Cerebrovascular Reactivity Assessment during Carbon Dioxide Inhalation Using SPECT
Abstract
:1. Introduction
2. Materials and Methods
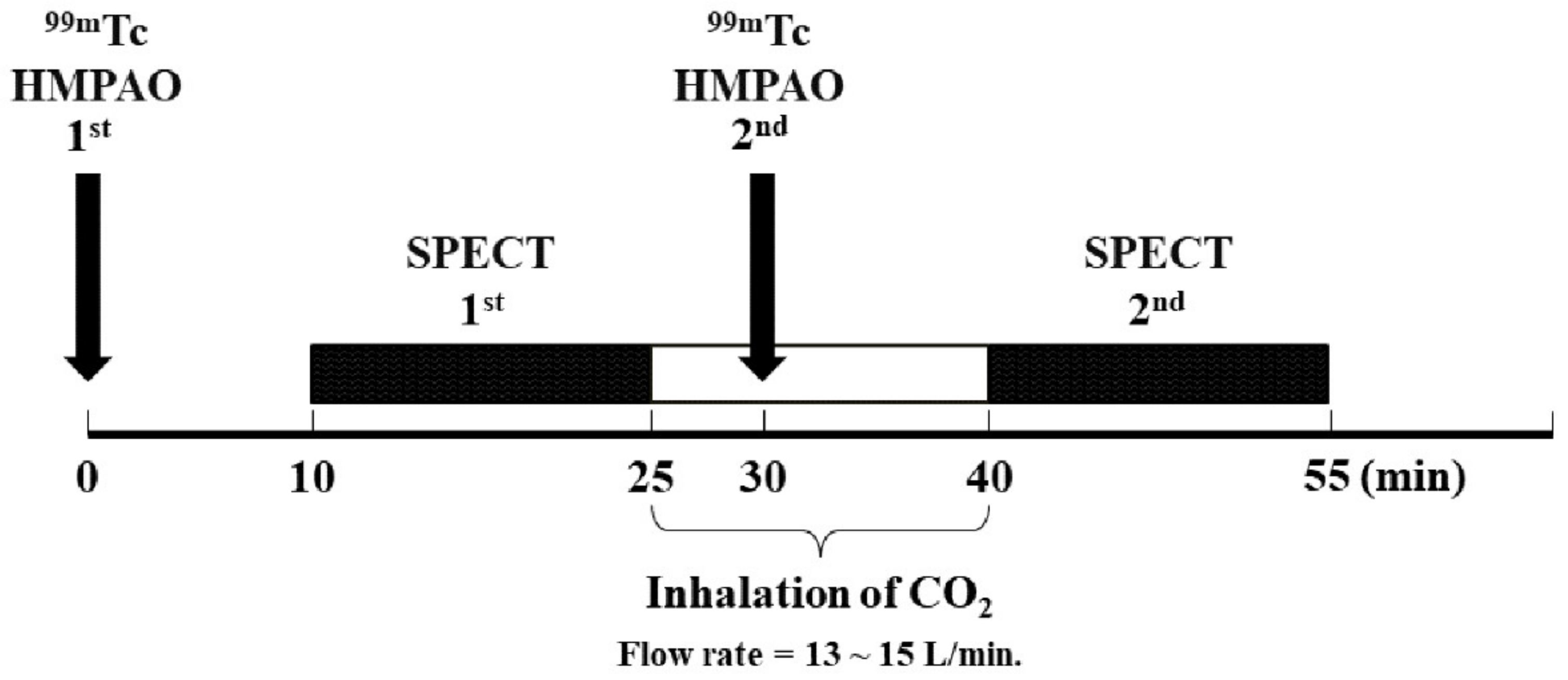
2.1. Subjects and SPECT Acquisition and Reconstruction
2.2. SPECT CVR Processing
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neirinckx, R.; Canning, L.; Piper, I.; Nowotnik, D.; Pickett, R.; Holmes, R.; Volkert, W.; Forster, A.; Weisner, P.; Marriott, J. Technetium-99m d,l-HM-PAO: A New Radiopharmaceutical for SPECT Imaging of Regional Cerebral Blood Perfusion. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1987, 28, 191–202. [Google Scholar]
- Anderson, A.R. 99mTc-D,L-Hexamethylene-Propyleneamine Oxime (99mTc-HMPAO): Basic Kinetic Studies of a Tracer of Cerebral Blood Flow. Cerebrovasc. Brain Metab. Rev. 1989, 1, 288–318. [Google Scholar]
- Abdel-Dayem, H.M.; Sadek, S.A.; Sayed, M.E.; Higazi, E. Technetium-99m HM-PAO Images (Planar and SPECT) of the Human Brain. Clin. Nucl. Med. 1988, 13, 139–143. [Google Scholar] [CrossRef]
- Burbridge, M.A.; Jaffe, R.A. Paradoxical Critical Hyperkalemia After Acetazolamide for Cerebrovascular Reactivity Study: A Case Report. A A Pract. 2019, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Vagal, A.S.; Leach, J.L.; Fernandez-Ulloa, M.; Zuccarello, M. The Acetazolamide Challenge: Techniques and Applications in the Evaluation of Chronic Cerebral Ischemia. Am. J. Neuroradiol. 2009, 30, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Holtby, H.; Bissonnette, B.; Wang, G.; Redington, A.N.; Van Arsdell, G.S. Carbon Dioxide—A Complex Gas in a Complex Circulation: Its Effects on Systemic Hemodynamics and Oxygen Transport, Cerebral, and Splanchnic Circulation in Neonates after the Norwood Procedure. J. Thorac. Cardiovasc. Surg. 2008, 136, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Juttukonda, M.R.; Donahue, M.J. Neuroimaging of Vascular Reserve in Patients with Cerebrovascular Diseases. NeuroImage 2019, 187, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Matsumoto, M.; Hashikawa, K.; Moriwaki, H.; Okazaki, Y.; Seike, Y.; Handa, N.; Uehara, T.; Kamada, T.; Nishimura, T. Carbon Dioxide Reactivity by Consecutive Technetium-99m-HMPAO SPECT in Patients with a Chronically Obstructed Major Cerebral Artery. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1994, 35, 32–40. [Google Scholar]
- Acker, G.; Lange, C.; Schatka, I.; Pfeifer, A.; Czabanka, M.A.; Vajkoczy, P.; Buchert, R. Brain Perfusion Imaging Under Acetazolamide Challenge for Detection of Impaired Cerebrovascular Reserve Capacity: Positive Findings with 15 O-Water PET in Patients with Negative 99mTc-HMPAO SPECT Findings. J. Nucl. Med. 2018, 59, 294–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, M.N.; Berry, N.M.; Cutting, M.A.; Keage, H.A.; Buckley, J.D.; Howe, P.R.C. Transcranial Doppler Ultrasound to Assess Cerebrovascular Reactivity: Reliability, Reproducibility and Effect of Posture. PeerJ 2013, 1, e65. [Google Scholar] [CrossRef]
- Sobczyk, O.; Crawley, A.P.; Poublanc, J.; Sam, K.; Mandell, D.M.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. Identifying Significant Changes in Cerebrovascular Reactivity to Carbon Dioxide. Am. J. Neuroradiol. 2016, 37, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; De Vis, J.B.; Lu, H. Cerebrovascular Reactivity (CVR) MRI with CO2 Challenge: A Technical Review. NeuroImage 2019, 187, 104–115. [Google Scholar] [CrossRef]
- Hou, X.; Liu, P.; Li, Y.; Jiang, D.; De Vis, J.B.; Lin, Z.; Sur, S.; Baker, Z.; Mao, D.; Ravi, H.; et al. The Association between BOLD-Based Cerebrovascular Reactivity (CVR) and End-Tidal CO2 in Healthy Subjects. NeuroImage 2020, 207, 116365. [Google Scholar] [CrossRef]
- Chan, S.; Evans, K.C.; Song, T.; Selb, J.; van der Kouwe, A.; Rosen, B.R.; Zheng, Y.; Ahn, A.; Kwong, K.K. Cerebrovascular Reactivity Assessment with O2-CO2 Exchange Ratio under Brief Breath Hold Challenge. PLoS ONE 2020, 15, e0225915. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z. Measurement of Cerebrovascular Reserve by Multimodal Imaging for Cerebral Arterial Occlusion or Stenosis Patients: Protocol of a Prospective, Randomized, Controlled Clinical Study. Trials 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Mountz, J.M.; Liu, H.-G.; Deutsch, G. Neuroimaging in Cerebrovascular Disorders: Measurement of Cerebral Physiology after Stroke and Assessment of Stroke Recovery. Semin. Nucl. Med. 2003, 33, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Shagera, Q.A.; Ryoo, H.G.; Ha, S.; Lee, D.S. Basal and Acetazolamide Brain Perfusion SPECT in Internal Carotid Artery Stenosis. Nucl. Med. Mol. Imaging 2020, 54, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yoo, M.Y.; Cheon, G.J.; Kang, K.W.; Chung, J.-K.; Lee, D.S. Parametric Cerebrovascular Reserve Images Using Acetazolamide 99mTc-HMPAO SPECT: A Feasibility Study of Quantitative Assessment. Nucl. Med. Mol. Imaging 2013, 47, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.-T. A Method for Attenuation Correction in Radionuclide Computed Tomography. IEEE Trans. Nucl. Sci. 1978, 25, 638–643. [Google Scholar] [CrossRef]
- Takeuchi, R.; Yonekura, Y.; Matsuda, H.; Konishi, J. Usefulness of a Three-Dimensional Stereotaxic ROI Template on Anatomically Standardised 99mTc-ECD SPET. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 331–341. [Google Scholar] [CrossRef]
- Imabayashi, E.; Soma, T.; Sone, D.; Tsukamoto, T.; Kimura, Y.; Sato, N.; Murata, M.; Matsuda, H. Validation of the Cingulate Island Sign with Optimized Ratios for Discriminating Dementia with Lewy Bodies from Alzheimer’s Disease Using Brain Perfusion SPECT. Ann. Nucl. Med. 2017, 31, 536–543. [Google Scholar] [CrossRef]
- Kunieda, T.; Miyake, K.; Sakamoto, H.; Iwasaki, Y.; Iida, S.; Morise, S.; Fujita, K.; Nakamura, M.; Kaneko, S.; Kusaka, H. Leptomeningeal Collaterals Strongly Correlate with Reduced Cerebrovascular Reactivity Measured by Acetazolamide-Challenged Single-Photon Emission Computed Tomography Using a Stereotactic Extraction Estimation Analysis in Patients with Unilateral Internal Carotid Artery Stenosis. Intern. Med. 2017, 56, 2857–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golestani, A.M.; Wei, L.L.; Chen, J.J. Quantitative Mapping of Cerebrovascular Reactivity Using Resting-State BOLD FMRI: Validation in Healthy Adults. NeuroImage 2016, 138, 147–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Kanno, I.; Ibaraki, M.; Hatazawa, J.; Miura, S. Changes in Human Cerebral Blood Flow and Cerebral Blood Volume during Hypercapnia and Hypocapnia Measured by Positron Emission Tomography. J. Cereb. Blood Flow Metab. 2003, 23, 665–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainslie, P.N.; Duffin, J. Integration of Cerebrovascular CO2 Reactivity and Chemoreflex Control of Breathing: Mechanisms of Regulation, Measurement, and Interpretation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1473–R1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battisti-Charbonney, A.; Fisher, J.; Duffin, J. The Cerebrovascular Response to Carbon Dioxide in Humans: Cerebrovascular Response to CO2. J. Physiol. 2011, 589, 3039–3048. [Google Scholar] [CrossRef]
- Bisschops, R.H.C.; Klijn, C.J.M.; Kappelle, L.J.; van Huffelen, A.C.; van der Grond, J. Association Between Impaired Carbon Dioxide Reactivity and Ischemic Lesions in Arterial Border Zone Territories in Patients with Unilateral Internal Carotid Artery Occlusion. Arch. Neurol. 2003, 60, 229. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.-W.; Zheng, J.; Shi, J.; Yin, Y.; Song, C.; Yang, F.; Zhang, Y.-Q.; Ma, L.-N. Assessment of the Cerebral Hemodynamic Benefits of Carotid Artery Stenting for Patients with Preoperative Hemodynamic Impairment Using Cerebral Single Photon Emission Computed Tomography (SPECT) and Carbon Dioxide Inhalation. Med. Sci. Monit. 2018, 24, 5398–5404. [Google Scholar] [CrossRef]
- Wilson, L.C.; Cotter, J.D.; Fan, J.-L.; Lucas, R.A.I.; Thomas, K.N.; Ainslie, P.N. Cerebrovascular Reactivity and Dynamic Autoregulation in Tetraplegia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R1035–R1042. [Google Scholar] [CrossRef]
- Yun, T.J.; Paeng, J.C.; Sohn, C.-H.; Kim, J.E.; Kang, H.-S.; Yoon, B.-W.; Choi, S.H.; Kim, J.; Lee, H.-Y.; Han, M.H.; et al. Monitoring Cerebrovascular Reactivity through the Use of Arterial Spin Labeling in Patients with Moyamoya Disease. Radiology 2016, 278, 205–213. [Google Scholar] [CrossRef]
- Xia, Y.; Shikii, S.; Shimomura, Y. Determining How Different Levels of Indoor Carbon Dioxide Affect Human Monotonous Task Performance and Their Effects on Human Activation States Using a Lab Experiment: A Tracking Task. Ergonomics 2020, 63, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.R.; Lesser, I.M.; Boone, K.B.; Miller, B.L.; Mena, I. Cerebral Blood Flow Changes in Normal Aging: Spect Measurements. Int. J. Geriatr. Psychiatry 1995, 10, 437–446. [Google Scholar] [CrossRef]
- Stefanidis, K.B.; Askew, C.D.; Klein, T.; Lagopoulos, J.; Summers, M.J. Healthy Aging Affects Cerebrovascular Reactivity and Pressure-Flow Responses, but Not Neurovascular Coupling: A Cross-Sectional Study. PLoS ONE 2019, 14, e0217082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.B.; Howery, A.J.; Rivera-Rivera, L.A.; Johnson, S.C.; Rowley, H.A.; Wieben, O.; Barnes, J.N. Age-Related Reductions in Cerebrovascular Reactivity Using 4D Flow MRI. Front. Aging Neurosci. 2019, 11, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Number | Sex | Age | Pre-Scan | Post-Scan | Count | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | MAP | HR | SBP | DBP | MAP | HR | 1st Scan | 2nd Scan | Ratio | |||
| #01 | M | 25 | 116 | 59 | 78 | 87 | 125 | 95 | 105 | 84 | 3593 | 10,599 | 2.95 |
| #02 | M | 23 | 116 | 65 | 82 | 60 | 131 | 67 | 88.33 | 67 | 3854 | 11,287 | 2.93 |
| #03 | F | 22 | 99 | 56 | 70.33 | 65 | 114 | 66 | 82 | 71 | 5223 | 15,440 | 2.96 |
| #04 | M | 23 | 119 | 60 | 79.67 | 60 | 122 | 67 | 85.33 | 56 | 3555 | 12,272 | 3.45 |
| #05 | M | 25 | 134 | 82 | 99.33 | 70 | 136 | 90 | 105.33 | 77 | 3543 | 10,453 | 2.95 |
| #06 | M | 26 | 143 | 69 | 93.67 | 61 | 153 | 66 | 95 | 62 | 3390 | 10,279 | 3.03 |
| #07 | M | 27 | 123 | 73 | 89.67 | 63 | 122 | 75 | 90.67 | 62 | 4222 | 12,963 | 3.07 |
| #08 | F | 21 | 91 | 52 | 65 | 51 | 98 | 60 | 72.67 | 61 | 3486 | 12,876 | 3.69 |
| #09 | F | 21 | 94 | 60 | 71.33 | 70 | 96 | 57 | 70 | 75 | 4441 | 13,696 | 3.08 |
| #10 | M | 25 | 123 | 67 | 85.67 | 65 | 130 | 76 | 94 | 66 | 4335 | 13,327 | 3.07 |
| Mean ± SD | 23.80 ± 2.10 | 115.80 ± 16.86 | 64.30 ± 8.85 | 81.47 ± 10.89 | 65.20 ± 9.43 | 122.70 ± 17.07 * | 71.90 ± 12.3 * | 88.83 ± 11.92 * | 68.10 ± 8.60 | 3964.20 ± 583.96 | 12,319.20 ± 1668.23 | 3.12 ± 0.25 | |
| ROI Number | Region Name | Hemisphere | # of ROIs | Relative Mean Voxel Based CVR Value (%) | p-Value |
|---|---|---|---|---|---|
| ROI #01 | Temporal | L | 10 | 0.756 | 0.285 |
| ROI #02 | Temporal | R | 10 | 1.849 | 0.047 * |
| ROI #03 | Vermis | M | 8 | 3.337 | 0.007 * |
| ROI #04 | Insula, Cingulate | L | 4 | 2.745 | 0.005 * |
| ROI #05 | Insula, Cingulate | R | 4 | 3.148 | 0.007 * |
| ROI #06 | Frontal | L | 15 | 1.862 | 0.005 * |
| ROI #07 | Frontal | R | 15 | 2.127 | 0.007 * |
| ROI #08 | Occipital | L | 6 | 0.586 | 0.508 |
| ROI #09 | Occipital | R | 6 | 0.597 | 0.285 |
| ROI #10 | Parietal | L | 6 | 2.274 | 0.017 * |
| ROI #11 | Parietal | R | 6 | 1.231 | 0.059 |
| ROI #12 | Central | L | 4 | 2.515 | 0.007 * |
| ROI #13 | Central | R | 4 | 1.463 | 0.285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-B.; Kang, C.-K. Cerebrovascular Reactivity Assessment during Carbon Dioxide Inhalation Using SPECT. Appl. Sci. 2021, 11, 1161. https://doi.org/10.3390/app11031161
Lee Y-B, Kang C-K. Cerebrovascular Reactivity Assessment during Carbon Dioxide Inhalation Using SPECT. Applied Sciences. 2021; 11(3):1161. https://doi.org/10.3390/app11031161
Chicago/Turabian StyleLee, Yeong-Bae, and Chang-Ki Kang. 2021. "Cerebrovascular Reactivity Assessment during Carbon Dioxide Inhalation Using SPECT" Applied Sciences 11, no. 3: 1161. https://doi.org/10.3390/app11031161






