Optimization of Extraction Conditions for Antioxidant Activity of Acer tegmentosum Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of A. tegmentosum
2.2. Chemicals and Materials
2.3. RSM
2.4. TPC
2.5. DPPH Activity
2.6. Total Flavonoid Content
2.7. Ferric Reducing Antioxidant Power (FRAP)
2.8. Trolox Equivalent Antioxidant Capacity (TEAC)
2.9. Oxygen Radical Absorbance Capacity (ORAC)
2.10. High-Pressure Liquid Chromatography and Quadrupole Time-of-Flight Spectrometry
2.11. Cell Culture
2.12. Cell Viability Assay and Assessment of ROS Formation in HepG2 Cells
2.13. Statistical Analysis
3. Results
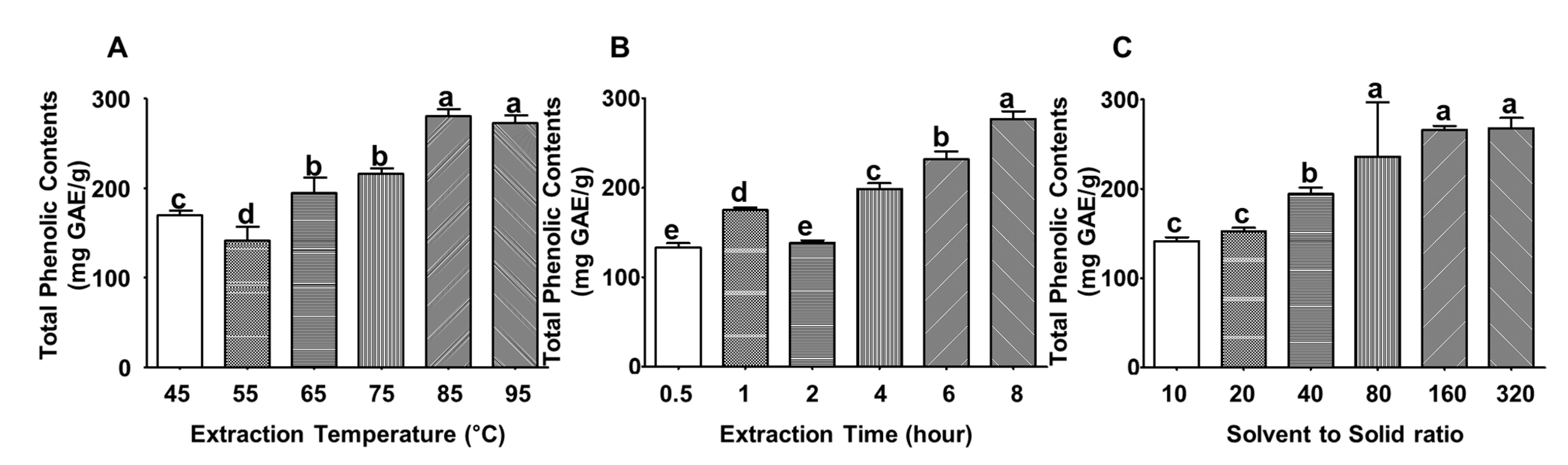
3.1. Determination of Experimental Conditions
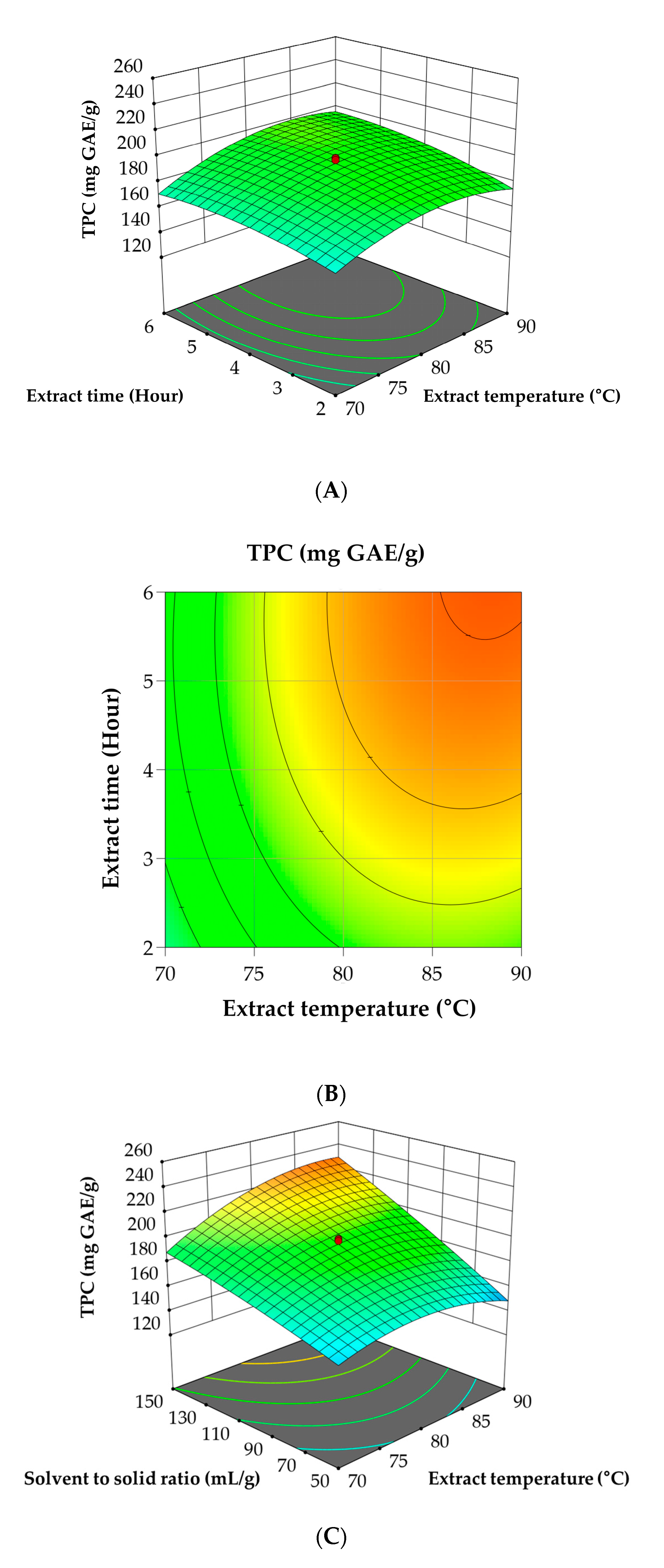
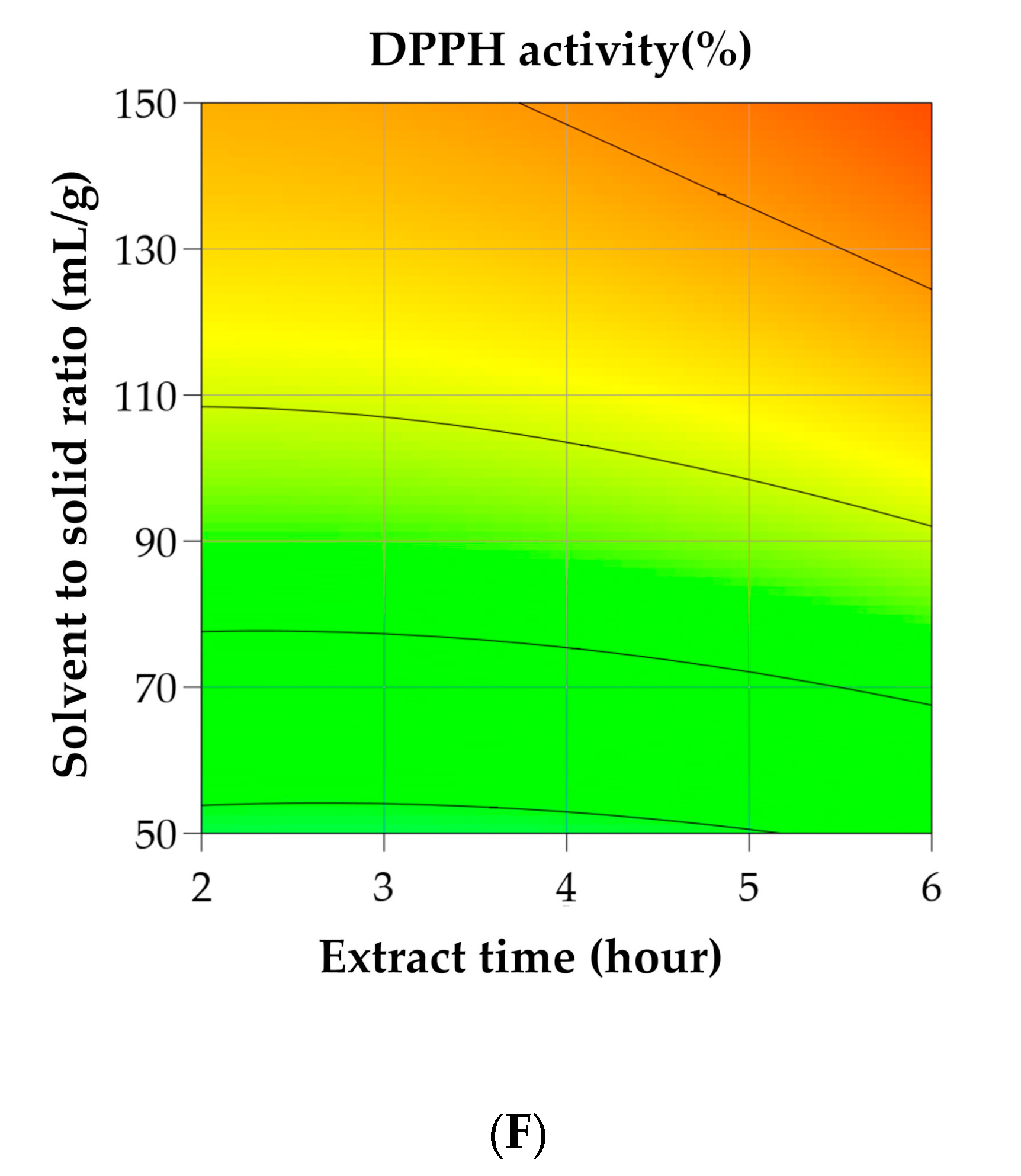
3.2. RSM for Optimization of A. tegmentosum
3.3. Antioxidant Activities of Hydrothermal A. tegmentosum Extract
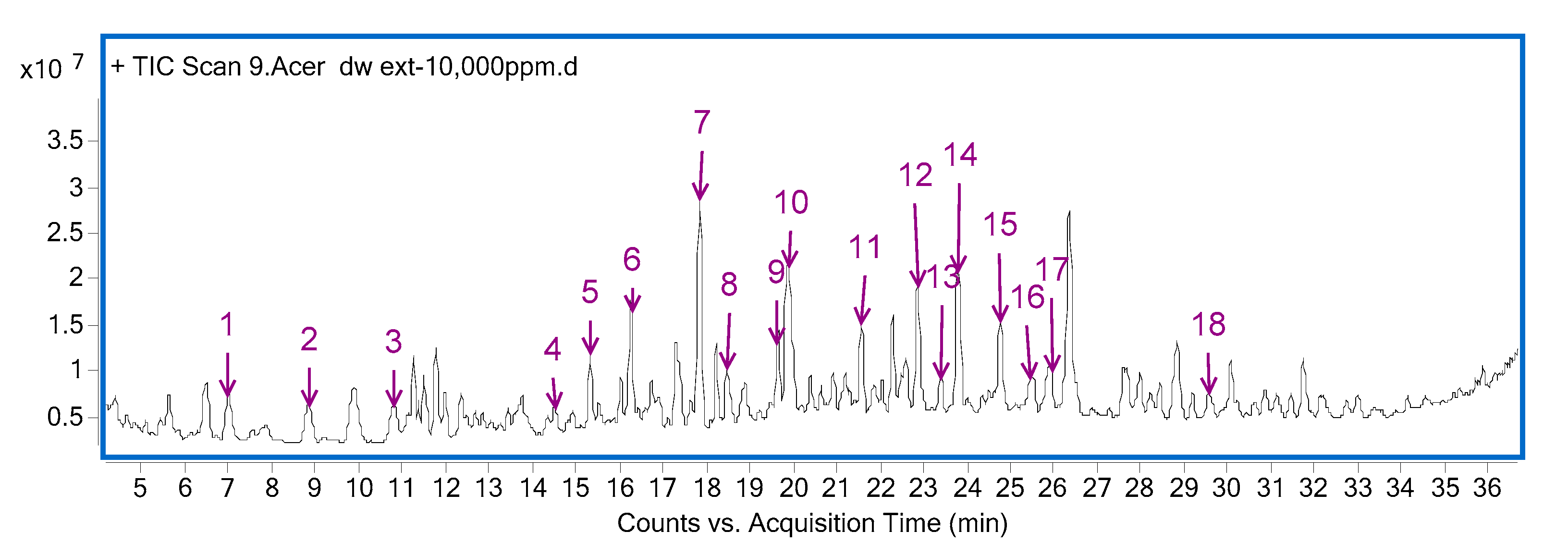
3.4. Identification of Functional Components in Hydrothermal A. tegmentosum Extract by HPLC-Q-TOF-MS
3.5. Antioxidant Activities of A. tegmentosum Extract in HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization Extraction Conditions for Improving Phenolic Content and Antioxidant Activity in Berberis asiatica Fruits Using Response Surface Methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Hong, B.K.; Eom, S.H.; Lee, C.O.; Lee, J.W.; Jeong, J.H.; Kim, J.K.; Cho, D.H.; Yu, C.Y.; Kwon, Y.S.; Kim, M.J. Biological Activities and Bioactive Compounds in the Extract of Acer tegmentosum Maxim. Stem. Korean J. Med. Crop Sci. 2007, 15, 296–303. [Google Scholar]
- Rhim, T.J. Anticytotoxic and Radical Scavenging Activities of Acer tegmentosum Maxim Stem Extracts. J. Environ. Sci. Int. 2015, 24, 1315–1329. [Google Scholar] [CrossRef]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef]
- Liu, Q.; Shin, E.J.; Ahn, M.J.; Hwang, B.Y.; Lee, M.K. Anti-Adipogenic Activity of Acer tegmentosum and Its Constituent, Catechin in 3T3-L1 Cells. Nat. Prod. Sci. 2011, 17, 212–215. [Google Scholar]
- Yu, T.; Lee, J.; Lee, Y.G.; Byeon, S.E.; Kim, M.H.; Sohn, E.-H.; Lee, Y.J.; Lee, S.G.; Cho, J.Y. In Vitro and In Vivo Anti-Inflammatory Effects of Ethanol Extract from Acer tegmentosum. J. Ethnopharmacol. 2010, 128, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Cytotoxic Phenolic Constituents of Acer tegmentosum Maxim. Arch. Pharmacal Res. 2006, 29, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Antioxidant Potential of Green and Black Tea Determined Using the Ferric Reducing Power (FRAP) Assay. Int. J. Food Sci. Nutr. 2000, 51, 181–188. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-Radical Absorbance Capacity Assay for Antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Katiki, L.M.; Ferreira, J.F.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F. Anthelmintic Effect of Plant Extracts Containing Condensed and Hydrolyzable Tannins on Caenorhabditis elegans, and Their Antioxidant Capacity. Vet. Parasitol. 2013, 192, 218–227. [Google Scholar] [CrossRef]
- Kong, K.W.; Mat-Junit, S.; Aminudin, N.; Ismail, A.; Abdul-Aziz, A. Antioxidant Activities and Polyphenolics from the Shoots of Barringtonia racemosa (L.) Spreng in a Polar to Apolar Medium System. Food Chem. 2012, 134, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Choi, S.W.; Chung, S.K. Antioxidative Flavonoids from the Garlic (Allium sativum L.) Shoot. Food Sci. Biotechnol. 2000, 9, 199–203. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B. Application of Response Surface Methodology (RSM) for Optimization of Leaching Parameters for Ash Reduction from Low-Grade Coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Nam, J.H.; Kim, M.Y.; Choi, J.; Lee, K.T.; Park, H.J. Analgesic and Anti-Gastropathic Effects of Salidroside Isolated from Heartwood. Open Bioact. Compd. J. 2009, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Park, H.S.; Jo, E.; Han, J.H.; Jung, S.H.; Lee, D.H.; Park, I.; Heo, K.S.; Na, M.; Myung, C.S. Hepatoprotective Effects of an Acer tegmentosum Maxim Extract Through Antioxidant Activity and the Regulation of Autophagy. J. Ethnopharmacol. 2019, 239, 111912. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, Y.J.; Han, Y.B.; Lee, J.C.; Lee, S.; Park, H.-J.; Lee, H.-J.; Kim, K.H. Isoamericanoic Acid B from Acer tegmentosum as a Potential Phytoestrogen. Nutrients 2018, 10, 1915. [Google Scholar] [CrossRef] [Green Version]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.; Schroeter, H.; Crozier, A. Absorption, Metabolism, Distribution and Excretion of (−)-Epicatechin: A Review of Recent Findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A. The Pharmacological Potential of Catechin. Indian J. Biochem. Biophys. 2020, 57, 505–511. [Google Scholar]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.; Heber, D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates with the Antioxidant Capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.J.; Park, G.H.; Kim, D.S.; Kang, Y.; Park, Y. Antioxidant and Anticancer Activities of Leaves Extracts from Acer tegmentosum. Korean J. Plant Res. 2020, 33, 551–557. [Google Scholar]
- Lee, C.-E.; Jeong, H.-H.; Cho, J.-A.; Ly, S.Y. In Vitro and In Vivo Anti-Oxidative and Anti-Inflammatory Activities of Acer tegmentosum Maxim Extracts. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1–9. [Google Scholar] [CrossRef]





| Independent Variables | Units | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | α | |||
| Extraction temperature | °C | X1 | 63.18 | 70 | 80 | 90 | 96.82 |
| Extraction time | h | X2 | 0.64 | 2 | 4 | 6 | 7.36 |
| Solvent-to-solid ratio | mL/g | X4 | 15.91 | 50 | 100 | 150 | 184.09 |
| Time (min) | Flow Rate (μL/min) | MP 1 A [2% Acetic Acid in DW 2 (v/v)] | MP B [0.1% Formic Acid in ACN 3] | Comment |
|---|---|---|---|---|
| 0–5 | 1000 | 100% | 0% | Isocratic flow |
| 5–30 | 1000 | 100–75% | 0–25% | Gradient flow |
| 30–40 | 1000 | 75–30% | 25–70% | Gradient flow |
| 40–41 | 1000 | 30–0% | 70–100% | Gradient flow |
| 41–50 | 1000 | 0% | 100% | Isocratic flow |
| 50–51 | 1000 | 0–100% | 100–0% | Gradient flow |
| 51–60 | 1000 | 100% | 0% | Isocratic flow |
| Acquisition Parameter | |
|---|---|
| Source type | Electrospray ionization |
| Ion polarity | Positive |
| Capillary voltage | 3500 V |
| Nozzle voltage | 1000 V |
| Fragmentor | 400 V |
| Skimmer | 65 V |
| OCT 1 RF Vpp | 750 V |
| Collision energy | 0 V |
| Nebulizer pressure | 45 psi |
| Sheath gas temperature | 350 °C |
| Sheath gas flow | 11 L/min |
| Drying gas (N2) temperature | 225 °C |
| Drying gas (N2) flow rate | 15 L/min |
| Experiment No. | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Solvent-to-Solid Ratio (mL/g) | TPC (mg GAE 1/g) | DPPH 2 (%) | |
| 1 | 70.00 | 2.00 | 50.00 | 153.68 | 19.82 |
| 2 | 90.00 | 2.00 | 50.00 | 136.32 | 20.20 |
| 3 | 70.00 | 2.00 | 150.00 | 149.52 | 20.25 |
| 4 | 90.00 | 2.00 | 150.00 | 159.26 | 20.37 |
| 5 | 70.00 | 6.00 | 50.00 | 172.17 | 21.24 |
| 6 | 90.00 | 6.00 | 50.00 | 212.88 | 24.80 |
| 7 | 70.00 | 6.00 | 150.00 | 199.66 | 22.32 |
| 8 | 90.00 | 6.00 | 150.00 | 240.36 | 25.06 |
| 9 | 63.18 | 4.00 | 100.00 | 136.28 | 17.73 |
| 10 | 96.82 | 4.00 | 100.00 | 168.37 | 20.91 |
| 11 | 80.00 | 4.00 | 15.91 | 165.93 | 22.33 |
| 12 | 80.00 | 4.00 | 184.09 | 180.99 | 23.30 |
| 13 | 80.00 | 0.64 | 100.00 | 130.82 | 18.59 |
| 14 | 80.00 | 7.36 | 100.00 | 233.48 | 23.82 |
| 15 | 80.00 | 4.00 | 100.00 | 199.20 | 23.08 |
| 16 | 80.00 | 4.00 | 100.00 | 198.58 | 22.30 |
| 17 | 80.00 | 4.00 | 100.00 | 179.56 | 23.23 |
| 18 | 80.00 | 4.00 | 100.00 | 198.31 | 23.01 |
| 19 | 80.00 | 4.00 | 100.00 | 198.87 | 23.02 |
| 20 | 80.00 | 4.00 | 100.00 | 197.60 | 22.73 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| P | 17544.67 | 9 | 1949.41 | 23.79 | <0.0001 |
| X1 | 1195.25 | 1 | 1195.25 | 14.58 | 0.0034 |
| X2 | 718.95 | 1 | 718.95 | 8.77 | 0.0142 |
| X3 | 11653.01 | 1 | 11653.01 | 142.18 | <0.0001 |
| X1 × X2 | 91.64 | 1 | 91.64 | 1.12 | 0.3152 |
| X1 × X3 | 990.69 | 1 | 990.69 | 12.09 | 0.006 |
| X2 × X3 | 163.74 | 1 | 163.74 | 2 | 0.1879 |
| X12 | 2454.68 | 1 | 2454.68 | 29.95 | 0.0003 |
| X22 | 448.25 | 1 | 448.25 | 5.47 | 0.0414 |
| X32 | 90.49 | 1 | 90.49 | 1.1 | 0.3181 |
| Residual error | 819.58 | 10 | 81.96 | ||
| Lack of fit | 518.92 | 5 | 103.78 | 1.73 | 0.2819 |
| Pure error | 300.66 | 5 | 60.13 | ||
| Correlation total | 18364.24 | 19 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 70.47 | 9 | 7.83 | 31.94 | <0.0001 |
| X1 | 10.8 | 1 | 10.8 | 44.03 | <0.0001 |
| X2 | 0.9426 | 1 | 0.9426 | 3.84 | 0.0783 |
| X3 | 34.04 | 1 | 34.04 | 138.85 | <0.0001 |
| X1 × X2 | 0.1456 | 1 | 0.1456 | 0.5938 | 0.4588 |
| X1 × X3 | 4.2 | 1 | 4.2 | 17.12 | 0.002 |
| X2 × X3 | 0.0689 | 1 | 0.0689 | 0.2811 | 0.6075 |
| X12 | 17.63 | 1 | 17.63 | 71.9 | <0.0001 |
| X22 | 0.2455 | 1 | 0.2455 | 1 | 0.3406 |
| X32 | 2.78 | 1 | 2.78 | 11.34 | 0.0072 |
| Residual error | 2.45 | 10 | 0.2452 | ||
| Lack of fit | 1.89 | 5 | 0.3777 | 3.35 | 0.1053 |
| Pure error | 0.5636 | 5 | 0.1127 | ||
| Correlation total | 72.93 | 19 |
| Total Flavonoid Content (mg QE/g) | FRAP 1 (μmol FeSO4/g) 4 | TEAC 2 (μmol TE/g) 5 | ORAC 3 (mmol TE/g) | |
|---|---|---|---|---|
| Acer tegmentosum | 263.31 ± 3.54 | 2094.39 ± 43.43 | 89.31 ± 0.32 | 3.80 ± 0.50 |
| Ascorbic acid | - | 18,750 ± 391.90 | 162.769 ± 5.71 | 4.20 ± 0.46 |
| No. | RT | Formula | Score | Diff (MFG, ppm) | [M + H]+ | Name |
|---|---|---|---|---|---|---|
| 1 | 7.01 | C10H10O3 | 99.93 | −0.01 | 179.07027 | p-Methoxycinnamic acid |
| 2 | 8.82 | C13H14O9 | 99.75 | 0 | 315.0711 | Unknown |
| 3 | 10.81 | C11H14O5 | 96.73 | 3.47 | 227.09065 | Genipin |
| 4 | 14.53 | C10H12O5 | 99.73 | 0.49 | 213.07567 | Unknown |
| 5 | 15.34 | C8H10O4 | 99.11 | 1.17 | 171.06496 | Unknown |
| 6 | 16.28 | C8H8O2 | 98.69 | 2.66 | 137.05934 | Benzyl formate |
| 7 | 17.85 | C9H10O3 | 99.34 | 1.63 | 167.06997 | D-(+)-3-Phenyllactic acid |
| 8 | 18.48 | C9H10O5 | 99.32 | 0.46 | 199.06001 | Unknown |
| 9 | 19.66 | C30H26O12 | 96.83 | 2.01 | 579.14845 | Procyanidin B |
| 10 | 19.94 | C20H20O14 | 98.18 | 1.47 | 485.09202 | 1, 6-digalloylglucose |
| 11 | 21.58 | C7H6O3 | 99.24 | 2.46 | 139.03862 | Tyrosol |
| 12 | 22.87 | C11H16O4 | 99.09 | 1.89 | 213.11171 | Dyhydrosinapyl alcohol |
| 13 | 23.40 | C13H20O5 | 99.76 | −0.31 | 257.13848 | Unknown |
| 14 | 23.81 | C10H8O5 | 99.36 | 0.54 | 209.04434 | Fraxetin |
| 15 | 24.77 | C15H14O6 | 98.61 | 1.31 | 291.0859 | Epicatechin |
| 16 | 25.50 | C11H10O5 | 99.8 | −0.07 | 223.0601 | 6-(2-Methoxyvinyl)benzo[1,3]dioxole-5-carboxylic acid |
| 17 | 25.94 | C37H30O16 | 98.12 | 1 | 731.15984 | Catechin-(4α->8)-epicatechin-3-O-gallate |
| 18 | 29.56 | C22H18O10 | 99.37 | 0.91 | 443.09687 | (-)-Epicatechin-3-O-gallate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Ha, J.-H.; Jeong, Y. Optimization of Extraction Conditions for Antioxidant Activity of Acer tegmentosum Using Response Surface Methodology. Appl. Sci. 2021, 11, 1134. https://doi.org/10.3390/app11031134
Kim I, Ha J-H, Jeong Y. Optimization of Extraction Conditions for Antioxidant Activity of Acer tegmentosum Using Response Surface Methodology. Applied Sciences. 2021; 11(3):1134. https://doi.org/10.3390/app11031134
Chicago/Turabian StyleKim, Inyong, Jung-Heun Ha, and Yoonhwa Jeong. 2021. "Optimization of Extraction Conditions for Antioxidant Activity of Acer tegmentosum Using Response Surface Methodology" Applied Sciences 11, no. 3: 1134. https://doi.org/10.3390/app11031134






