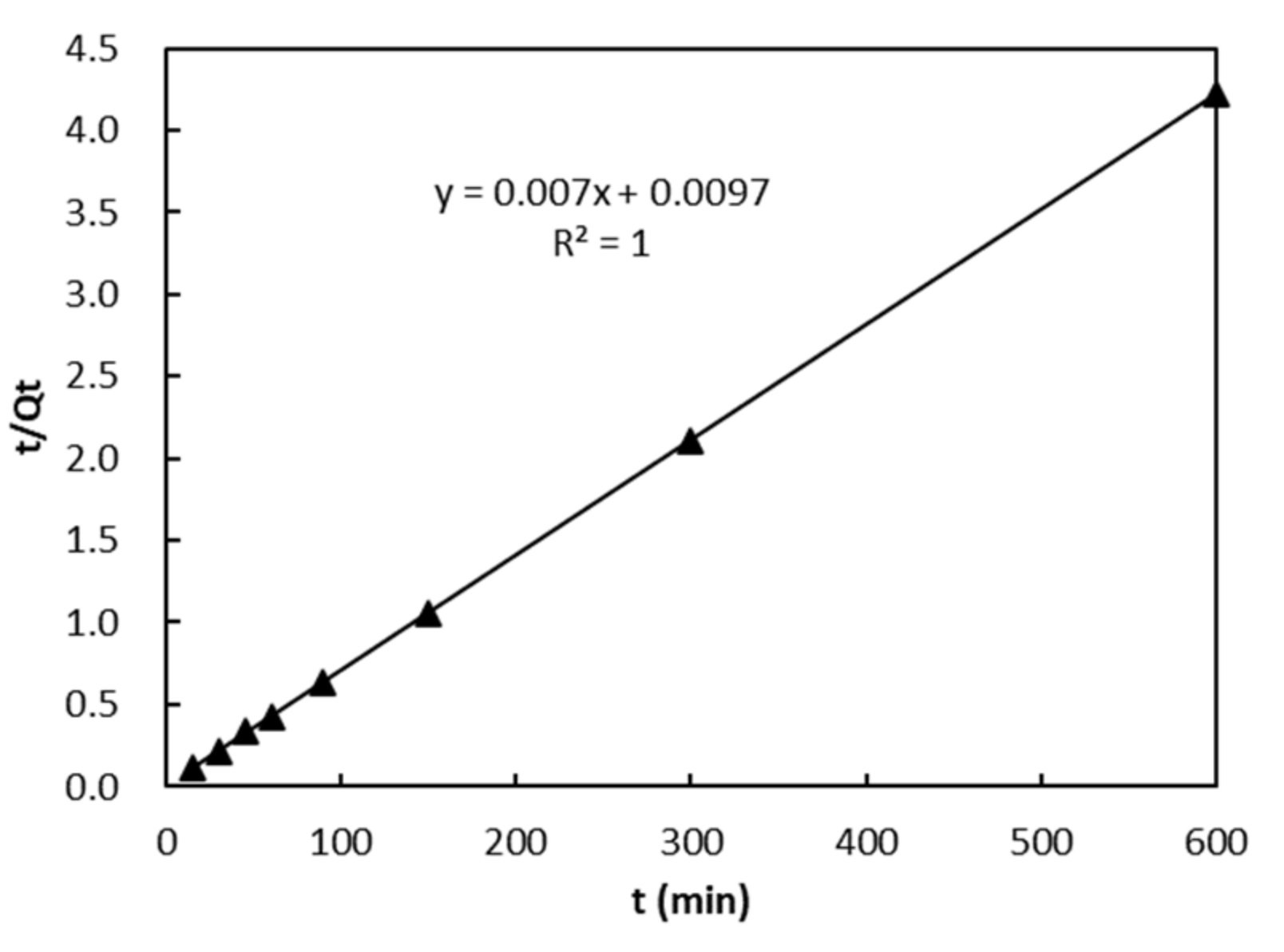
3.6. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.-J.; Di, L.; Ren, Q.-S.; Wang, J.-Y. Applications and degradation of proteins used as tissue engineering materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef] [Green Version]
- Compassion in World Farming. Statistics: Broiler Chickens Welfare Problems; Compassion in World Farming: Surrey, UK, 2013; Volume 8, pp. 1–11. [Google Scholar]
- Kormanjos, S.M.; Filipovic, S.S.; Radovic, V.A.; Okanovic, D.G.; Njezic, Z.B. Influence of the applied pressure of processing upon biocative comonents of diets made of feathers. Hem. Ind. 2013, 67, 135–138. [Google Scholar] [CrossRef]
- Kucinska, J.K.; Magnucka, E.G.; Oksinska, M.P.; Pietr, S.J. Bioefficacy of hen feather keratin hydrolysate and compost on vegetable plant growth. Compost. Sci. Util. 2014, 22, 179–187. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Insam, H. Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: A review reatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: A review. Crit. Rev. Microbiol. 2013, 39, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.B.; Townend, F. The titration curve of feather keratin. Trans. Faraday Soc. 1936, 32, 897. [Google Scholar] [CrossRef]
- Lundgrcn, H.P.; Stein, A.M.; Koorn, V.M.; O’Connell, R.A. Stability of synthetic keratin fibers in alcohol–water mixtures. Theoretical basis for a new method for solubilizing feather keratin. J. Phys. Colloid Chem. 1948, 52, 180–206. [Google Scholar] [CrossRef]
- Yin, X.-C.; Li, F.-Y.; He, Y.-F.; Wang, Y.; Wang, R.-M. Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater. Sci. 2013, 1, 528–536. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y. Controlled de-cross-linking and disentanglement of feather keratin for fiber preparation via a novel process. ACS Sustain. Chem. Eng. 2014, 2, 1404–1410. [Google Scholar] [CrossRef]
- Barati, D.; Kader, S.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Sawyer, R.H.; Jabbari, E. Synthesis and Characterization of Photo-Cross-Linkable Keratin Hydrogels for Stem Cell Encapsulation. Biomacromolecules 2017, 18, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cai, S.; Xu, L.; Yang, Y. Water-stable three-dimensional ultrafine fibrous scaffolds from keratin for cartilage tissue engineering. Langmuir 2014, 30, 8461–8470. [Google Scholar] [CrossRef] [Green Version]
- Mu, B.; Hassan, F.; Yang, Y. Controlled assembly of secondary keratin structures for continuous and scalable production of tough fibers from chicken feathers. Green Chem. 2020, 22, 1726–1734. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.; Tanideh, N.; Zare, S.; Sari Aslani, F.; Koohi-Hosseinabadi, O.; Rowshanghias, A.; Pourjavaheri, F.; Mehryar, P.; Muthuraj, R. Electrospun poly(3-hydroxybutyrate)/chicken feather-derived keratin scaffolds: Fabrication, in vitro and in vivo biocompatibility evaluation. J. Biomater. Appl. 2020, 34, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Matyasovsky, J.; Sedliacik, J.; Matyasovsky, J., Jr.; Jurkovic, P.; Duchovic, P. Collagen and keratin colloid systems with a multifunctional effect for cosmetic and technical applications. J. Am. Leather Chem. Assoc. 2014, 109, 284–295. [Google Scholar]
- Wrześniewska-Tosik, K.; Marcinkowska, M.; Niekraszewicz, A.; Potocka, D.A.; Mik, T. Fibrous Composites Based on Keratin from Chicken Feathers. FIBRES Text. East. Eur. 2011, 19, 118–123. [Google Scholar]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Grupta, M.; Mukherjee, A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int. J. Biolog. Macromol. 2021, 180, 339–354. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Z.-T.; Liu, Z.-W. Chemically modified chicken feather as sorbent for removing toxic chromium(VI) ions. Ind. Eng. Chem. Res. 2009, 48, 6882–6889. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. RSC Advances Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Hager, A.S.; Vallons, K.J.R.; Arendt, E.K. Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J. Agric. Food Chem. 2012, 60, 6157–6163. [Google Scholar] [CrossRef]
- Orliac, O.; Rouilly, A.; Silvestre, F.; Rigal, L. Effects of additives on the mechanical properties, hydrophobicity and water uptake of thermo-moulded films produced from sunflower protein isolate. Polymer 2002, 43, 5417–5425. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Selvakumar, R.; Sastry, T.P.; Sadulla, S.; Mandal, A.B.; Doble, M. Experimental and theoretical studies on gallic acid assisted EDC/NHS initiated crosslinked collagen scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Chien, W.; Tseng, S.-J.; Tang, S.-C. EDC/NHS-mediated heparinization of small intestinal submucosa for recombinant adeno-associated virus serotype 2 binding and transduction. Biomaterials 2007, 28, 2350–2357. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Finfrock, Y.Z.; Liu, Y.Y. Characterization of chromium species and distribution during Cr(VI) removal by biochar using confocal micro-X-ray fluorescence redox mapping and X-ray absorption spectroscopy. Environ. Int. 2020, 134, 105216. [Google Scholar] [CrossRef]
- Dong, N.; He, F.; Xin, J.; Wang, Q.; Lei, Z.; Su, B. A novel one-step hydrothermal method to prepare CoFe2O4/graphene-like carbons magnetic separable adsorbent. Mater. Res. Bull. 2016, 80, 186–190. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Arai, K.M.; Takahashi, R.; Yokote, Y.; Akahane, K. Amino-acid sequence of feather keratin from fowl. Eur. J. Biochem. 1983, 132, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.D.; Bridgen, J. The Keratin Chains of Avian Scale Tissue. Sequence Heterogeneity and the Number of Scale Keratin Genes. Eur. J. Biochem. 1976, 67, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.D.; Rogers, G.E. The Structural Basis for the Heterogeneity of Chick down Feather Keratin. The Partial Amino Acid Sequence of Down Feather Kreatin. Eur. J. Biochem. 1976, 69, 341–350. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. The role of β-sheets in the structure and assembly of keratins. Biophys. Rev. 2009, 1, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, R.D.B.; Parry, D.A.D. Reprint of: Keratin intermediate filaments: Differences in the sequences of the Type I and Type II chains explain the origin of the stability of an enzyme-resistant four-chain fragment. J. Struct. Biol. 2014, 186, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, M.; Eckhart, L.; Alibardi, L. The molecular organization of the beta-sheet region in Corneous beta-proteins (beta-keratins) of sauropsids explains its stability and polymerization into filaments. J. Struct. Biol. 2016, 194, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.B.; Parry, D.A.D. Molecular packing in the feather keratin filament. J. Struct. Biol. 2008, 162, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhou, H.; Lian, J.; Chen, H.; Xu, H.; Zhou, X. Preparation of pH-responsive avermectin/feather keratin-hyaluronic acid with anti-UV and sustained-release properties. Colloids Surf. B Biointerfaces 2019, 175, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Bio. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Yu, F.; Ma, L.; Pan, X.; Luo, G.; Lin, S.; Mo, X.; He, C.; Wang, H. Hyaluronic acid/EDC/NHS-crosslinked green electrospun silk fibroin nanofibrous scaffolds for tissue engineering. Rsc. Adv. 2016, 6, 99720–99728. [Google Scholar] [CrossRef]
- Zou, X.; Pan, J.; Ou, H.; Wang, X.; Guan, W.; Li, C.; Yan, Y.; Duan, Y. Adsorptive removal of Cr(III) and Fe(III) from aqueous solution by chitosan/attapulgite composites: Equilibrium, thermodynamics and kinetics. Chem. Eng. J. 2011, 167, 112–121. [Google Scholar] [CrossRef]

| Mn (g/mol) | Mw (g/mol) | Mw/Mn | |
|---|---|---|---|
| FK | 21,910 (20%) | 46,390 (18%) | 2.117 (27%) |
| T (K) | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF (L·mg−1) | 1/n | R2 | Qm,cal (mg·g−1) | KL (L·mg−1) | R2 | |
| 303 | 40.516 | 0.4433 | 0.9855 | 178.6 | 0.201 | 0.9253 |
| T(K) | Qe,exp | Pseudo First Order Kinetic Model | Pseudo Second Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | Qe1,cal | R2 | k2 | Qe2,cal | R2 | ||
| 303 | 142.2 | 0.155 | 38.7 | 0.8199 | 0.005 | 142.9 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Wang, C.; Wang, Y.; Wang, R. From Feather to Adsorbent: Keratin Extraction, Chemical Modification, and Fe(III) Removal from Aqueous Solution. Appl. Sci. 2021, 11, 12163. https://doi.org/10.3390/app112412163
Pan S, Wang C, Wang Y, Wang R. From Feather to Adsorbent: Keratin Extraction, Chemical Modification, and Fe(III) Removal from Aqueous Solution. Applied Sciences. 2021; 11(24):12163. https://doi.org/10.3390/app112412163
Chicago/Turabian StylePan, Sujuan, Changqing Wang, Yibo Wang, and Rongmin Wang. 2021. "From Feather to Adsorbent: Keratin Extraction, Chemical Modification, and Fe(III) Removal from Aqueous Solution" Applied Sciences 11, no. 24: 12163. https://doi.org/10.3390/app112412163
APA StylePan, S., Wang, C., Wang, Y., & Wang, R. (2021). From Feather to Adsorbent: Keratin Extraction, Chemical Modification, and Fe(III) Removal from Aqueous Solution. Applied Sciences, 11(24), 12163. https://doi.org/10.3390/app112412163






