Evaluation of Automated Segmentation Algorithm for Macular Volumetric Measurements of Eight Individual Retinal Layer Thickness
Abstract
:1. Introduction
2. Methods and Materials
2.1. Participants
2.2. OCT Scans
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, D.; Duguid, G. Optical coherence tomography—A review of the principles and contemporary uses in retinal investigation. Eye 2004, 18, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwanza, J.-C.; Budenz, D.L. New developments in optical coherence tomography imaging for glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.R.; Wollstein, G.; Kim, N.R.; Na, J.H.; Nevins, J.E.; Kim, C.Y.; Schuman, J.S. Macular assessment using optical coherence tomography for glaucoma diagnosis. Br. J. Ophthalmol. 2012, 96, 1452–1455. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.M.; Mowatt, G.; Elders, A.; Lois, N.; Fraser, C.; Hernández, R.; Amoaku, W.; Burr, J.M.; Lotery, A.; Ramsay, C.R.; et al. Optical coherence tomography for the monitoring of neovascular age-related macular degeneration: A systematic review. Ophthalmology 2015, 122, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzold, A.; de Boer, J.F.; Schippling, S.; Vermersch, P.; Kardon, R.; Green, A.; Calabresi, P.A.; Polman, C. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Pierro, L.; Gagliardi, M.; Iuliano, L.; Ambrosi, A.; Bandello, F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5912–5920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, V.; Armstrong, J.J.; Pintwala, R.; Hutnik, C. Optical coherence tomography for glaucoma diagnosis: An evidence based meta-analysis. PLoS ONE 2018, 13, e0190621. [Google Scholar] [CrossRef]
- De Clerck, E.E.B.; Schouten, J.S.A.G.; Berendschot, T.T.J.M.; Kessels, A.G.H.; Nuijts, R.M.M.A.; Beckers, H.J.M.; Schram, M.T.; Stehouwer, C.D.A.; Webers, C.A.B. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015, 3, 653–663. [Google Scholar] [CrossRef]
- Saidha, S.; Al-Louzi, O.; Ratchford, J.N.; Bhargava, P.; Oh, J.; Newsome, S.D.; Prince, J.L.; Pham, D.; Roy, S.; van Zijl, P.; et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann. Neurol. 2015, 78, 801–813. [Google Scholar] [CrossRef]
- Graham, E.C.; You, Y.; Yiannikas, C.; Garrick, R.; Parratt, J.; Barnett, M.H.; Klistorner, A. Progressive Loss of Retinal Ganglion Cells and Axons in Nonoptic Neuritis Eyes in Multiple Sclerosis: A Longitudinal Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2311–2317. [Google Scholar] [CrossRef]
- Chen, T.C.; Hoguet, A.; Junk, A.K.; Nouri-Mahdavi, K.; Radhakrishnan, S.; Takusagawa, H.L.; Chen, P.P. Spectral-Domain OCT: Helping the Clinician Diagnose Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmology 2018, 125, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Birkeldh, U.; Manouchehrinia, A.; Hietala, M.A.; Hillert, J.; Olsson, T.; Piehl, F.; Kockum, I.S.; Brundin, L.; Zahavi, O.; Wahlberg-Ramsay, M.; et al. The Temporal Retinal Nerve Fiber Layer Thickness Is the Most Important Optical Coherence Tomography Estimate in Multiple Sclerosis. Front. Neurol. 2017, 8, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayhan, H.A.; Aslan Bayhan, S.; Tanık, N.; Gürdal, C. The association of spectral-domain optical coherence tomography determined ganglion cell complex parameters and disease severity in Parkinson’s disease. Curr. Eye Res. 2014, 39, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Ahn, J.; Kim, T.W.; Jeon, B.S. Optical coherence tomography in Parkinson’s disease: Is the retina a biomarker? J. Parkinsons Dis. 2014, 4, 197–204. [Google Scholar] [CrossRef]
- Tao, L.W.; Wu, Z.; Guymer, R.H.; Luu, C.D. Ellipsoid zone on optical coherence tomography: A review. Clin. Experiment. Ophthalmol. 2016, 44, 422–430. [Google Scholar] [CrossRef]
- Hood, D.C.; Zhang, X.; Ramachandran, R.; Talamini, C.L.; Raza, A.; Greenberg, J.P.; Sherman, J.; Tsang, S.H.; Birch, D.G. The inner segment/outer segment border seen on optical coherence tomography is less intense in patients with diminished cone function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9703–9709. [Google Scholar] [CrossRef]
- Spaide, R.F. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina 2013, 33, 1800–1808. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sato, T.; Kishi, S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am. J. Ophthalmol. 2009, 148, 105–110. [Google Scholar] [CrossRef]
- Lamin, A.; Oakley, J.D.; Dubis, A.M.; Russakoff, D.B.; Sivaprasad, S. Changes in volume of various retinal layers over time in early and intermediate age-related macular degeneration. Eye 2019, 33, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Matlach, J.; Wagner, M.; Malzahn, U.; Gobel, W. Repeatability of peripapillary retinal nerve fiber layer and inner retinal thickness among two spectral domain optical coherence tomography devices. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6536–6546. [Google Scholar] [CrossRef] [Green Version]
- Toteberg-Harms, M.; Sturm, V.; Knecht, P.B.; Funk, J.; Menke, M.N. Repeatability of nerve fiber layer thickness measurements in patients with glaucoma and without glaucoma using spectral-domain and time-domain OCT. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vicent, A.; Brautaset, R.; Venkataraman, A.P. Repeatability of quantitative measurements of retinal layers with SD-OCT and agreement between vertical and horizontal scan protocols in healthy eyes. PLoS ONE 2019, 14, e0221466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ctori, I.; Huntjens, B. Repeatability of Foveal Measurements Using Spectralis Optical Coherence Tomography Segmentation Software. PLoS ONE 2015, 10, e0129005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shen, M.; Huang, S.; Leng, L.; Zhu, D.; Lu, F. Repeatability and reproducibility of eight macular intra-retinal layer thicknesses determined by an automated segmentation algorithm using two SD-OCT instruments. PLoS ONE 2014, 9, e87996. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.; Cassels, N.; Lu, K.; Acton, J.H.; Margrain, T.H.; North, R.V.; Fergusson, J.; White, N.; Wood, A. Automated Retinal Layer Segmentation Using Spectral Domain Optical Coherence Tomography: Evaluation of Inter-Session Repeatability and Agreement between Devices. PLoS ONE 2016, 11, e0162001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlinden, C.; Khadka, J.; Pesudovs, K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol. Opt. 2011, 31, 330–338. [Google Scholar] [CrossRef]
- McAlinden, C.; Khadka, J.; Pesudovs, K. Precision (repeatability and reproducibility) studies and sample-size calculation. J. Cataract Refract. Surg. 2015, 41, 2598–2604. [Google Scholar] [CrossRef]
- Oberwahrenbrock, T.; Weinhold, M.; Mikolajczak, J.; Zimmermann, H.; Paul, F.; Beckers, I.; Brandt, A.U. Reliability of Intra-Retinal Layer Thickness Estimates. PLoS ONE 2015, 10, e0137316. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.H.; Han, J.Y.; Kang, H.G.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Ki, M. Comparison of Individual Retinal Layer Thicknesses between Highly Myopic Eyes and Normal Control Eyes Using Retinal Layer Segmentation Analysis. Sci. Rep. 2019, 9, 14000. [Google Scholar] [CrossRef]
- Otani, T.; Yamaguchi, Y.; Kishi, S. Improved visualization of Henle fiber layer by changing the measurement beam angle on optical coherence tomography. Retina 2011, 31, 497–501. [Google Scholar] [CrossRef]
- Ouyang, Y.; Walsh, A.C.; Keane, P.A.; Heussen, F.M.; Pappuru, R.K.R.; Sadda, S.R. Different phenotypes of the appearance of the outer plexiform layer on optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. Albr. von Graefes Arch. fur Klin. und Exp. Ophthalmol. 2013, 251, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Lujan, B.J.; Roorda, A.; Knighton, R.W.; Carroll, J. Revealing Henle’s fiber layer using spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1486–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrejen, S.; Gallego-Pinazo, R.; Freund, K.B.; Paques, M. Recognition of Henle’s fiber layer on OCT images. Ophthalmology 2013, 120, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Caldito, N.; Antony, B.; He, Y.; Lang, A.; Nguyen, J.; Rothman, A.; Ogbuokiri, E.; Avornu, A.; Balcer, L.; Frohman, E.; et al. Analysis of Agreement of Retinal-Layer Thickness Measures Derived from the Segmentation of Horizontal and Vertical Spectralis OCT Macular Scans. Curr. Eye Res. 2018, 43, 415–423. [Google Scholar] [CrossRef] [PubMed]
- ISO Probability and General Statistical Terms. Statistics: Vocabulary and symbols. Int. Organ. Stand. 2006. ISO 3534-1:2006. [Google Scholar]
- ISO Accuracy (trueness and precision) of measurement methods and results—Part 1: General principles and definitions. Int. Organ. Stand. 1994. ISO 5725-1:1994.
- Muftuoglu, I.K.; Ramkumar, H.L.; Bartsch, D.-U.; Meshi, A.; Gaber, R.; Freeman, W.R. Quantitative analysis of the inner retinal layer thicknesses in age-related macular degeneration using corrected optical coherence tomography segmentation. Retina 2018, 38, 1478–1484. [Google Scholar] [CrossRef]
- Witkin, A.J.; Ko, T.H.; Fujimoto, J.G.; Chan, A.; Drexler, W.; Schuman, J.S.; Reichel, E.; Duker, J.S. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am. J. Ophthalmol. 2006, 142, 945–952. [Google Scholar] [CrossRef] [Green Version]
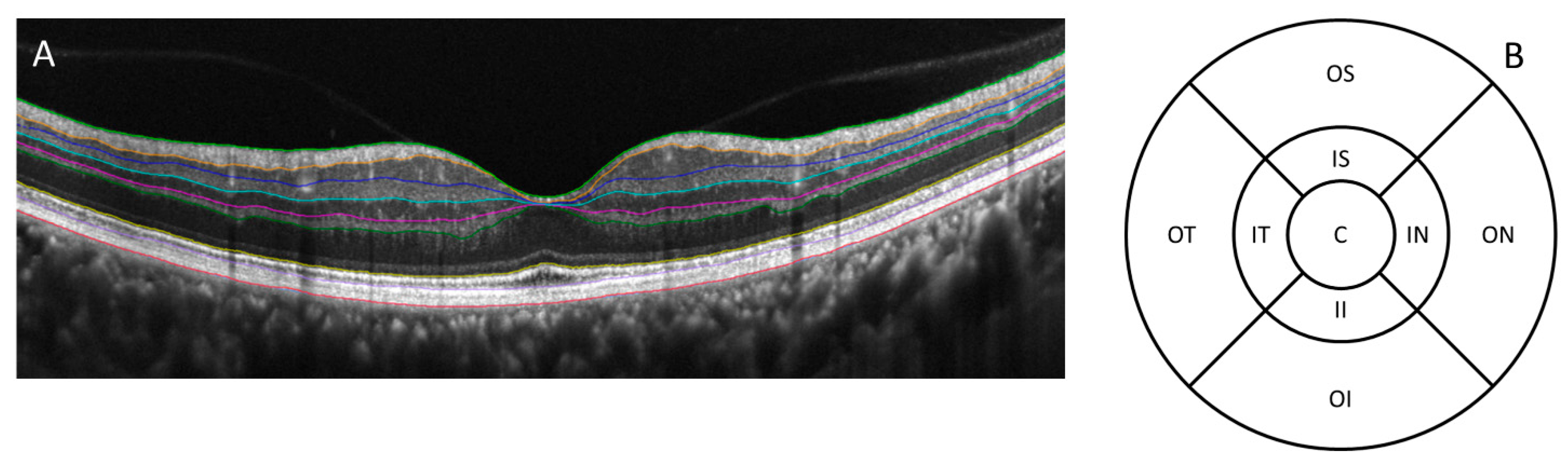
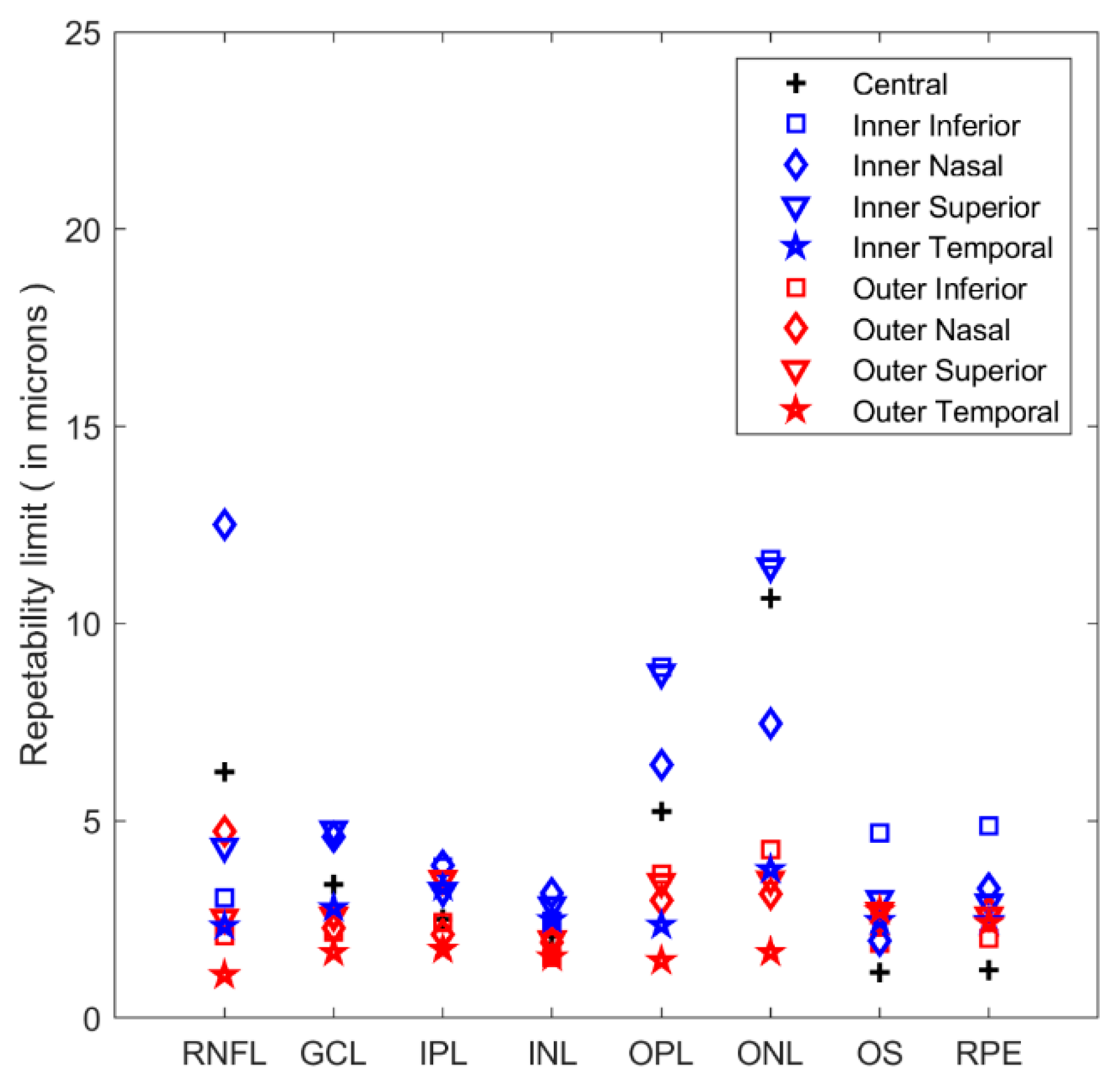
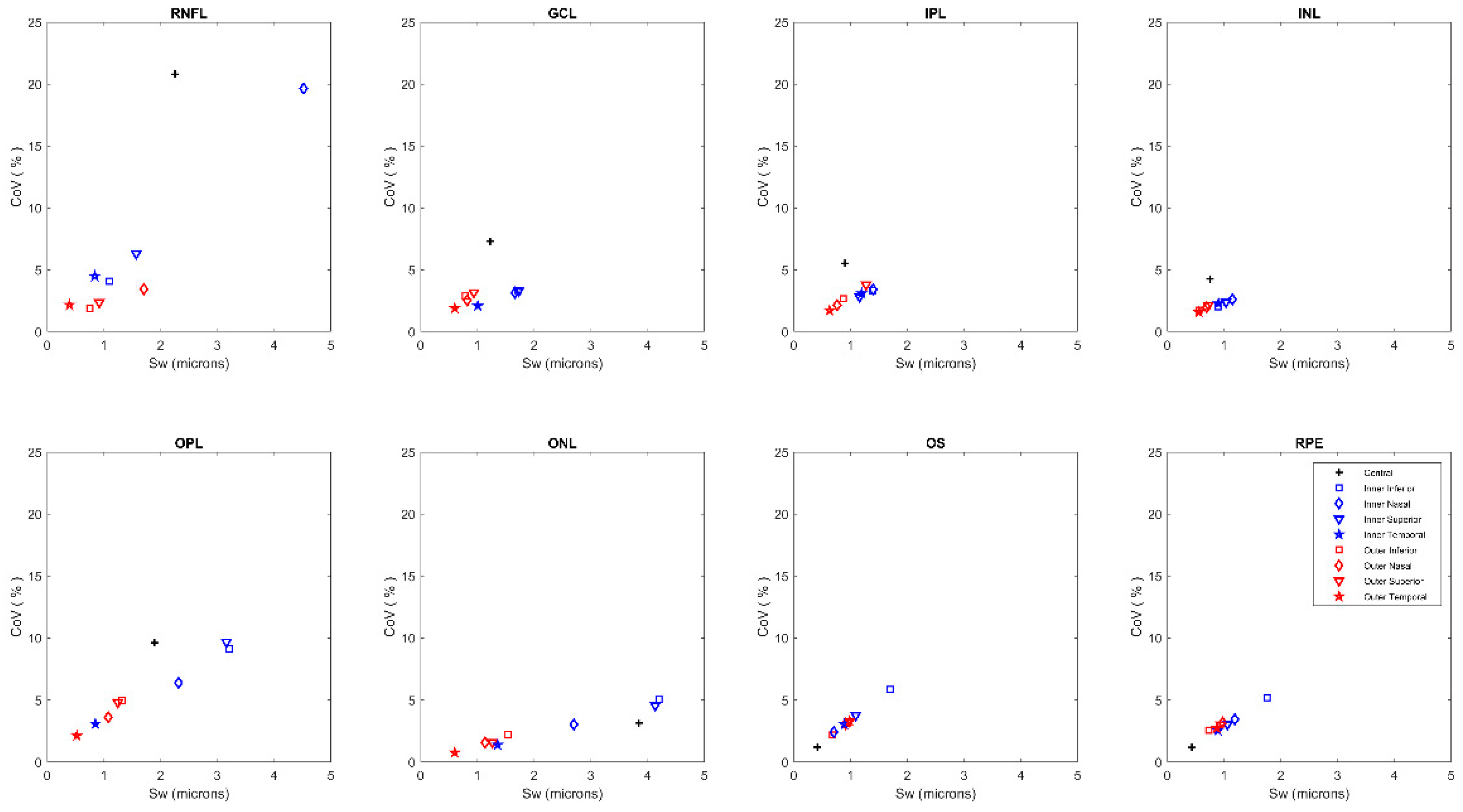
| Central | Inner Inferior | Outer Inferior | Inner Nasal | Outer Nasal | Inner Superior | Outer Superior | Inner Temporal | Outer Temporal | |
|---|---|---|---|---|---|---|---|---|---|
| RNFL | 11 ± 6 | 27 ± 3 | 40 ± 5 | 23 ± 6 | 50 ± 4.9 | 25 ± 3 | 39 ± 5 | 19 ± 5 | 18 ± 1 |
| GCL | 17 ± 4 | 51 ± 5 | 27 ± 3 | 53 ± 5 | 32 ± 4 | 52 ± 4 | 30 ± 3 | 48 ± 5 | 32 ± 3 |
| IPL | 16 ± 3 | 42 ± 3 | 33 ± 3 | 41 ± 3 | 35 ± 4 | 41 ± 3 | 33 ± 3 | 38 ± 3 | 36 ± 3 |
| INL | 18 ± 3 | 44 ± 2 | 32 ± 3 | 44 ± 3 | 35 ± 3 | 43 ± 2 | 34 ± 3 | 39 ± 2 | 35 ± 2 |
| OPL | 10 ± 5 | 35 ± 8 | 27 ± 4 | 36 ± 9 | 30 ± 4 | 33 ± 5 | 26 ± 2 | 28 ± 3 | 25 ± 2 |
| ONL | 122 ± 10 | 83 ± 12 | 69 ± 6 | 89 ± 13 | 73 ± 8 | 91 ± 9 | 80 ± 7 | 98 ± 8 | 79 ± 6 |
| OS | 35 ± 1 | 29 ± 3 | 31 ± 3 | 29 ± 2 | 29 ± 3 | 29 ± 2 | 30 ± 4 | 29 ± 2 | 30 ± 4 |
| RPE | 37 ± 2 | 34 ± 3 | 28 ± 3 | 34 ± 3 | 31 ± 3 | 35 ± 3 | 31 ± 4 | 35 ± 2 | 32 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahavi, O.; Domínguez-Vicent, A.; Brautaset, R.; Venkataraman, A.P. Evaluation of Automated Segmentation Algorithm for Macular Volumetric Measurements of Eight Individual Retinal Layer Thickness. Appl. Sci. 2021, 11, 1250. https://doi.org/10.3390/app11031250
Zahavi O, Domínguez-Vicent A, Brautaset R, Venkataraman AP. Evaluation of Automated Segmentation Algorithm for Macular Volumetric Measurements of Eight Individual Retinal Layer Thickness. Applied Sciences. 2021; 11(3):1250. https://doi.org/10.3390/app11031250
Chicago/Turabian StyleZahavi, Ori, Alberto Domínguez-Vicent, Rune Brautaset, and Abinaya Priya Venkataraman. 2021. "Evaluation of Automated Segmentation Algorithm for Macular Volumetric Measurements of Eight Individual Retinal Layer Thickness" Applied Sciences 11, no. 3: 1250. https://doi.org/10.3390/app11031250
APA StyleZahavi, O., Domínguez-Vicent, A., Brautaset, R., & Venkataraman, A. P. (2021). Evaluation of Automated Segmentation Algorithm for Macular Volumetric Measurements of Eight Individual Retinal Layer Thickness. Applied Sciences, 11(3), 1250. https://doi.org/10.3390/app11031250






