Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
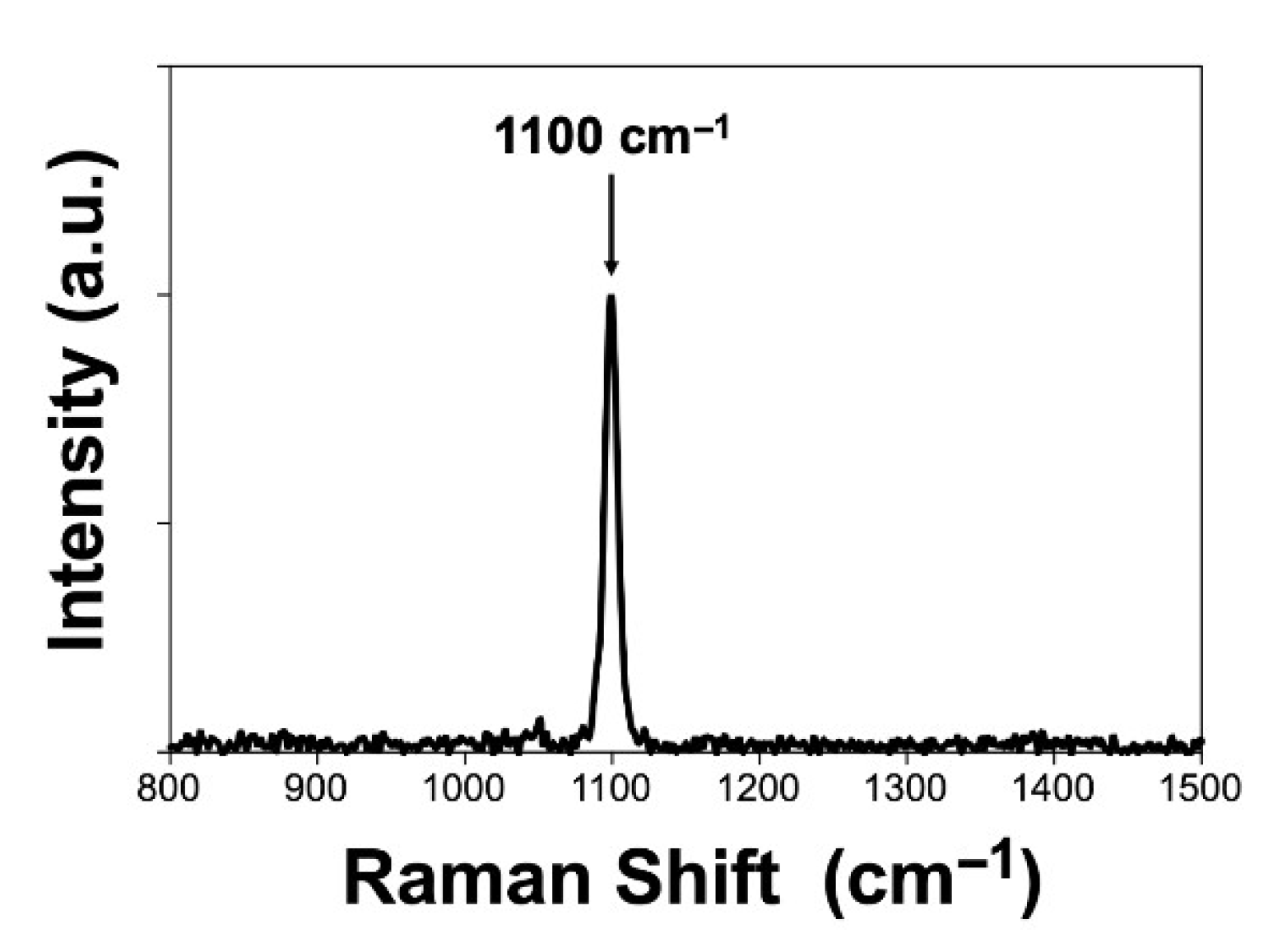
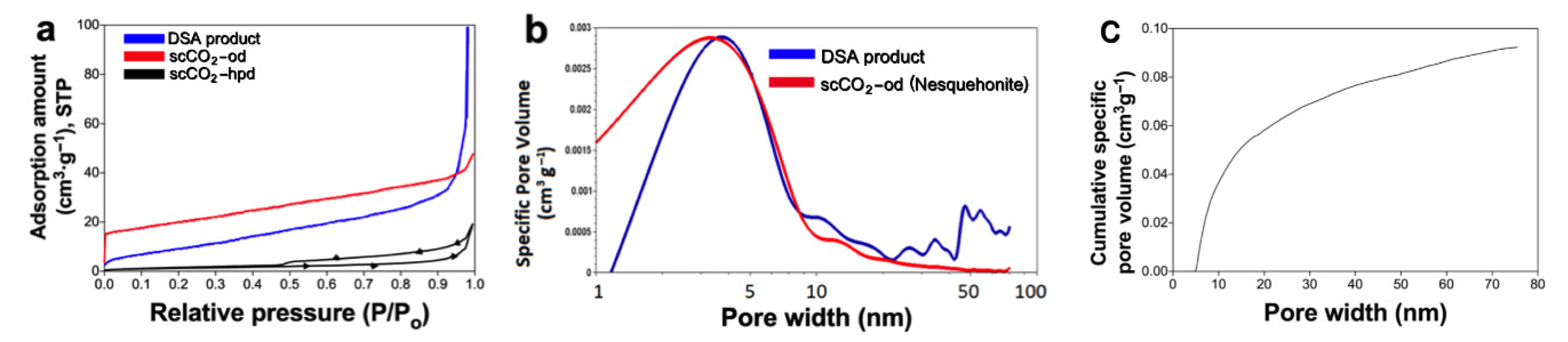
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [PubMed] [Green Version]
- Unluer, C.; Al-Tabbaa, A. Characterization of Light and Heavy Hydrated Magnesium Carbonates Using Thermal Analysis. J. Therm. Anal. Calorim. 2014, 115, 595–607. [Google Scholar]
- Farhang, F.; Oliver, T.K.; Rayson, M.; Brent, G.; Stockenhuber, M.; Kennedy, E. Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2016, 303, 439–449. [Google Scholar]
- Glasser, F.P.; Jauffret, G.; Morrison, J.; Galvez-Martos, J.-L.; Patterson, N.; Imbabi, M.S.-E. Sequestering CO2 by Mineralization into Useful Nesquehonite-Based Products. Front. Energy Res. 2013, 4, 3. [Google Scholar]
- Frykstrand, S.; Forsgren, J.; Mihranyan, A.; Strømme, M. On the pore forming mechanism of Upsalite, a micro- and mesoporous magnesium carbonate. Microporous Mesoporous Mater. 2014, 190, 99–104. [Google Scholar]
- Yang, J.; Alvebratt, C.; Lu, X.; Bergström, C.A.S.; Strömme, M.; Welch, K. Amorphous magnesium carbonate nanoparticles with strong stabilizing capability for amorphous ibuprofen. Int. J. Pharm. 2018, 548, 515–521. [Google Scholar]
- Cheung, O.; Zhang, P.; Frykstrand, S.; Zheng, H.; Yang, T.; Sommariva, M.; Zou, X.; Strömme, M. Nanostructure and pore size control of template-free synthesised mesoporous magnesium carbonate. RSC Adv. 2016, 6, 74241–74249. [Google Scholar]
- Frykstrand, S.; Forsgren, J.; Cheung, O.; Zhang, P.; Hong, J.; Strømme, M.; Ferraz, N. Study of mesoporous magnesium carbonate in contact with whole human blood. RSC Adv. 2016, 6, 52810–52816. [Google Scholar]
- Vall, M.; Hultberg, J.; Strömme, M.; Cheung, O. Carbon dioxide adsorption on mesoporous magnesium carbonate. Energy Procedia 2019, 158, 4671–4676. [Google Scholar]
- Shahwan, T.; Zünbül, B.; Eroğlu, A.E.; Yılmaz, S. Effect of magnesium carbonate on the uptake of aqueous zinc and lead ions by natural kaolinite and clinoptilolite. Appl. Clay Sci. 2005, 30, 209–218. [Google Scholar]
- Wang, P.; Shen, T.; Li, X.; Tang, Y.; Li, Y. Magnetic Mesoporous Calcium Carbonate-Based Nanocomposites for the Removal of Toxic Pb(II) and Cd(II) Ions from Water. ACS Appl. Nano Mater. 2020, 3, 1272–1281. [Google Scholar]
- Shan, Q.; Zhang, Y.; Xue, X. Removal of copper from wastewater by using the synthetic nesquehonite. Environ. Prog. Sustain. Energy 2013, 3, 543–546. [Google Scholar]
- Castilleja-Escobedo, O.; Sánchez-García, R.E.; Nigama, K.D.P.; López-Salinas, J.L. Directional displacement of non-aqueous fluids through spontaneous aqueous imbibition in porous structures. Chem. Eng. Sci. 2020, 228, 115959. [Google Scholar]
- Kim, K.D.; Kim, Y.D.; Kim, S.W. Synthesis of Porous Magnesium Oxide Cubes with Nano-Grain Structure in Supercritical CO2/Ethanol Solution. J. Nanosci. Nanotechnol. 2002, 11, 5723–5728. [Google Scholar]
- de Vito, C.; Ferrini, V.; Mignardi, S.; Cagnetti, M.; Leccese, F. Progress in carbon dioxide sequestration via carbonation of aqueous saline wastes. Period. Mineral. 2012, 81, 333–344. [Google Scholar]
- Nakashima, Y.; Takai, C.; Razavi-Khosroshahi, H.; Suthabanditpong, W.; Fuji, M. Synthesis of ultra-small hollow silica nanoparticles using the prepared amorphous calcium carbonate in one-pot process. Adv. Powder Technol. 2018, 29, 904–908. [Google Scholar]
- DeSimone, J.M. Practical Approaches to Green Solvents. Science 2002, 297, 799–803. [Google Scholar]
- Yoo, Y.; Kang, D.; Choi, E.; Park, J.; Hugh, I. Morphology control of magnesium carbonate for CO2 utilization using Mg2+ ions in industrial wastewater depending on length of alkyl chain of primary alkanolamine, reaction temperature, CO2 concentration, and Mg2+/Na+ ratio. Chem. Eng. J. 2019, 370, 237–250. [Google Scholar]
- Sim, S.; Cole, I.S.; Choi, Y.S.; Birbilis, N. A review of the protection strategies against internal corrosion for the safe transport of supercritical CO2 via steel pipelines for CCS purposes. Int. J. Greenh. Gas Control 2014, 29, 185–199. [Google Scholar]
- Langmuir, D. Stability of Carbonates in the system MgO-CO2-H2O. J. Geol. 2015, 73, 730–754. [Google Scholar]
- Perry, C.T.; Salter, M.A.; Harborne, A.R.; Crowley, S.F.; Jelks, H.L.; Wilson, R.W. Fish as major carbonate mud producers and missing components of the tropical carbonate factory. Proc. Natl. Acad. Sci. USA 2011, 108, 3865–3869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Ouyang, J.; Yang, H. Synthesis and characterization of nesquehonite (MgCO3·3H2O) powders from natural talc. Powder Technol. 2016, 292, 169–175. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Botha, A. DTA and FT-IR analysis of the rehydration of basic magnesium carbonate. J. Therm. Anal. Calorim. 2003, 71, 987–996. [Google Scholar] [CrossRef]
- Forsgren, J.; Frykstrand, S.; Grandfield, K.; Mihranyan, A.; Strömme, M. A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. PLoS ONE 2013, 8, e68486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Freeman, J.J.; Jolliff, B.L.; Chou, I. Sulfates on Mars: A systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochim. Cosmochim. Acta 2006, 70, 6118–6135. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials (Basel) 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ferrini, V.; de Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef]

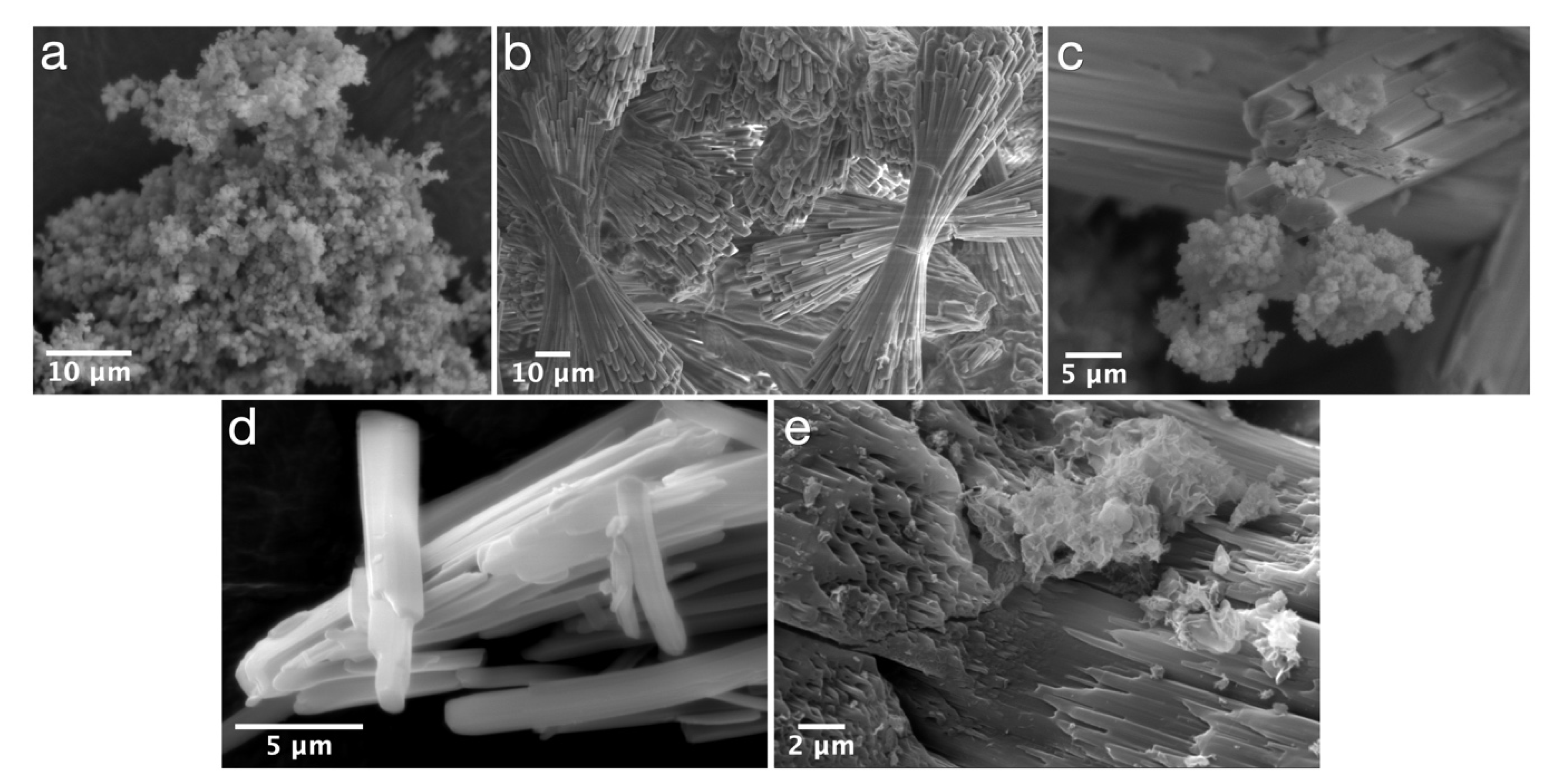
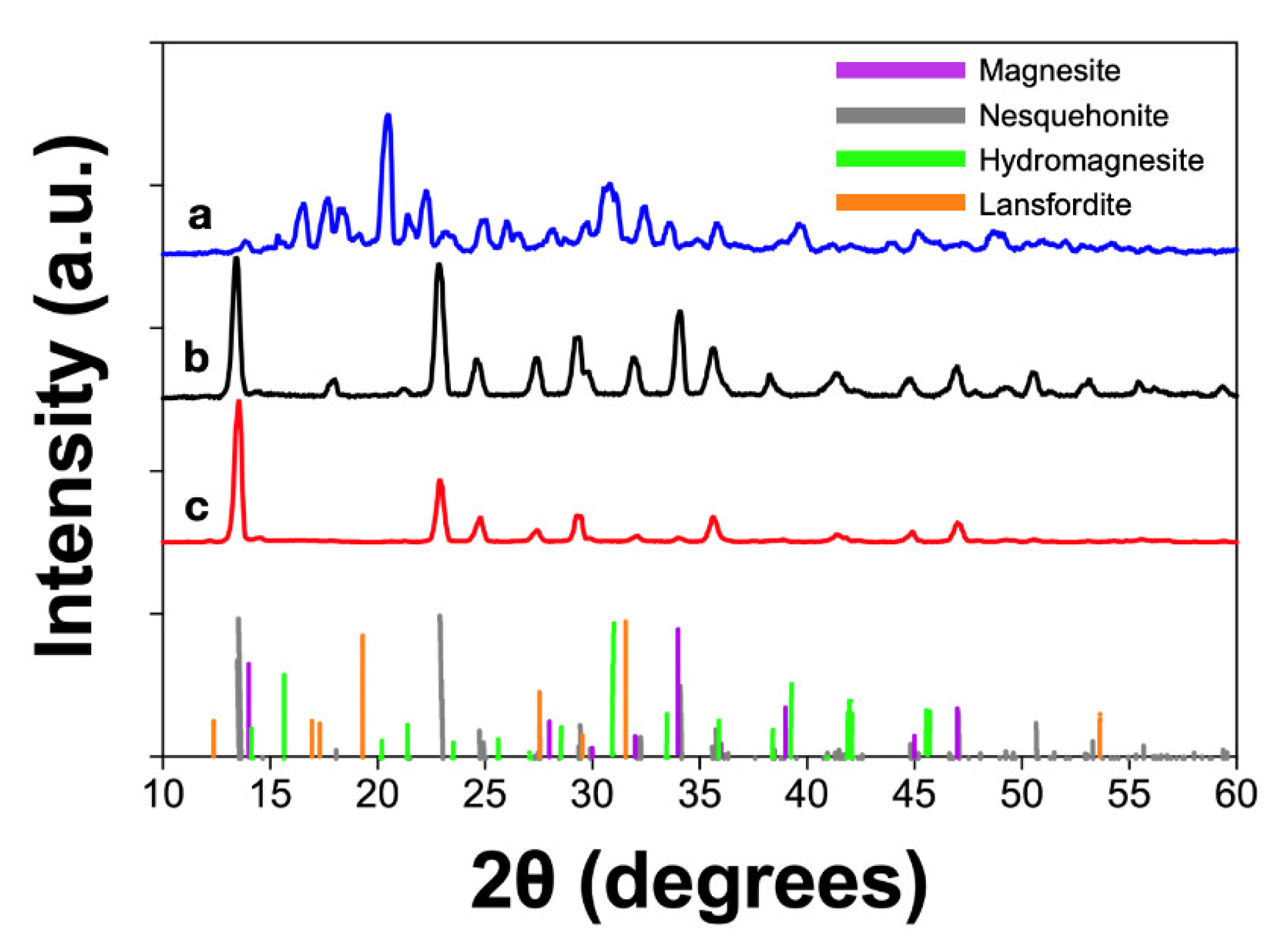
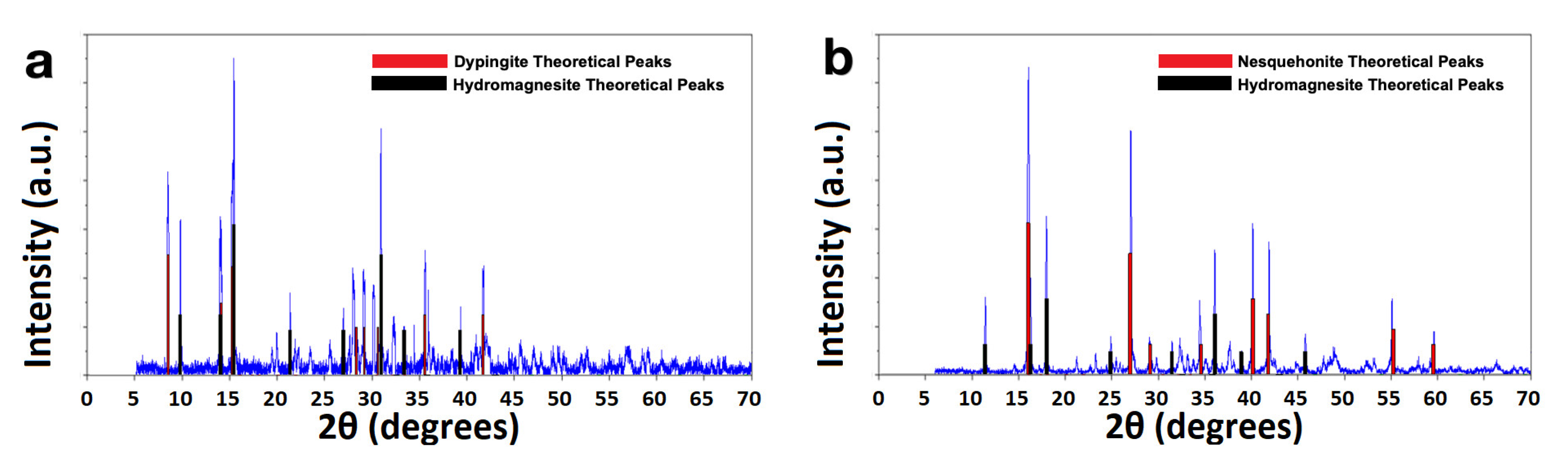
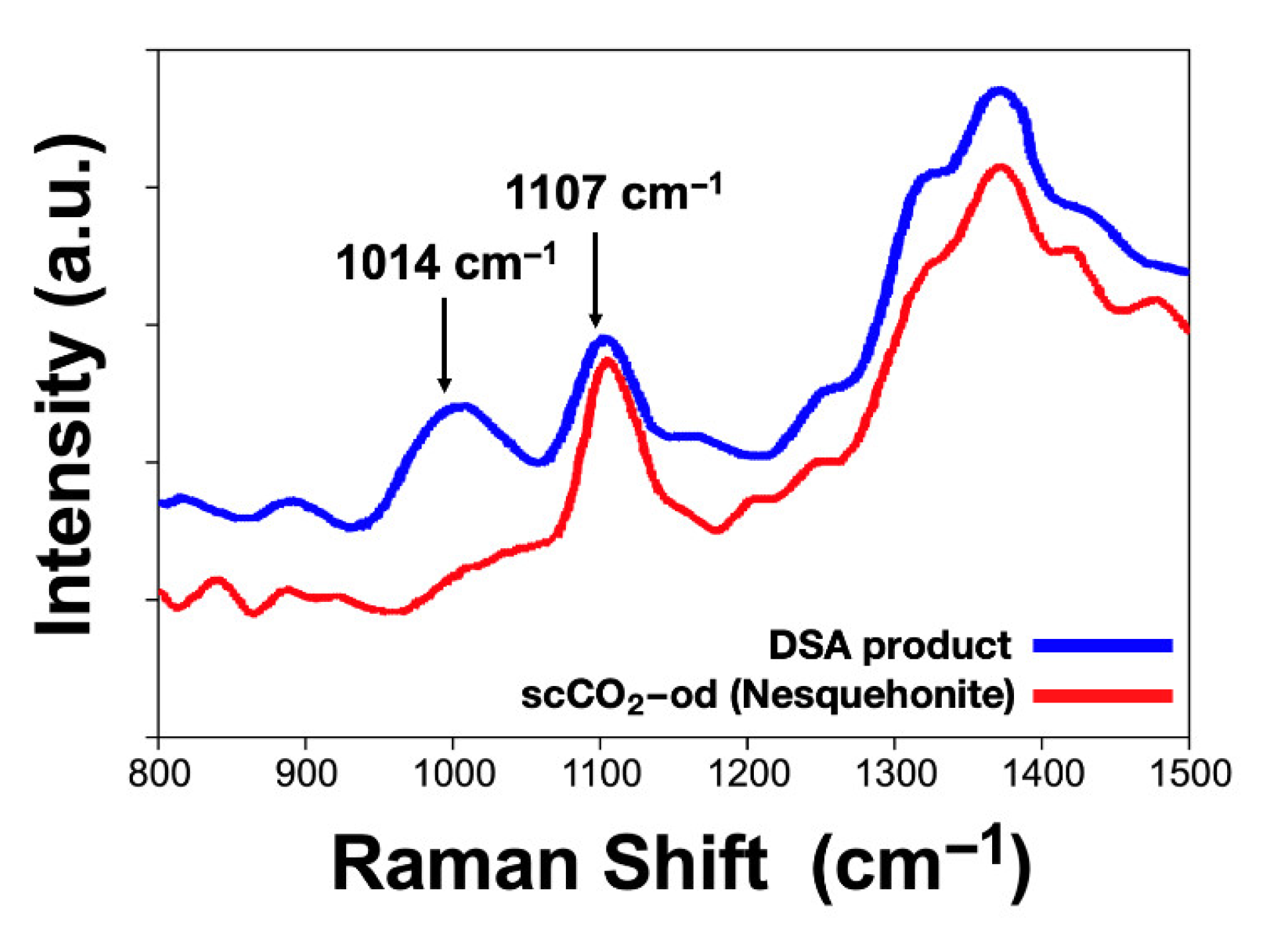
| Experimental Method | Precursors Salts | Reaction Time | Reaction Temperature | Drying Temperature |
|---|---|---|---|---|
| Aqueous Synthesis | MgCl2·6H2O | 25 and 60 min | 5, 21, and 70 °C | 40 °C |
| Mg(NO3)2·6H2O | ||||
| MgSO4·7H2O | ||||
| scCO2 Synthesis | Mg(NO3)2·6H2O | 12 h | 35–40 °C | 40 °C |
| 100 °C |
| Phase | scCO2-od | scCO2-hpd | DSA |
|---|---|---|---|
| Magnesite | 0% | 20% | 0% |
| Nesquehonite | 65% | 0% | 54% |
| Lansfordite | 27% | 60% | 40% |
| Hydromagnesite | 8% | 20% | 6% |
| Sample | Specific Surface Area (m2g−1) | Average Pore Size (nm) |
|---|---|---|
| scCO2-od nesquehonite | 70.4 | 1.02 |
| DSA | 39.1 | 3.78 |
| MgCl2 + NaHCO3 + KOH | 11.8 | 50 |
| Commercial carbonate | 18.1 | 4.89 |
| scCO2 -hpd | 19.8 | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Macías, F.J.; Ortiz-Castillo, J.E.; López-Lara, E.; García-Cuéllar, A.J.; López-Salinas, J.L.; García-Pérez, C.A.; Castilleja-Escobedo, O.; Vega-Cantú, Y.I. Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Appl. Sci. 2021, 11, 1141. https://doi.org/10.3390/app11031141
Rodríguez-Macías FJ, Ortiz-Castillo JE, López-Lara E, García-Cuéllar AJ, López-Salinas JL, García-Pérez CA, Castilleja-Escobedo O, Vega-Cantú YI. Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Applied Sciences. 2021; 11(3):1141. https://doi.org/10.3390/app11031141
Chicago/Turabian StyleRodríguez-Macías, Fernando J., José E. Ortiz-Castillo, Erika López-Lara, Alejandro J. García-Cuéllar, José L. López-Salinas, César A. García-Pérez, Orlando Castilleja-Escobedo, and Yadira I. Vega-Cantú. 2021. "Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide" Applied Sciences 11, no. 3: 1141. https://doi.org/10.3390/app11031141
APA StyleRodríguez-Macías, F. J., Ortiz-Castillo, J. E., López-Lara, E., García-Cuéllar, A. J., López-Salinas, J. L., García-Pérez, C. A., Castilleja-Escobedo, O., & Vega-Cantú, Y. I. (2021). Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Applied Sciences, 11(3), 1141. https://doi.org/10.3390/app11031141







