The Development of Current Collection in Micro-Tubular Solid Oxide Fuel Cells—A Review
Abstract
Featured Application
Abstract
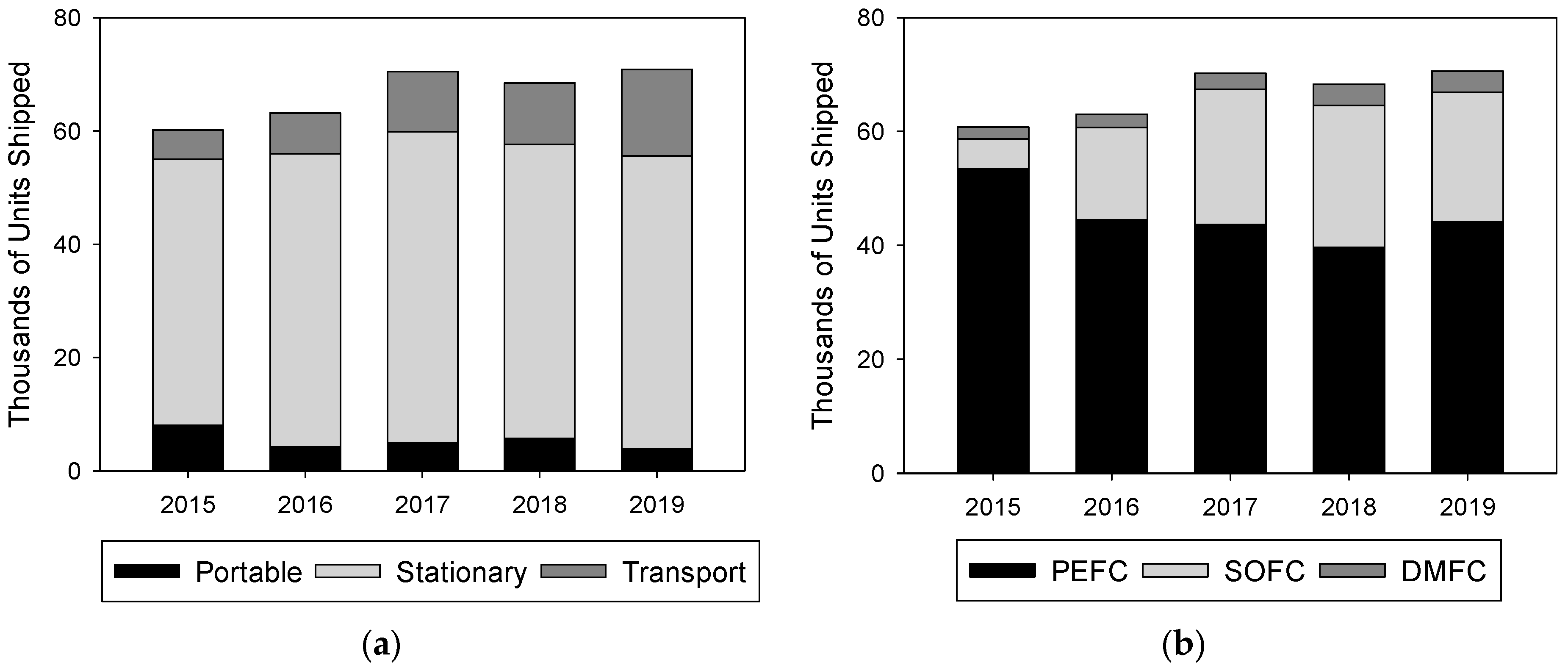
1. Introduction to Fuel Cell Technology
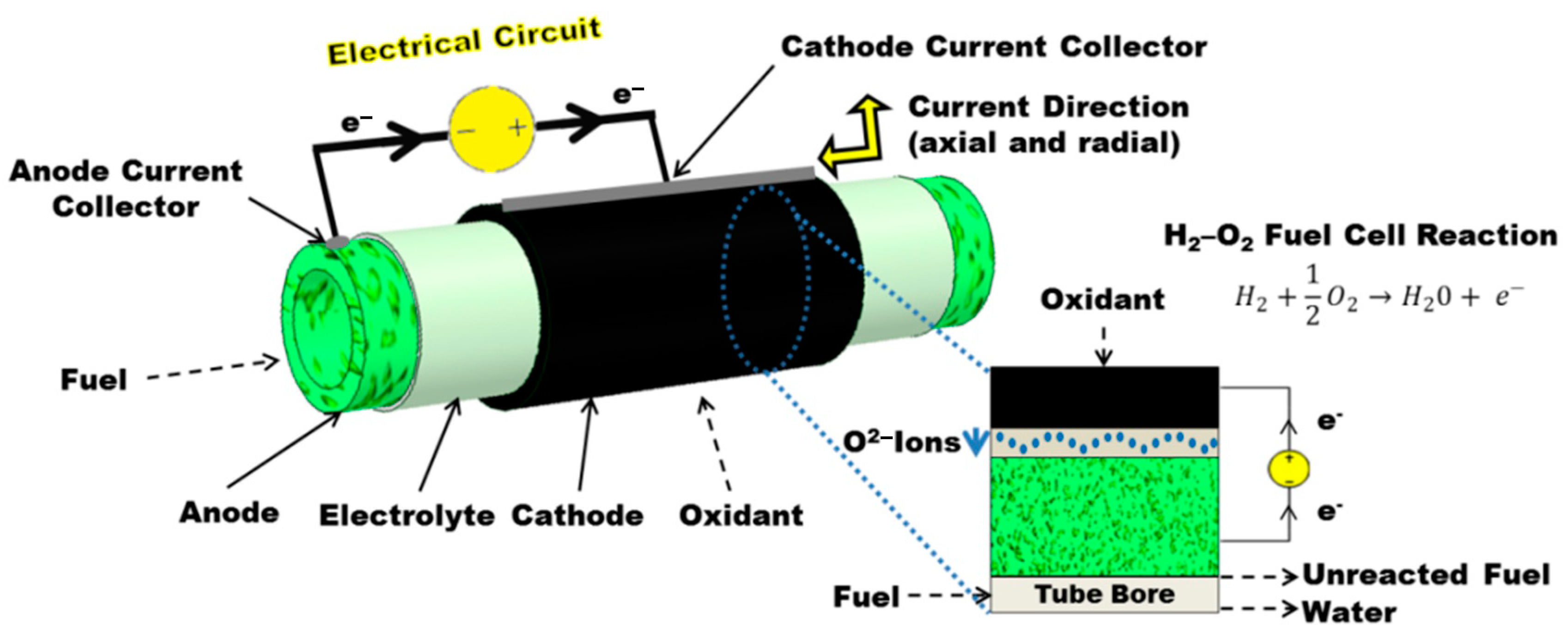
2. Tubular Solid Oxide Fuel Cells
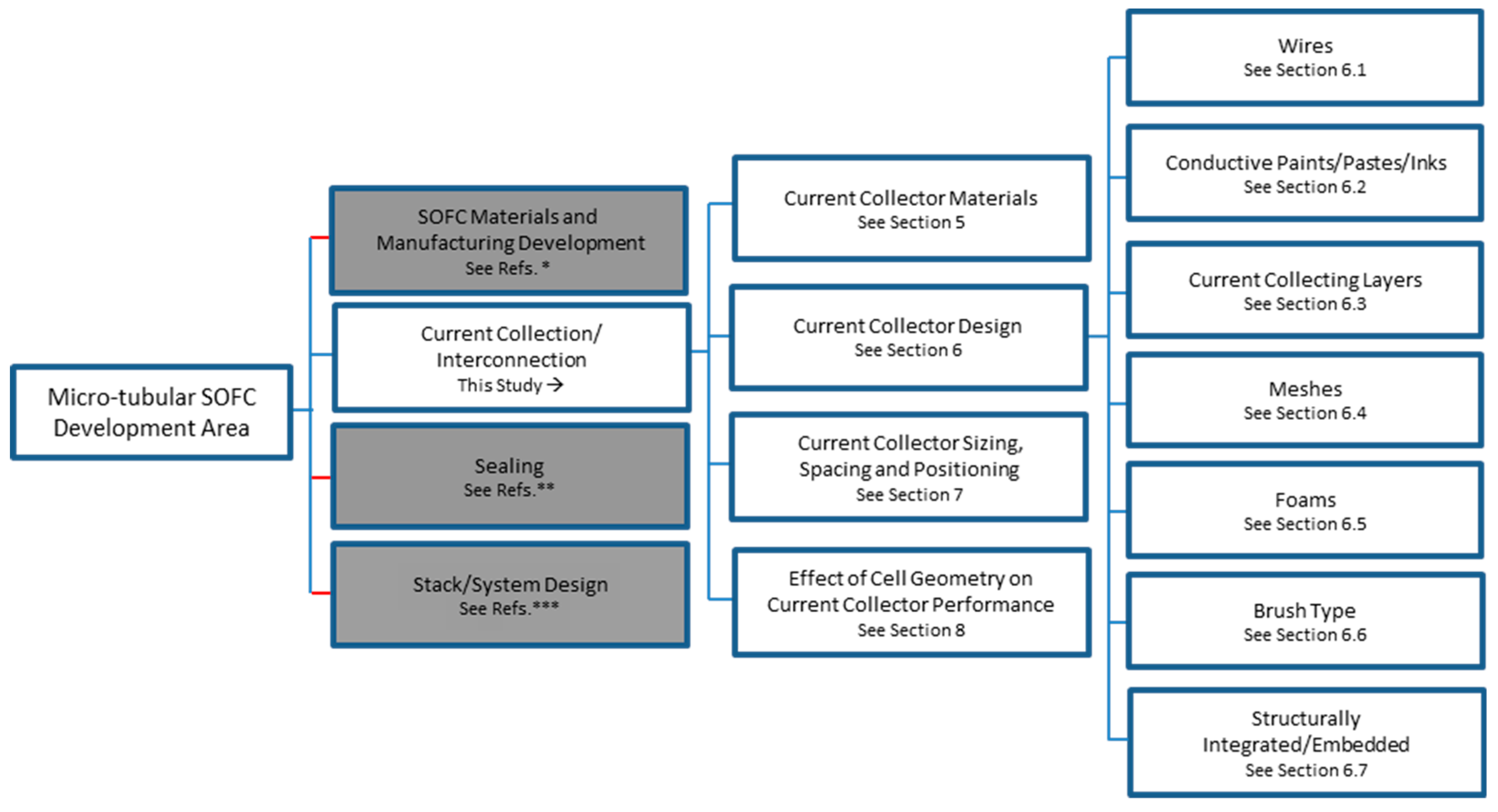
3. Current Collection in µT-SOFCs
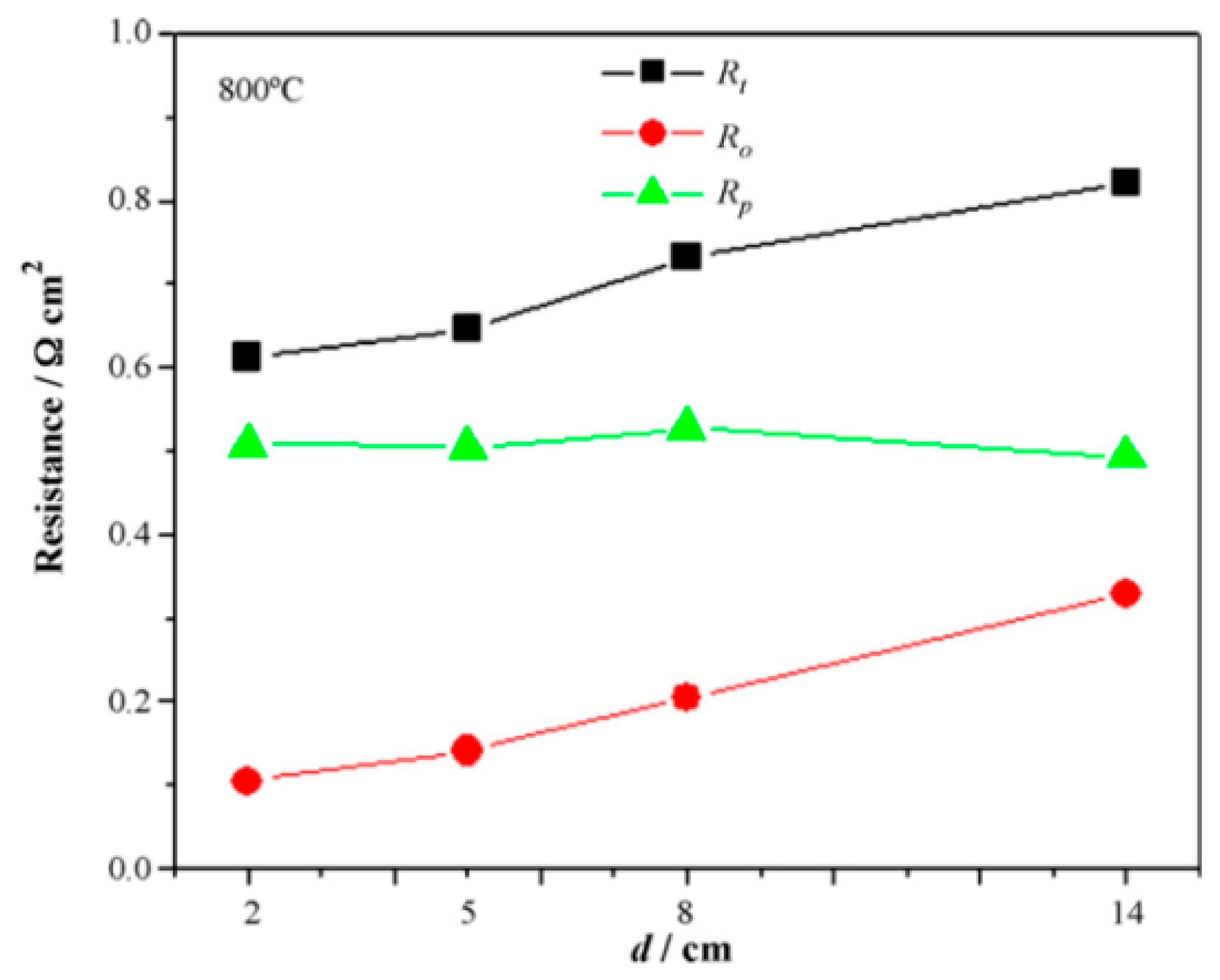
4. Current Collector Performance
5. Materials for Current Collection
5.1. Historical Trend in SOFC Interconnect Materials
5.2. Metallic Interconnects
5.3. Micro-Tubular SOFC Geometry-Specific Current Collector Material Trend
6. Current Collector Shape and Structure
6.1. Wires
6.2. Conductive Pastes, Paints and Inks
6.3. Current Collecting Layers
6.4. Meshes
6.5. Foams
6.6. Brush Type
6.7. Structurally Integrated/Embedded
7. Current Collector Sizing, Spacing and Positioning
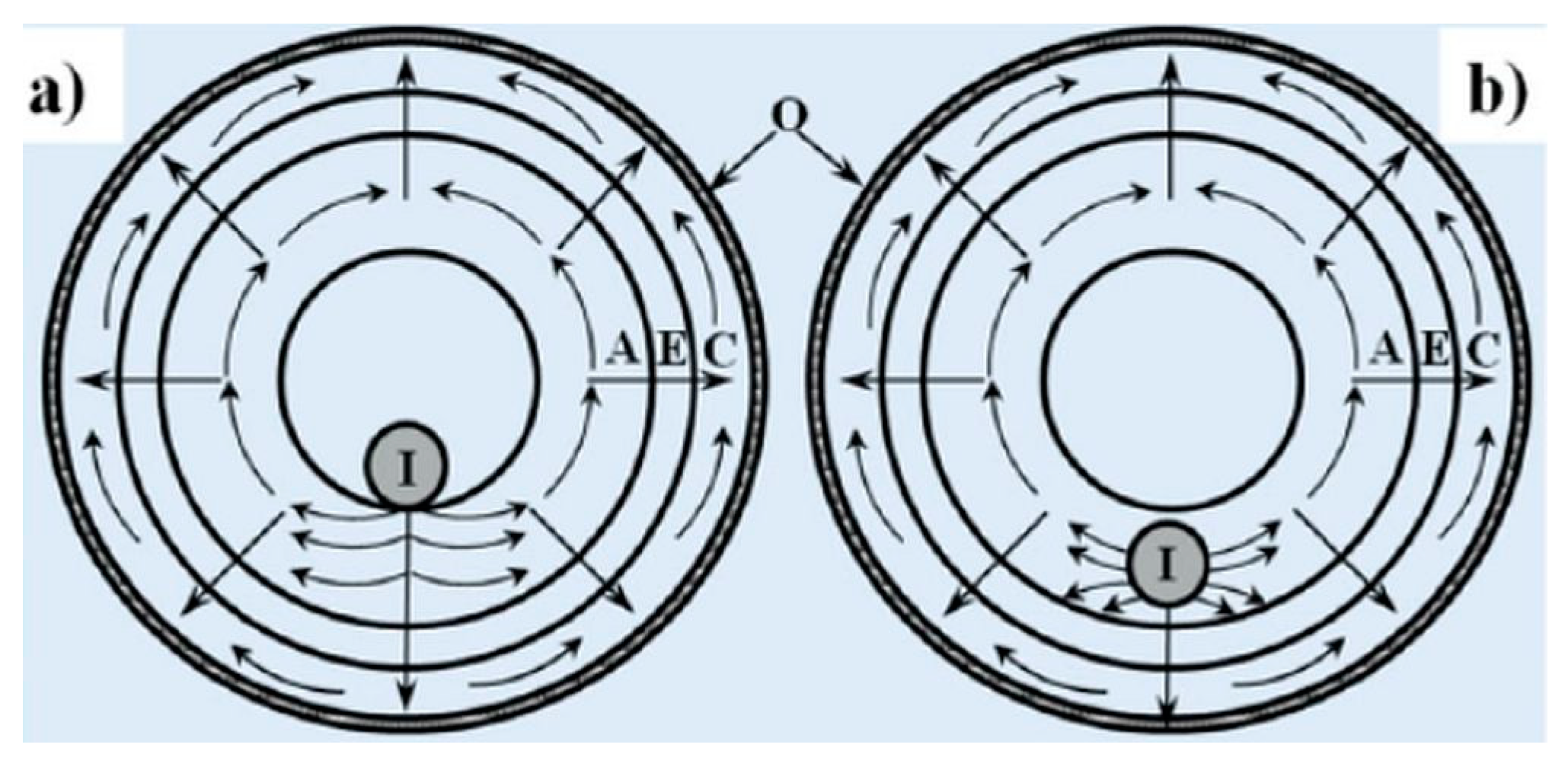
8. Effect of Cell Geometry on Current Collection
9. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bujalski, W.; Dikwal, C.M.; Kendall, P.K. Cycling of three solid oxide fuel cell types. J. Power Sources 2007, 171, 96–100. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M. High-Temperature Solid Oxide Fuel Cells for the 21st Century: Fundamentals, Design and Applications; Elsevier: London, UK, 2015. [Google Scholar]
- Mench, M.M. Fuel Cell Engines; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Boersma, R.J.; Sammes, N.M.; Fee, C.J. Losses resulting from in-plane electricity conduction in tubular solid oxide fuel cells. Solid State Ion. 2000, 135, 493–502. [Google Scholar] [CrossRef]
- Bove, R.; Sammes, N.M. The Effect of Current Collectors Configuration on the Performance of a Tubular SOFC. Proc. Electrochem. Soc. 2005, 2005–2007, 780–789. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M.; Niewolak, L.; Tietz, F.; Quadakkers, W.J. Interconnects. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: London, UK, 2016; pp. 195–254. [Google Scholar]
- Larmine, J.; Dicks, A. Fuel Cell Systems Explained; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Chen, W. Mobile Applications: Cars, Trucks, locomotives, Marine Vehicles, and Aircraft; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Singhal, S.C. Solid oxide fuel cells for stationary, mobile, and military applications. Solid State Ion. 2002, 152, 405–410. [Google Scholar] [CrossRef]
- Nissan Motor Corporation. Nissan Announces Development of the World’s First SOFC-Powered Vehicle System that Runs on Bioethanol Electric Power. 2016. Available online: https://global.nissannews.com/en/releases/160614-01-e?source=nng (accessed on 3 October 2020).
- Hart, D.; Lehner, F.; Jones, S.; Lewis, J. The Fuel Cell Industry Review 2019. 2019. Available online: www.FuelCellIndustryReview.com (accessed on 1 May 2020).
- Watanabe, C. Ene-Farms Use Hydrogen to Power Homes but Don’t Come Cheap. Bloom. Technol. 2015. Available online: https://www.bloomberg.com/news/articles/2015-01-15/fuel-cells-for-homes-japanese-companies-pitch-clean-energy (accessed on 1 August 2020).
- Plug Power. GENDRIVE Fuel Cells. 2017. Available online: http://www.plugpower.com/products/gendrive/ (accessed on 8 May 2017).
- Intelligent Energy. Intelligent Energy’s Fuel Cells Stacks to be Used in Met Police Zero Emission Scooter Trial. Intell. Energy 2017. Available online: http://www.intelligent-energy.com/news-and-events/company-news/2017/02/07/intelligent-energys-fuel-cells-stacks-to-be-used-in-met-police-zero-emission-scooter-trial/ (accessed on 8 May 2017).
- Barley, S. Hydrogen Bus Launched on London Tourist Route, Guard. 2010. Available online: https://www.theguardian.com/environment/2010/dec/10/hydrogen-bus-london (accessed on 5 August 2020).
- Hyundai. Hyundai ix35 Hydrogen Fuel Cell Vehicle. 2017. Available online: http://www.hyundai.co.uk/about-us/environment/hydrogen-fuel-cell (accessed on 8 May 2017).
- Toyota. Mirai—Bringing the Future into the Present. 2017. Available online: https://www.toyota.co.uk/new-cars/new-mirai/landing.json (accessed on 8 May 2017).
- Fernandes, M.; Andrade, S.D.P.; Bistritzki, V.; Fonseca, R.; Zacarias, L.; Gonçalves, H.; De Castro, A.; Domingues, R.; Matencio, T. SOFC-APU systems for aircraft: A review. Int. J. Hydrog. Energy 2018, 43, 16311–16333. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Jamil, S.M.; Othman, M.H.D.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Li, K. Recent fabrication techniques for micro-tubular solid oxide fuel cell support: A review. J. Eur. Ceram. Soc. 2015, 35, 1–22. [Google Scholar] [CrossRef]
- Bianco, M.; Linder, M.; Larring, Y.; Greco, F.; Van Herle, J. Lifetime Issues for Solid Oxide Fuel Cell Interconnects; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Zhu, J.H.; Ghezel-Ayagh, H. Cathode-side electrical contact and contact materials for solid oxide fuel cell stacking: A review. Int. J. Hydrog. Energy 2017, 42, 24278–24300. [Google Scholar] [CrossRef]
- Shi, H.; Su, C.; Yang, G.; Ran, R.; Hao, Y.; Tadé, M.O.; Shao, Z. Fabrication and operation of flow-through tubular SOFCs for electric power and synthesis gas cogeneration from methane. AIChE J. 2014, 60, 1036–1044. [Google Scholar] [CrossRef]
- Lessing, P.A. A review of sealing technologies applicable to solid oxide electrolysis cells. J. Mater. Sci. 2007, 42, 3465–3476. [Google Scholar] [CrossRef]
- Singh, R.N. Sealing Technology for Solid Oxide Fuel Cells (SOFC). Int. J. Appl. Ceram. Technol. 2007, 4, 134–144. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A review on cell/stack designs for high performance solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Lawlor, V.; Griesser, S.S.; Buchinger, G.; Olabi, A.; Cordiner, S.; Meissner, D. Review of the micro-tubular solid oxide fuel cell Part I. Stack design issues and research activities. J. Power Sources 2009, 193, 387–399. [Google Scholar] [CrossRef]
- Howe, K.S.; Thompson, G.J.; Kendall, K. Micro-tubular solid oxide fuel cells and stacks. J. Power Sources 2011, 196, 1677–1686. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M.; Minh, N.Q. Cell and stack design, fabrication and performance. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: London, UK, 2016; pp. 255–282. [Google Scholar]
- Wachsman, E.D.; Lee, K. Lowering the Temperature of Solid Oxide Fuel Cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef]
- Zhu, W.; Deevi, S. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. A 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, L.M.; Rivas, M.; Otaegi, L.; Gomez, N.; Alvarez, M.A.; Sarasketa-Zabala, E.; Manzanedo, J.; Burgos, N.; Castro, F.; Laresgoiti, A.; et al. Tubular Metal Support Solid Oxide Fuel Cell Manufacturing and Characterization. ECS Trans. 2011, 35, 445–450. [Google Scholar] [CrossRef]
- Haberman, B.A.; Marquis, A.J. A Numerical Investigation into the Interaction between Current Flow and Fuel Consumption in a Segmented-in-Series Tubular SOFC. J. Fuel Cell Sci. Technol. 2009, 6, 031002. [Google Scholar] [CrossRef]
- Vora, S.D. SECA Program at Siemens Westinghouse, Presented at SECA Annual Workshop and Peer Review Meeting. 2004. Available online: https://www.osti.gov/scitech/servlets/purl/834189#page=41 (accessed on 20 August 2020).
- Kendall, M.; Meadowcroft, A.D.; Kendall, K. Microtubular Solid Oxide Fuel Cells (mSOFCs). ECS Trans. 2013, 57, 123–131. [Google Scholar] [CrossRef]
- Kendall, K. Introduction to SOFCs. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: London, UK, 2016; pp. 1–24. [Google Scholar]
- Sin, Y.; Galloway, K.; Roy, B.; Sammes, N.M.; Song, J. The properties and performance of micro-tubular (less than 2.0 mm O.D.) anode suported solid oxide fuel cell (SOFC). Int. J. Hydrog. Energy 2011, 36, 1882–1889. [Google Scholar] [CrossRef]
- Ren, C.; Gan, Y.; Yang, C.; Lee, M.; Xue, X. Fabrication and Characterization of Direct Methane Fueled Thin Film SOFCs Supported by Microchannel-Structured Microtubular Substrates. ACS Appl. Energy Mater. 2020, 3, 1831–1841. [Google Scholar] [CrossRef]
- Kendall, K.; Meadowcroft, A. Improved ceramics leading to microtubular Solid Oxide Fuel Cells (mSOFCs). Int. J. Hydrog. Energy 2013, 38, 1725–1730. [Google Scholar] [CrossRef]
- Mukerjee, S.; Leah, R.; Selby, M.; Stevenson, G.; Brandon, N.P. Life and Reliability of Solid Oxide Fuel Cell-Based Products. In Solid Oxide Fuel Cell Lifetime and Reliability; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 173–192. [Google Scholar] [CrossRef]
- Hart, D.; Lehner, F.; Rose, R.; Lewis, J.; Klippenstein, M. The Fuel Cell Industry Review 2015; E4Ttech: London, UK, 2015. [Google Scholar]
- Kraftwek. 2017. Available online: https://www.kickstarter.com/projects/ezelleron/kraftwerk-highly-innovative-portable-power-plant (accessed on 4 April 2017).
- Kendall, K.; Kendall, M.; Adler, S.B. Sources of cell and electrode polarisation losses in SOFCs. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: London, UK, 2016; pp. 357–381. [Google Scholar] [CrossRef]
- Hatchwell, C.; Sammes, N.; Kendall, K. Cathode current-collectors for a novel tubular SOFC design. J. Power Sources 1998, 70, 85–90. [Google Scholar] [CrossRef]
- Hatchwell, C.; Sammes, N.M.; Brown, I.W.M.; Kendall, K. Current collectors for a novel tubular design of solid oxide fuel cell. J. Power Sources 1999, 77, 64–68. [Google Scholar] [CrossRef]
- Kilbride, I.P. Preparation and properties of small diameter tubular solid oxide fuel cells for rapid start-up. J. Power Sources 1996, 61, 167–171. [Google Scholar] [CrossRef]
- Kendall, K.; Palin, M. A small solid oxide fuel cell demonstrator for microelectronic applications. J. Power Sources 1998, 71, 268–270. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Improvement of SOFC Performance Using a Microtubular, Anode-Supported SOFC. J. Electrochem. Soc. 2006, 153, 925–928. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamaguchi, T.; Fujishiro, Y.; Awano, M. Fabrication and characterization of micro tubular SOFCs for operation in the intermediate temperature. J. Power Sources 2006, 160, 73–77. [Google Scholar] [CrossRef]
- Dhir, A.; Kendall, K. Microtubular SOFC anode optimisation for direct use on methane. J. Power Sources 2008, 181, 297–303. [Google Scholar] [CrossRef]
- Dhir, A. Improved Microtubular Solid Oxide Fuel Cells. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2008. [Google Scholar]
- Dhir, A.; Kendall, K. Improving Reliability of Microtubular SOFCs for Direct Use on Methane. ECS Trans. 2007, 7, 823–828. [Google Scholar] [CrossRef]
- Kendall, K. Progress in solid oxide fuel cell materials. Int. Mater. Rev. 2005, 50, 257–264. [Google Scholar] [CrossRef]
- Fu, Y.; Bazant, M.Z. Theoretical and Experimental Study of Solid Oxide Fuel Cell (SOFC) Using Impedance Spectra. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2014. [Google Scholar]
- Wu, J.; Liu, X. Recent Development of SOFC Metallic Interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Fergus, J.W. Metallic interconnects for solid oxide fuel cells. Mater. Sci. Eng. A 2005, 397, 271–283. [Google Scholar] [CrossRef]
- Kong, W.; Li, J.; Liu, S.; Lin, Z. The influence of interconnect ribs on the performance of planar solid oxide fuel cell and formulae for optimal rib sizes. J. Power Sources 2012, 204, 106–115. [Google Scholar] [CrossRef]
- Fergus, J.W. Lanthanum chromite-based materials for solid oxide fuel cell interconnects. Solid State Ion. 2004, 171, 1–15. [Google Scholar] [CrossRef]
- Badwal, S.P.; Zhang, J. Interaction between chromia forming alloy interconnects and air. Solid State Ion. 1997, 99, 297–310. [Google Scholar] [CrossRef]
- Brandner, L.S.S.M.; Bienertb, C.; Megela, S.; Kusnezoffa, M.; Trofimenkoa, N.; Sauchuka, V.; Venskutonisb, A.; Krausslerb, W.; Michaelis, A. Long Term Performance of Stacks with Chromium-based Interconnects (CFY). ECS Trans. 2013, 57, 2235–2244. [Google Scholar] [CrossRef]
- Niewolak, L.; Wessel, E.; Singheiser, L.; Quadakkers, W.J. Potential suitability of ferritic and austenitic steels as interconnect materials for solid oxide fuel cells operating at 600 °C. J. Power Sources 2010, 195, 7600–7608. [Google Scholar] [CrossRef]
- Casteel, M. Hybrid Materials Design for SOFC Interconnect Applications. Ph.D. Thesis, Rensselaer Polytechnic Institute, Troy, NY, USA, 2012. [Google Scholar]
- Hammer, J.E.; Laney, S.J.; Jackson, R.W.; Coyne, K.; Pettit, F.S. The Oxidation of Ferritic Stainless Steels in Simulated Solid-Oxide Fuel-Cell Atmospheres. Oxid. Met. 2007, 67, 1–38. [Google Scholar] [CrossRef]
- Jablonski, P.D.; Alman, D.E. Oxidation resistance and mechanical properties of experimental low coefficient of thermal expansion (CTE) Ni-base alloys. Int. J. Hydrog. Energy 2007, 32, 3705–3712. [Google Scholar] [CrossRef]
- Sakai, N.; Yokokawa, H.; Horita, T.; Yamaji, K. Lanthanum Chromite-Based Interconnects as Key Materials for SOFC Stack Development. Int. J. Appl. Ceram. Technol. 2005, 1, 23–30. [Google Scholar] [CrossRef]
- Linder, M.; Hocker, T.; Holzer, L.; Friedrich, K.A.; Iwanschitz, B.; Mai, A.; Schuler, J.A. Model-based prediction of the ohmic resistance of metallic interconnects from oxide scale growth based on scanning electron microscopy. J. Power Sources 2014, 272, 595–605. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Ramousse, S.; Kromp, A.; Leonide, A.; Weber, A. Manufacturing and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2011, 196, 7117–7125. [Google Scholar] [CrossRef]
- Zhao, F.; Virkar, A.V. Dependence of polarization in anode-supported solid oxide fuel cells on various cell parameters. J. Power Sources 2005, 141, 79–95. [Google Scholar] [CrossRef]
- Finsterbusch, M. Degradation Mechanisms of Solid Oxide Fuel Cell Cathodes. Ph.D. Thesis, Technische Universität Ilmenau, Ilmenau, Germany, 2011. [Google Scholar]
- Froitzheim, J.; Canovic, S.; Nikumaa, M.; Sachitanand, R.; Johansson, L.; Svensson, J.-E. Long term study of Cr evaporation and high temperature corrosion behaviour of Co coated ferritic steel for solid oxide fuel cell interconnects. J. Power Sources 2012, 220, 217–227. [Google Scholar] [CrossRef]
- Singh, P.; Yang, Z.; Viswanathan, V.; Stevenson, J.W. Observations on the Structural Degradation of Silver during Simultaneous Exposure to Oxidizing and Reducing Environments. J. Mater. Eng. Perform. 2004, 13, 287–294. [Google Scholar] [CrossRef]
- Wu, J.; Johnson, C.D.; Gemmen, R.S.; Liu, X. The performance of solid oxide fuel cells with Mn–Co electroplated interconnect as cathode current collector. J. Power Sources 2009, 189, 1106–1113. [Google Scholar] [CrossRef]
- Mukerjee, S.; Haltiner, K.; Kerr, R.; Chick, L.; Sprenkle, V.; Meinhardt, K.; Lu, C.; Kim, J.Y.; Weil, K.S. Solid Oxide Fuel Cell Development: Latest Results. ECS Trans. 2007, 7, 59–65. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Aydin, O.; Nakajima, H.; Kitahara, T. Processes Involving in the Temperature Variations in Solid Oxide Fuel Cells In-Situ Analyzed through Electrode-Segmentation Method. J. Electrochem. Soc. 2015, 163, F216–F224. [Google Scholar] [CrossRef]
- Compson, C.; Songho, C.; Abermathy, H.; Choi, Y.-M.; Meilin, L. Stability and performance of silver in an SOFC interconnect environment. In Proceedings of the 31st International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 21–26 January 2007; pp. 301–312. [Google Scholar]
- Akhtar, N.; Decent, S.P.; Kendall, K. Structural stability of silver under single-chamber solid oxide fuel cell conditions. Int. J. Hydrog. Energy 2009, 34, 7807–7810. [Google Scholar] [CrossRef]
- Majewski, A.J.; Dhir, A. Application of silver in microtubular solid oxide fuel cells. Mater. Renew. Sustain. Energy 2018, 7, 16. [Google Scholar] [CrossRef]
- Cambridge Press. Materials Data Book, 2003 Editi; Cambridge University Engineering Department: Cambridge, UK, 2003. [Google Scholar]
- Ding, J.; Zhou, X.; Liu, Q.; Yin, G. Development of tubular anode-supported solid oxide fuel cell cell and 4-cell-stack based on lanthanum gallate electrolyte membrane for mobile application. J. Power Sources 2018, 401, 336–342. [Google Scholar] [CrossRef]
- Zhu, H.; Kee, R.J.; Janardhanan, V.M.; Deutschmann, O.; Goodwin, D.G. Modeling Elementary Heterogeneous Chemistry and Electrochemistry in Solid-Oxide Fuel Cells. J. Electrochem. Soc. 2005, 152, A2427–A2440. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Parkinson, R. Properties and Applications of Electroless Nickel. Nickel Dev. Inst. 1997, 37, 1–33. Available online: https://www.nickelinstitute.org/media/1769/propertiesandapplicationsofelectrolessnickel_10081_.pdf (accessed on 5 February 2020).
- Curtis, L. Electroforming—Jewellery Handbooks; Bloomsbury Publishing PLC: London, UK, 2004. [Google Scholar]
- Fan, P.; Li, G.; Zeng, Y.; Zhang, X. Numerical study on thermal stresses of a planar solid oxide fuel cell. Int. J. Therm. Sci. 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Lin, C.; Chen, T.; Chyou, Y.; Chiang, L. Thermal stress analysis of a planar SOFC stack. J. Power Sources 2007, 164, 238–251. [Google Scholar] [CrossRef]
- ThyssenKrupp, V.D.M. Crofer 22 APU—Material Data Sheet No. 4046; ThyssenKrupp VDM: Hongkong, China, 2010. [Google Scholar]
- Ottersted, R. Electrolyte for Cost-Effective, Electrolyte-Supported High-Temperature Fuel Cell Having High Performance and High Mechanical Strength. U.S. Patent 9,136,553 B2, 15 September 2015. [Google Scholar]
- Haynes International. HAYNES® 230® Alloy, Data Sheet. 2020. Available online: http://haynesintl.com/docs/default-source/pdfs/new-alloy-brochures/high-temperature-alloys/brochures/230-brochure.pdf (accessed on 1 January 2020).
- Linderoth, S.; Hendriksen, P.V.; Mogensen, M.; Langvad, N. Investigations of metallic alloys for use as interconnects in solid oxide fuel cell stacks. J. Mater. Sci. 1996, 31, 5077–5082. [Google Scholar] [CrossRef]
- Greiner, H. Chromium based Alloys for High Temperature SOFC Applications. ECS Proc. 1995, 1995–1, 879–888. [Google Scholar] [CrossRef]
- Sammes, N.M.; Du, Y.; Bove, R. Design and fabrication of a 100 W anode supported micro-tubular SOFC stack. J. Power Sources 2005, 145, 428–434. [Google Scholar] [CrossRef]
- AZO Materials, Azo Materials, Silver—Applications and Properties of Silver. 2001. Available online: https://www.azom.com/properties.aspx?ArticleID=600 (accessed on 1 January 2021).
- The Alloys Network, Properties, Fabrication and Applications of Commercially Pure Nickel. 2020. Available online: https://www.nickel-alloys.net/article/commercially-pure-properties.html (accessed on 18 September 2020).
- Merker, J.; Lupton, D.; Töpfer, M.; Knake, H. High temperature mechanical properties of the platinum group metals: Elastic properties of platinum, rhodium and iridium and their alloys at high temperatures. Platin. Met. Rev. 2001, 45, 74–82. [Google Scholar]
- AZO Materials, AZO Materials, Gold—Properties and Applications of Gold. 2001. Available online: https://www.azom.com/properties.aspx?ArticleID=598 (accessed on 1 January 2021).
- AZO Materials, AZO Materials, an Introduction to Palladium. 2001. Available online: https://www.azom.com/properties.aspx?ArticleID=1339 (accessed on 1 January 2021).
- Jiang, S.P.; Love, J.G.; Apateanu, L. Effect of contact between electrode and current collector on the performance of solid oxide fuel cells. Solid State Ion. 2003, 160, 15–26. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M.; Kendall, K. Portable early market SOFCs. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: London, UK, 2016; pp. 329–356. [Google Scholar]
- Rabuni, M.F.; Vatcharasuwan, N.; Li, T.; Li, K. High performance micro-monolithic reversible solid oxide electrochemical reactor. J. Power Sources 2020, 458, 228026. [Google Scholar] [CrossRef]
- Hornes, A.; Torrell, M.; Morata, A.; Kendall, M.; Kendall, K.; Tarancon, A.; Road, P.H. Towards a high fuel utilization and low degradation of micro-tubular solid oxide fuel cells. Int. J. Hydrog. Energy 2017, 42, 13889–13901. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M.; Cassidy, M.; Connor, P.A.; Irvine, J.T.S.; Savaniu, C.D. Anodes. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: Amsterdam, The Netherlands, 2016; pp. 133–160. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M.; Kawada, T.; Horita, T. Cathodes. In High-Temperature Solid Oxide Fuel Cells 21st Century; Elsevier: Amsterdam, The Netherlands, 2016; pp. 161–193. [Google Scholar]
- Durango-Petro, J.; Usuba, J.; Valle, H.; Abarzua, G.; Flies, H.; Udayabhaskar, R.; Mangalaraja, R.V. Ascendable method for the fabrication of micro-tubular solid oxide fuel cells by ram-extrusion technique. Ceram. Int. 2020, 46, 2602–2611. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Monzón, H.; Larrea, A.; Orera, V. Improved stability of reversible solid oxide cells with a nickelate-based oxygen electrode. J. Phys. Chem. A 2015, 4, 1446–1453. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Luebbe, H.; Silva, J.; Van Herle, J. Electrochemical Performance of Nd1.95NiO4+δCathode supported Microtubular Solid Oxide Fuel Cells. Fuel Cells 2014, 15, 98–104. [Google Scholar] [CrossRef]
- Panthi, D.; Choi, B.; Tsutsumi, A. Direct methane operation of a micro-tubular solid oxide fuel cell with a porous zirconia support. J. Solid State Electrochem. 2016, 21, 255–262. [Google Scholar] [CrossRef]
- Panthi, D.; Choi, B.; Tsutsumi, A. A Novel Micro-Tubular Solid Oxide Fuel Cell with a Porous Zirconia Support for Intermediate-Temperature Operation. ECS Trans. 2015, 68, 2259–2265. [Google Scholar] [CrossRef]
- Zhao, K.; Kim, B.H.; Xu, Q.; Du, Y.; Ahn, B.G. Redox cycling performance of inert-substrate-supported tubular single cells with nickel anode current collector. J. Power Sources 2015, 293, 336–342. [Google Scholar] [CrossRef]
- Kikuta, K.; Yasue, K.; Suzuki, M.; Kanehira, S.; Kirihara, S. Design and fabrication of micro close end tubular SOFC with internal conduction layer. J. Ceram. Soc. Jpn. 2016, 124, 360–364. [Google Scholar] [CrossRef]
- Li, T.; Wu, Z.; Li, K. A dual-structured anode/Ni-mesh current collector hollow fibre for micro-tubular solid oxide fuel cells (SOFCs). J. Power Sources 2014, 251, 145–151. [Google Scholar] [CrossRef]
- Kendall, P.K.; Slinn, M.; Preece, J. Formulating liquid ethers for microtubular SOFCs. J. Power Sources 2006, 157, 750–753. [Google Scholar] [CrossRef]
- Liu, C.; Pu, J.; Chen, X.; Ma, Z.; Ding, X.; Zhou, J.; Wang, S. Influence of anode’s microstructure on electrochemical performance of solid oxide direct carbon fuel cells. Int. J. Hydrog. Energy 2020, 45, 11784–11790. [Google Scholar] [CrossRef]
- Hanifi, A.R.; Paulson, S.; Torabi, A.; Shinbine, A.; Tucker, M.C.; Birss, V.; Etsell, T.H.; Sarkar, P. Slip-cast and hot-solution infiltrated porous yttria stabilized zirconia (YSZ) supported tubular fuel cells. J. Power Sources 2014, 266, 121–131. [Google Scholar] [CrossRef]
- Park, B.; Song, R.; Lee, S.; Lim, T.; Park, S.; Jung, W.; Lee, J. Conformal bi-layered perovskite/spinel coating on a metallic wire network for solid oxide fuel cells via an electrodeposition-based route. J. Power Sources 2017, 348, 40–47. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A.; Hanifi, A.R.; Etsell, T.H.; Sarkar, P.; Orera, V.M. Microtubular solid oxide fuel cells with lanthanum strontium manganite infiltrated cathodes. Int. J. Hydrog. Energy 2015, 40, 5469–5474. [Google Scholar] [CrossRef]
- Khan, M.Z.; Song, R.-H.; Hussain, A.; Lee, S.-B.; Lim, T.-H.; Hong, J.-E. Effect of applied current density on the degradation behavior of anode-supported flat-tubular solid oxide fuel cells. J. Eur. Ceram. Soc. 2020, 40, 1407–1417. [Google Scholar] [CrossRef]
- Cui, D.; Ji, Y.; Chang, C.; Wang, Z.; Xiao, X. Influence of fuel flow rate on the performance of micro tubular solid oxide fuel cell. Int. J. Hydrog. Energy 2020, 45, 13459–13468. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Li, L.; Wang, Z.; Liu, M.; Rainwater, B.H.; Bai, Y. Electrochemical properties of micro-tubular intermediate temperature solid oxide fuel cell with novel asymmetric structure based on BaZr0.1Ce0.7Y0.1Yb0.1O3−δ proton conducting electrolyte. Int. J. Hydrog. Energy 2019, 44, 16887–16897. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Lee, I.-S.; Park, M.-H.; Jun, J.-H.; Park, C.-R.; Kim, B.-S.; Lee, C.-W.; Choi, S.-H. Cathode Current Collector for Solid Oxide Fuel Cell, and Solid Oxide Fuel Cell Compromising same. U.S. Patent 2017/0005345 A1, 5 January 2017. [Google Scholar]
- Huang, W.; Finnerty, C.; Sharp, R.; Wang, K.; Balili, B. High-Performance 3D Printed Microtubular Solid Oxide Fuel Cells. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Troskialina, L.; Dhir, A.; Steinberger-Wilckens, R. Improved Performance and Durability of Anode Supported SOFC Operating on Biogas. ECS Trans. 2015, 68, 2503–2513. [Google Scholar] [CrossRef]
- Fu, X.Z.; Melnik, J.; Low, Q.X.; Luo, J.; Chuang, K.T.; Sanger, A.R.; Yang, Q.M. Surface modified Ni foam as current collector for syngas solid oxide fuel cells with perovskite anode catalyst. Int. J. Hydrog. Energy 2010, 35, 11180–11187. [Google Scholar] [CrossRef]
- Yan, N.; Fu, X.-Z.; Luo, J.; Chuang, K.T.; Sanger, A.R. Ni–P coated Ni foam as coking resistant current collector for solid oxide fuel cells fed by syngas. J. Power Sources 2012, 198, 164–169. [Google Scholar] [CrossRef]
- Crumm, A.; Shuck, Q.; Rice, J. Solid Oxide Fuel Cell with Improved Current Collection. U.S. Patent 8,343,689 B2, 1 January 2013. [Google Scholar]
- Helgadóttir, Á.; Lalot, S.; Beaubert, F.; Pálsson, H. Mesh Twisting Technique for Swirl Induced Laminar Flow Used to Determine a Desired Blade Shape. Appl. Sci. 2018, 8, 1865. [Google Scholar] [CrossRef]
- Naga Sarada, S.; Radha, K.K.; Raju, A.V.S. Experimental investigations in a circular tube to enhance turbulent heat transfer using mesh inserts. J. Eng. Appl. Sci. 2009, 4, 53–60. [Google Scholar]
- Rahimi, M.; Aghel, B.; Alsairafi, A. Chemical Engineering and Processing: Process Intensification Experimental and CFD studies on using coil wire insert in a proton exchange membrane fuel cell. Chem. Eng. Process. Process Intensif. 2010, 49, 688–695. [Google Scholar] [CrossRef]
- De la Torre, R.; Sglvado, V. Fabrication of Innovative Compliant Current Collector-Supported Microtubular Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2011, 9, 1058–1063. [Google Scholar] [CrossRef]
- De La Torre, R.; Avila-Paredes, H.J.; Sglavo, V.M. Comparative Performance Analysis of Anode-Supported Micro-Tubular SOFCs with Different Current-Collection Architectures. Fuel Cells 2013, 13. [Google Scholar] [CrossRef]
- De la Torre García, R. Production of Micro-Tubular Solid Oxide Fuel Cells. Ph.D. Thesis, University of Trento, Trento, Italy, 2011. [Google Scholar]
- Casarin, M.; Sglavo, V.M. Effect of the Current Collector on Performance of Anode-Supported Microtubular Solid Oxide Fuel Cells. J. Fuel Cell Sci. Technol. 2015, 12, 031005. [Google Scholar] [CrossRef]
- Celik, A.N. Three-dimensional multiphysics model of a planar solid oxide fuel cell using computational fluid dynamics approach. Int. J. Hydrog. Energy 2018, 43, 19730–19748. [Google Scholar] [CrossRef]
- Jeon, D.H. A comprehensive CFD model of anode-supported solid oxide fuel cells. Electrochim. Acta 2009, 54, 2727–2736. [Google Scholar] [CrossRef]
- Navasa, M.; Graves, C.; Chatzichristodoulou, C.; Løye, T.; Sund, B. A three dimensional multiphysics model of a solid oxide electrochemical cell: A tool for understanding degradation. Int. J. Hydrog. Energy 2018, 43, 11913–11931. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, C.; Jin, C.; Liu, J. Anode Current Collecting Efficiency of Tubular Anode-supported Solid Oxide Fuel Cells. Fuel Cells 2011, 11, 465–468. [Google Scholar] [CrossRef]
- Meadowcroft, A.; Howe, K.; Dhir, A.; Steinberger-Wilckens, R. Connection Optimisation for Micro-Tubular Solid Oxide Fuel Cells (A1507). In Proceedings of the 11th European SOFC & SOE Forum, Lucerne, Switzerland, 1–7 July 2014. [Google Scholar]
- Shimizu, A.; Nakajima, H.; Kitahara, T. Current Distribution Measurement of a Microtubular Solid Oxide Fuel Cell. ECS Trans. 2013, 57, 727–732. [Google Scholar] [CrossRef]
- Jin, C.; Liu, J.; Li, L.; Bai, Y. Electrochemical properties analysis of tubular NiO–YSZ anode-supported SOFCs fabricated by the phase-inversion method. J. Membr. Sci. 2009, 341, 233–237. [Google Scholar] [CrossRef]














| Characteristic | Microtubular | Planar |
|---|---|---|
| Power Density | Medium | High |
| Mechanical Strength | High | Lower |
| Start-up | Fast | Slower |
| Interconnect | Difficult/Cumbersome | Straightforward |
| Sealing | Facile | Complex |
| Manifold | Simple | Simple |
| Stack Power Density | Medium | High |
| System Compactness | High | High |
| Manufacturing Cost | Medium-High | Lower |
| Material | CTE ×10−6 [K] | Conductivity [S.cm−1} | Melting Point [°C] | Young’s Modulus [GPa] | Ref(s). |
|---|---|---|---|---|---|
| Ni-YSZ | 11–13 | 3 × 102 (800 °C) | - | 57–58 (800 °C) | [6,20,88] |
| LaCrO3 | 9.5 | 0.34 (700 °C) | 2510 | - | [20,24,77] |
| Crofer 22-APU (ferritic) | 11.5–12.5 | 8.3 × 104 (1000 °C) 8.7 × 103 (800 °C) | 1510–1530 | 216 (750 °C) | [88,89,90,91] |
| Haynes 23 (nickel-based) | 15.2 (800 °C) | 7.7 × 103 (800 °C) | 1301–1371 | 159 (800 °C) | [67,92] |
| Ducrolloy (Cr-based) | 11.8–12 (800 °C) | 1 × 104 (1000 °C) | 1700 | - | [93,94] |
| Silver (99.9%) | 18.9, 22 (800 °C) | 1.6 × 105 (800 °C) | 961 | 69–74 (800 °C) | [24,95,96] |
| Nickel | 12–13.5 (800 °C) | 2.5 × 104 (800 °C) | 1455 | 190–220 (800 °C) | [97] |
| Platinum | 10 (800 °C) | 2.3 × 104 (800 °C) | 1769 | 127 (800 °C) | [24,98] |
| Gold | 16.6 (800 °C) | 1.1 × 105 (800 °C) | 1064 | 76–81 (800 °C) | [24,99] |
| Palladium | 12.3(800 °C) | 2.55 × 104 (800 °C) | 1552 | 118–124 (800 °C) | [24,100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodjati-Pugh, O.; Dhir, A.; Steinberger-Wilckens, R. The Development of Current Collection in Micro-Tubular Solid Oxide Fuel Cells—A Review. Appl. Sci. 2021, 11, 1077. https://doi.org/10.3390/app11031077
Hodjati-Pugh O, Dhir A, Steinberger-Wilckens R. The Development of Current Collection in Micro-Tubular Solid Oxide Fuel Cells—A Review. Applied Sciences. 2021; 11(3):1077. https://doi.org/10.3390/app11031077
Chicago/Turabian StyleHodjati-Pugh, Oujen, Aman Dhir, and Robert Steinberger-Wilckens. 2021. "The Development of Current Collection in Micro-Tubular Solid Oxide Fuel Cells—A Review" Applied Sciences 11, no. 3: 1077. https://doi.org/10.3390/app11031077
APA StyleHodjati-Pugh, O., Dhir, A., & Steinberger-Wilckens, R. (2021). The Development of Current Collection in Micro-Tubular Solid Oxide Fuel Cells—A Review. Applied Sciences, 11(3), 1077. https://doi.org/10.3390/app11031077







