Interactions between Phase-Separated Liquids and Membrane Surfaces
Abstract
:1. Introduction
2. Physical Properties of Phase-Separating Molecules and Biomolecular Condensates
2.1. Biophysical Drivers of Liquid-Liquid Phase Separation
2.2. Stimulus Responsiveness of Biomolecular Condensates
3. Contact with Liquid Phases that Separate from Solution Reshapes Membranes at Phase Boundaries
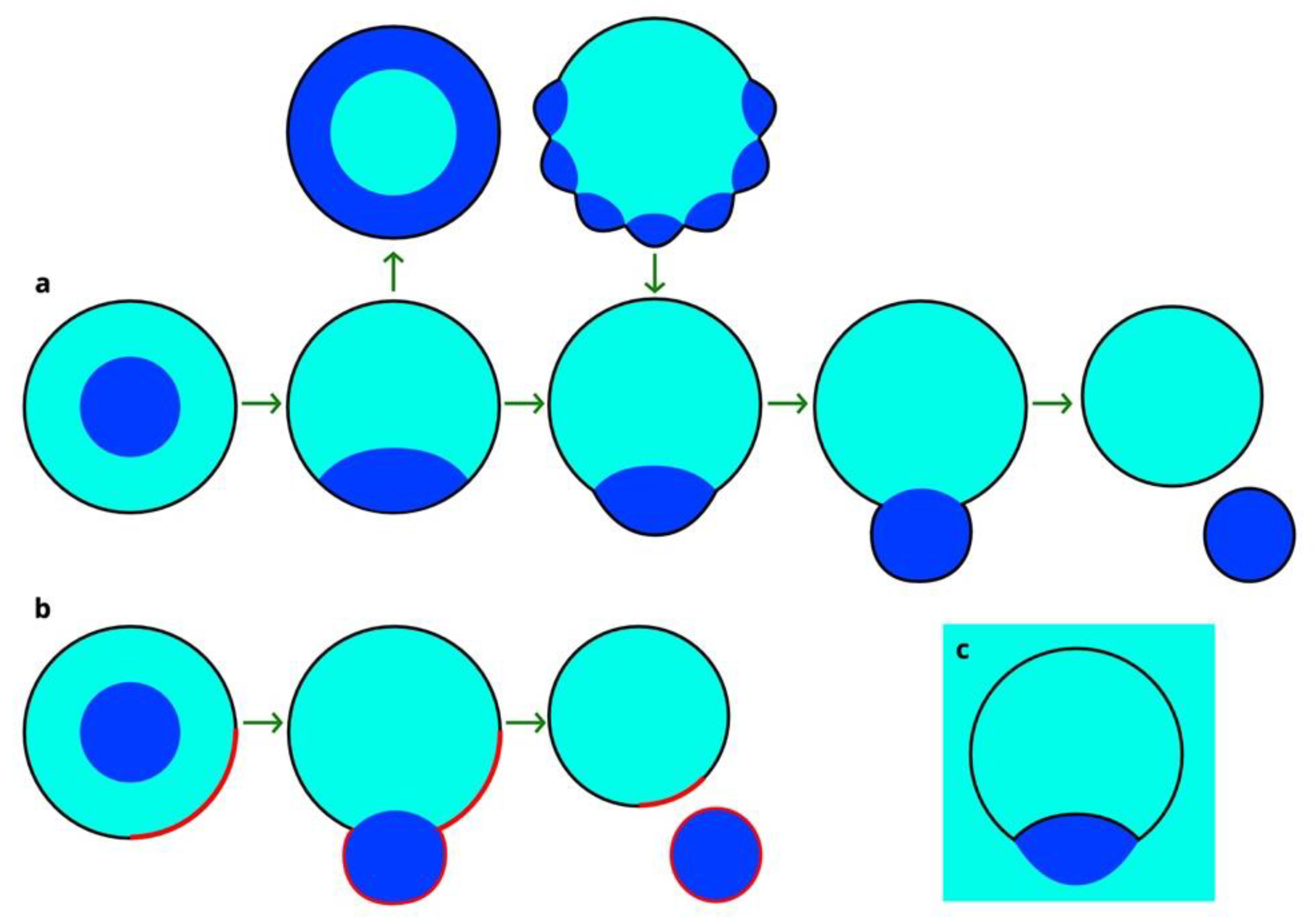
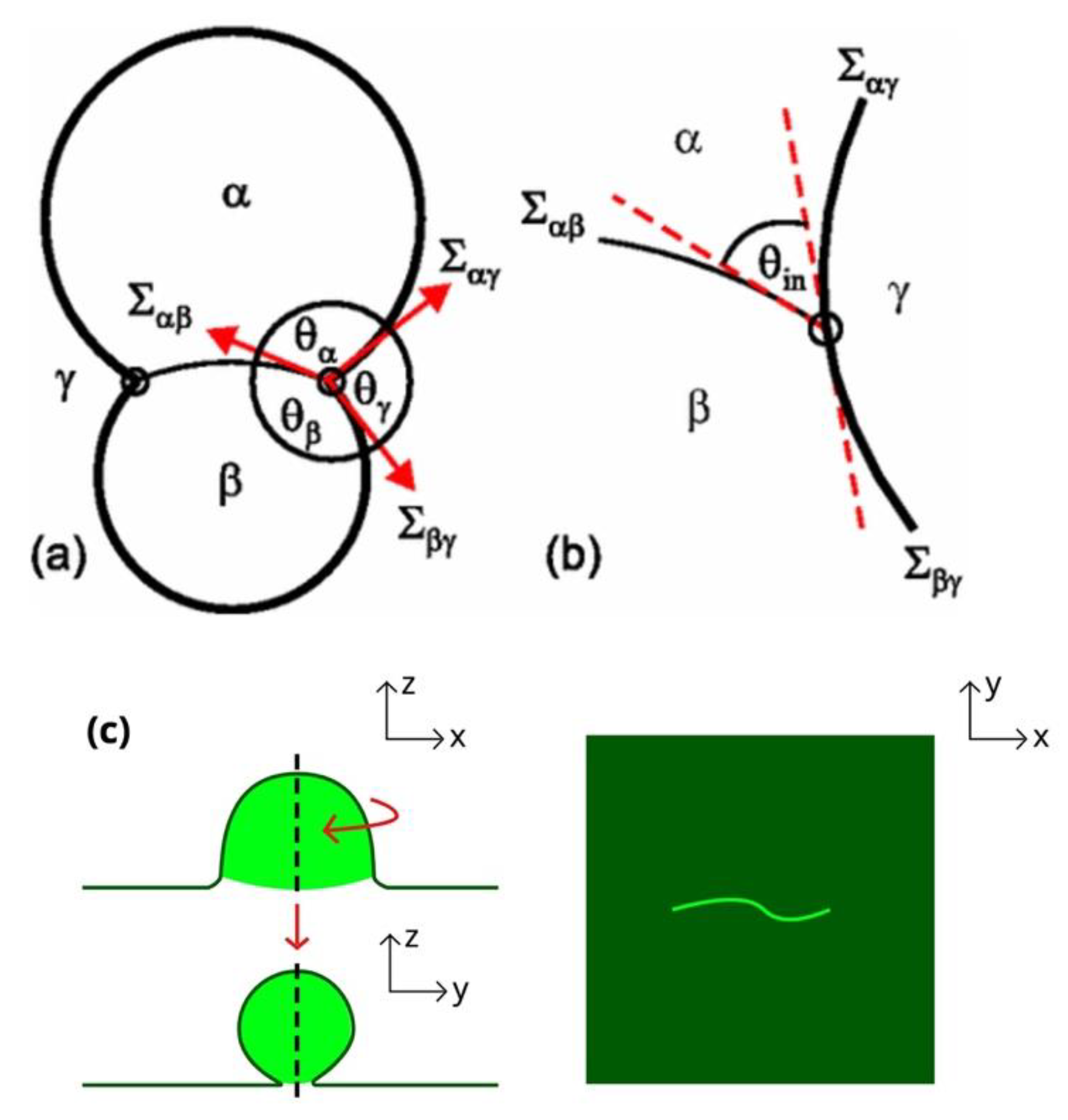
3.1. Wetting Transition & Budding in Vesicle-Enclosed ATPSs
3.2. Deviations from Budding: Nanotubes and Nanodroplets
4. Phase Separation Mediated by Membranes Can Reshape and Laterally Reorganize Membranes
4.1. The Formation of Some Biomolecular Condensates is Mediated by the Membrane
4.2. Phase-Separated Protein Domains Modify the Properties of Membranes and Reshape Them
4.3. Membrane Reorganization by Phase-Separated Protein Domains Contributes to Biological Structures
5. Biomolecular Condensates Promoting Membrane Protein Clustering Influence Protein Function
5.1. Phase Separation in LAT Clusters Has a Functional Contribution to T Cell Signaling
5.2. Phase Separation Contributes to Nephrin Signaling
5.3. The Synapse Is Organized by Multiple Membrane-Mediated Biomolecular Condensates
6. Small Vesicles May Surround-or Act as Components of-Phase-Separated Condensates
6.1. Vesicles Can Partition into a Liquid Phase of an ATPS or to the Phase Boundary
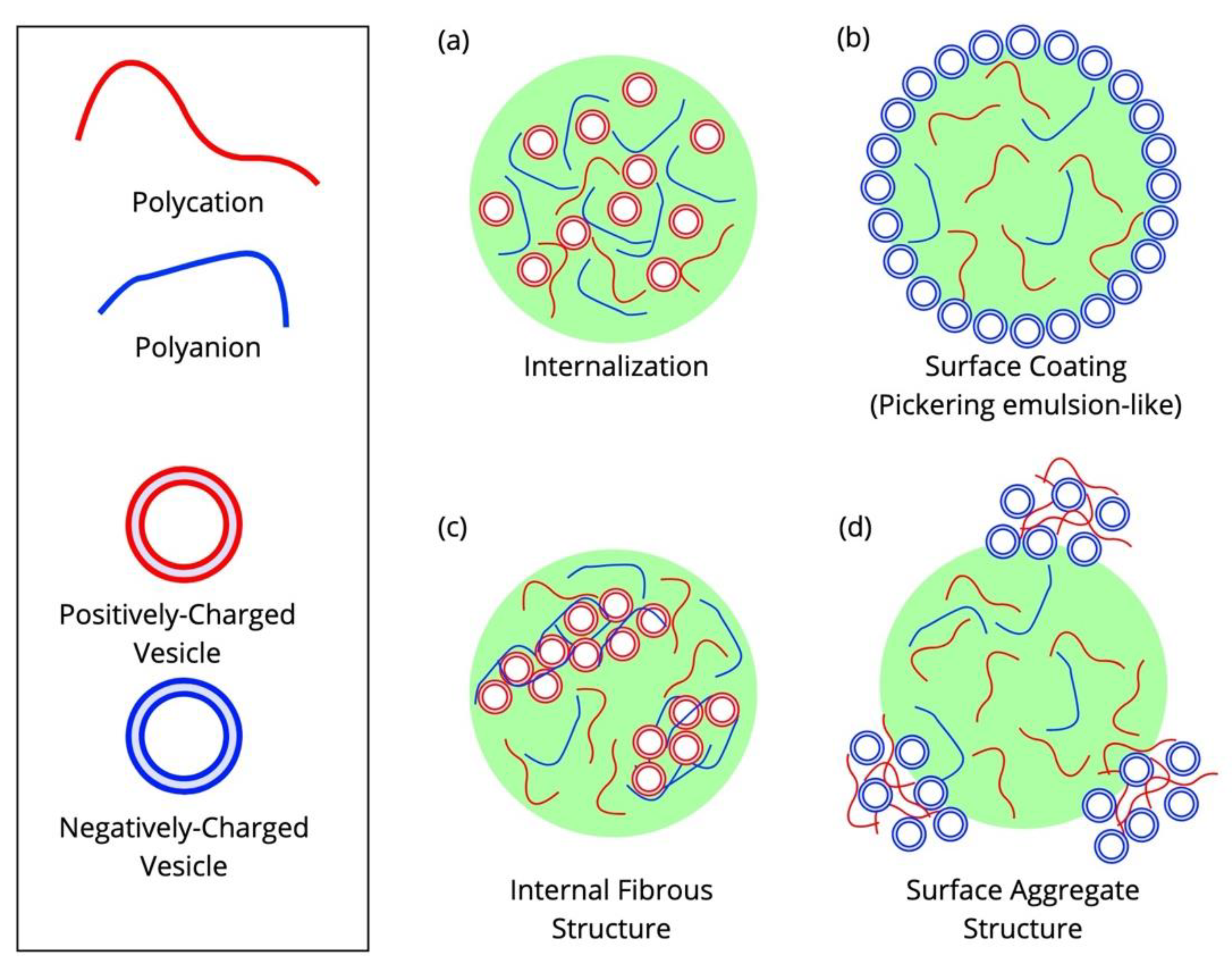
6.2. Vesicle Interactions with Complex Coacervates Depend on Several Physical Factors
6.3. Vesicle Organization by Biomolecular Condensates Is Biologically Relevant
7. Active Processes and Nonequilibrium States in Cells Modify Biomolecular Condensate Properties
7.1. Artificial Regulatory Mechanisms Reveal Biomolecular Condensate Responses to Nonequilibrium Processes
7.2. Regulation of Cellular Processes Impacts Biomolecular Condensates
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATPS | aqueous two-phase system; |
| BAR | Bin, amphiphysin and Rvs161/167; |
| BuGZ | BUB3-interacting and GLEBS motif-containing protein ZNF207; |
| chol-polyU | cholesterol-polyU; |
| CIN85 | Cbl-interacting protein of 85 kDa; |
| FUS | Fused in Sarcoma protein; |
| FUS LC | FUS low-complexity domain; |
| IDR | intrinsically disordered region; |
| LAT | linker for the activation of T cells; |
| LCST | lower critical solution temperature; |
| LUV | large unilamellar vesicle; |
| NICD | nephrin intracellular domain; |
| N-WASP | neuronal Wiskott-Aldrich syndrome protein; |
| PEG | polyethylene glycol; |
| PSD | postsynaptic density; |
| Rh-PE | 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); |
| SLP65 | Src homology (SH) 2 domain-containing leukocyte protein of 65 kDa; |
| SOS | son of sevenless; |
| SUV | small unilamellar vesicle; |
| TCR | T cell receptor; |
| UCST | upper critical solution temperature |
References
- Case, L.B.; Ditlev, J.A.; Rosen, M.K. Regulation of Transmembrane Signaling by Phase Separation. Annu. Rev. Biophys. 2019, 48, 465–494. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Banjade, S.; Cheng, H.C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ditlev, J.A.; Hui, E.; Xing, W.; Banjade, S.; Okrut, J.; King, D.S.; Taunton, J.; Rosen, M.K.; Vale, R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, J.; Weber, S.C.; Vaidya, N.; Haataja, M.; Brangwynne, C.P. RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E5237–E5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef] [Green Version]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.H.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef] [Green Version]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Nott, T.J.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.D.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.B.; Görlich, D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem. Sci. 2016, 41, 46–61. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-M.; Holehouse, A.S.; Pappu, R.V. Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu. Rev. Biophys. 2020, 49, 107–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brangwynne, C.P.; Tompa, P.; Pappu, R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015, 11, 899–904. [Google Scholar] [CrossRef]
- Huggins, M.L. Some Properties of Solutions of Long-chain Compounds. J. Phys. Chem. 1942, 46, 151–158. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Overbeek, J.T.G.; Voorn, M.J. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Comp. Physiol. 1957, 49, 7–26. [Google Scholar] [CrossRef]
- Burke, K.A.; Janke, A.M.; Rhine, C.L.; Fawzi, N.L. Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol. Cell 2015, 60, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Pak, C.W.; Kosno, M.; Holehouse, A.S.; Padrick, S.B.; Mittal, A.; Ali, R.; Yunus, A.A.; Liu, D.R.; Pappu, R.V.; Rosen, M.K. Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein. Mol. Cell 2016, 63, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Elbaum-Garfinkle, S.; Langdon, E.M.; Taylor, N.; Occhipinti, P.; Bridges, A.A.; Brangwynne, C.P.; Gladfelter, A.S. RNA Controls PolyQ Protein Phase Transitions. Mol. Cell 2015, 60, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjade, S.; Wu, Q.; Mittal, A.; Peeples, W.B.; Pappu, R.V.; Rosen, M.K. Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proc. Natl. Acad. Sci. USA 2015, 112, E6426–E6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, T.S.; Holehouse, A.S.; Rosen, M.K.; Pappu, R.V. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. Elife 2017, 6, e30294. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N.; Rubinstein, M. Thermoreversible gelation in solutions of associative polymers. 1. Statics. Macromolecules 1998, 31, 1373–1385. [Google Scholar] [CrossRef]
- Ruff, K.M.; Roberts, S.; Chilkoti, A.; Pappu, R.V. Advances in Understanding Stimulus-Responsive Phase Behavior of Intrinsically Disordered Protein Polymers. J. Mol. Biol. 2018, 430, 4619–4635. [Google Scholar] [CrossRef]
- Martin, E.W.; Mittag, T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry 2018, 57, 2478–2487. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Huang, Y.; He, X.; Cui, H.; Zhu, X.; Zheng, Y. Phase Transition of Spindle-Associated Protein Regulate Spindle Apparatus Assembly. Cell 2015, 163, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Murthy, A.C.; Dignon, G.L.; Kan, Y.; Zerze, G.H.; Parekh, S.H.; Mittal, J.; Fawzi, N.L. Molecular interactions underlying liquid−liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 2019, 26, 637–648. [Google Scholar] [CrossRef]
- Reichheld, S.E.; Muiznieks, L.D.; Keeley, F.W.; Sharpe, S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proc. Natl. Acad. Sci. USA 2017, 114, E4408–E4415. [Google Scholar] [CrossRef] [Green Version]
- Helfrich, M.R.; Mangeney-Slavin, L.K.; Long, M.S.; Djoko, K.Y.; Keating, C.D. Aqueous phase separation in giant vesicles. J. Am. Chem. Soc. 2002, 124, 13374–13375. [Google Scholar] [CrossRef]
- Long, M.S.; Jones, C.D.; Helfrich, M.R.; Mangeney-Slavin, L.K.; Keating, C.D. Dynamic microcompartmentation in synthetic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5920–5925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lipowsky, R.; Dimova, R. Transition from Complete to Partial Wetting within Membrane Compartments. J. Am. Chem. Soc. 2008, 130, 12252–12253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lipowsky, R.; Dimova, R. Membrane nanotubes induced by aqueous phase separation and stabilized by spontaneous curvature. Proc. Natl. Acad. Sci. USA 2011, 108, 4731–4736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.S.; Cans, A.-S.; Keating, C.D. Budding and Asymmetric Protein Microcompartmentation in Giant Vesicles Containing Two Aqueous Phases. J. Am. Chem. Soc. 2008, 130, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Andes-Koback, M.; Keating, C.D. Complete Budding and Asymmetric Division of Primitive Model Cells To Produce Daughter Vesicles with Different Interior and Membrane Compositions. J. Am. Chem. Soc. 2011, 133, 9545–9555. [Google Scholar] [CrossRef]
- Li, Y.; Kusumaatmaja, H.; Lipowsky, R.; Dimova, R. Wetting-Induced Budding of Vesicles in Contact with Several Aqueous Phases. J. Phys. Chem. B 2012, 116, 1819–1823. [Google Scholar] [CrossRef]
- Liu, Y.; Agudo-Canalejo, J.; Grafmüller, A.; Dimova, R.; Lipowsky, R. Patterns of flexible nanotubes formed by liquid-ordered and liquid-disordered membranes. ACS Nano 2016, 10, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Kusumaatmaja, H.; Li, Y.; Dimova, R.; Lipowsky, R. Intrinsic Contact Angle of Aqueous Phases at Membranes and Vesicles. Phys. Rev. Lett. 2009, 103, 238103. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Xiao, W.; Yoshikawa, K. Wetting transitions within membrane compartments. Soft Matter 2014, 10, 5311–5317. [Google Scholar] [CrossRef] [Green Version]
- Fan, H. Liquid droplet spreading with line tension effect. J. Phys. Condens. Matter 2006, 18, 4481–4488. [Google Scholar] [CrossRef]
- Satarifard, V.; Grafmüller, A.; Lipowsky, R. Nanodroplets at Membranes Create Tight-Lipped Membrane Necks via Negative Line Tension. ACS Nano 2018, 12, 12424–12435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrocki, G.; Im, W.; Sugita, Y.; Feig, M. Clustering and dynamics of crowded proteins near membranes and their influence on membrane bending. Proc. Natl. Acad. Sci. USA 2019, 116, 24562–24567. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, B.N.; Hoek, J.B.; Westerhoff, H.V. Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends Cell Biol. 2000, 10, 173–178. [Google Scholar] [CrossRef]
- Gallop, J.L.; McMahon, H.T. BAR domains and membrane curvature: Bringing your curves to the BAR. Biochem. Soc. Symp. 2005, 72, 223–231. [Google Scholar] [CrossRef]
- Robles-Ramos, M.Á.; Zorrilla, S.; Alfonso, C.; Margolin, W.; Rivas, G.; Monterroso, B. Assembly of bacterial cell division protein FtsZ into dynamic biomolecular condensates. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, I.-H.; Imanaka, M.Y.; Modahl, E.H.; Torres-Ocampo, A.P. Lipid Raft Phase Modulation by Membrane-Anchored Proteins with Inherent Phase Separation Properties. ACS Omega 2019, 4, 6551–6559. [Google Scholar] [CrossRef]
- Banjade, S.; Rosen, M.K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 2014, 3, e04123. [Google Scholar] [CrossRef]
- Alimohamadi, H.; Rangamani, P. Modeling Membrane Curvature Generation due to Membrane–Protein Interactions. Biomolecules 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Tsafrir, I.; Sagi, D.; Arzi, T.; Guedeau-Boudeville, M.-A.; Frette, V.; Kandel, D.; Stavans, J. Pearling Instabilities of Membrane Tubes with Anchored Polymers. Phys. Rev. Lett. 2001, 86, 1138–1141. [Google Scholar] [CrossRef] [Green Version]
- Chaïeb, S.; Rica, S. Spontaneous curvature-induced pearling instability. Phys. Rev. E 1998, 58, 7733–7737. [Google Scholar] [CrossRef] [Green Version]
- Alimohamadi, H.; Ovryn, B.; Rangamani, P. Modeling membrane nanotube morphology: The role of heterogeneity in composition and material properties. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.; Alimohamadi, H.; Bakka, B.; Trementozzi, A.N.; Fawzi, N.L.; Rangamani, P.; Stachowiak, J.C. Membrane bending by protein phase separation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Last, M.G.F.; Deshpande, S.; Dekker, C. pH-Controlled Coacervate–Membrane Interactions within Liposomes. ACS Nano 2020, 14, 4487–4498. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, O.; Michalak, K.; Maniewska, J.; Hendrich, A.B. Giant unilamellar vesicles—A perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol. 2009, 56, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Veatch, S.L.; Keller, S.L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.K.; Huang, W.Y.C.; Carbone, C.B.; Nocka, L.M.; Parikh, A.N.; Vale, R.D.; Groves, J.T. Coupled membrane lipid miscibility and phosphotyrosine-driven protein condensation phase transitions. Biophys. J. 2020. [Google Scholar] [CrossRef]
- Bergeron-Sandoval, L.-P.; Michnick, S.W. Mechanics, Structure and Function of Biopolymer Condensates. J. Mol. Biol. 2018, 430, 4754–4761. [Google Scholar] [CrossRef]
- Bergeron-Sandoval, L.-P.; Heris, H.K.; Hendricks, A.G.; Ehrlicher, A.J.; Francois, P.; Pappu, R.V.; Michnick, S.W. Endocytosis caused by liquid-liquid phase separation of proteins. bioRxiv 2017, 145664. [Google Scholar] [CrossRef] [Green Version]
- Weikl, T.R. Membrane-Mediated Cooperativity of Proteins. Annu. Rev. Phys. Chem. 2018, 69, 521–539. [Google Scholar] [CrossRef]
- Prévost, C.; Zhao, H.; Manzi, J.; Lemichez, E.; Lappalainen, P.; Callan-Jones, A.; Bassereau, P. IRSp53 senses negative membrane curvature and phase separates along membrane tubules. Nat. Commun. 2015, 6, 8529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.G.; Zhang, H. Phase Separation in Membrane Biology: The Interplay between Membrane-Bound Organelles and Membraneless Condensates. Dev. Cell 2020, 55, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Wange, R.L. LAT, the Linker for Activation of T Cells: A Bridge Between T Cell-Specific and General Signaling Pathways. Sci. Signal. 2000, 2000, re1. [Google Scholar] [CrossRef] [PubMed]
- Douglass, A.D.; Vale, R.D. Single-Molecule Microscopy Reveals Plasma Membrane Microdomains Created by Protein-Protein Networks that Exclude or Trap Signaling Molecules in T Cells. Cell 2005, 121, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Ditlev, J.A.; Vega, A.R.; Köster, D.V.; Su, X.; Tani, T.; Lakoduk, A.M.; Vale, R.D.; Mayor, S.; Jaqaman, K.; Rosen, M.K. A composition-dependent molecular clutch between T cell signaling condensates and actin. Elife 2019, 8, e42695. [Google Scholar] [CrossRef]
- Gureasko, J.; Kuchment, O.; Makino, D.L.; Sondermann, H.; Bar-Sagi, D.; Kuriyan, J. Role of the histone domain in the autoinhibition and activation of the Ras activator Son of Sevenless. Proc. Natl. Acad. Sci. USA 2010, 107, 3430–3435. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.Y.C.; Alvarez, S.; Kondo, Y.; Lee, Y.K.; Chung, J.K.; Lam, H.Y.M.; Biswas, K.H.; Kuriyan, J.; Groves, J.T. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019, 363, 1098–1103. [Google Scholar] [CrossRef]
- Martin, E.W.; Mittag, T. Dwelling at membranes promotes decisive signaling. Science 2019, 363, 1036–1037. [Google Scholar] [CrossRef]
- Verma, R.; Kovari, I.; Soofi, A.; Nihalani, D.; Patrie, K.; Holzman, L.B. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Investig. 2006, 116, 1346–1359. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoodi, J.; Sigmundsson, K.; Öfverstedt, L.-G.; Skoglund, U.; Öbrink, B.; Wartiovaara, J.; Tryggvason, K. Nephrin Promotes Cell-Cell Adhesion through Homophilic Interactions. Am. J. Pathol. 2003, 163, 2337–2346. [Google Scholar] [CrossRef] [Green Version]
- Case, L.B.; Zhang, X.; Ditlev, J.A.; Rosen, M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Wu, H.; Zhang, M. Phase separation at the synapse. Nat. Neurosci. 2020, 23, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Shang, Y.; Araki, Y.; Guo, T.; Huganir, R.L.; Zhang, M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 2016, 166, 1163–1175.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.; Chen, X.; Guan, D.; Xu, J.; Wu, H.; Tong, P.; Zhang, M. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 2018, 174, 1172–1187.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, G.; Wang, Y.; Zhang, M. Gephyrin-mediated formation of inhibitory postsynaptic density sheet via phase separation. Cell Res. 2020. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Shen, Z.; Chen, X.; Zeng, M.; Du, S.; Zhang, M. RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Mol. Cell 2019, 73, 971–984.e5. [Google Scholar] [CrossRef] [Green Version]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607. [Google Scholar] [CrossRef] [Green Version]
- Pechstein, A.; Tomilin, N.; Fredrich, K.; Vorontsova, O.; Sopova, E.; Evergren, E.; Haucke, V.; Brodin, L.; Shupliakov, O. Vesicle Clustering in a Living Synapse Depends on a Synapsin Region that Mediates Phase Separation. Cell Rep. 2020, 30, 2594–2602.e3. [Google Scholar] [CrossRef]
- Dewey, D.C.; Strulson, C.A.; Cacace, D.N.; Bevilacqua, P.C.; Keating, C.D. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat. Commun. 2014, 5, 4670. [Google Scholar] [CrossRef] [Green Version]
- Aumiller, W.M.; Pir Cakmak, F.; Davis, B.W.; Keating, C.D. RNA-Based Coacervates as a Model for Membraneless Organelles: Formation, Properties, and Interfacial Liposome Assembly. Langmuir 2016, 32, 10042–10053. [Google Scholar] [CrossRef] [Green Version]
- Tilcock, C.; Chin, R.; Veiro, J.; Cullis, P.; Fisher, D. Detection of surface charge-related properties in model membrane systems by aqueous two-phase partition. Biochim. Biophys. Acta Biomembr. 1989, 986, 167–171. [Google Scholar] [CrossRef]
- Moldavski, N.; Cohen, S. Determinants of liposome partitioning in aqueous two-phase systems: Evaluation by means of a factorial design. Biotechnol. Bioeng. 1996, 52, 529–537. [Google Scholar] [CrossRef]
- Tilcock, C.; Cullis, P.; Dempsey, T.; Youens, B.N.; Fisher, D. Aqueous two-phase polymer partitioning of lipid vesicles of defined size and composition. Biochim. Biophys. Acta Biomembr. 1989, 979, 208–214. [Google Scholar] [CrossRef]
- Moribe, K.; Maruyama, K.; Iwatsuru, M. Estimation of surface state of poly(ethylene glycol)-coated liposomes using an aqueous two-phase partitioning technique. Chem. Pharm. Bull. 1997, 45, 1683–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kırbaş, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B.; Ozdemir, B.; Öztürkoğlu, D.; Ölmez, H.; İşlek, Z.; Şahin, F.; Taşlı, P.N. Optimized Isolation of Extracellular Vesicles From Various Organic Sources Using Aqueous Two-Phase System. Sci. Rep. 2019, 9, 19159. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.U. Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Veis, A. A review of the early development of the thermodynamics of the complex coacervation phase separation. Adv. Colloid Interface Sci. 2011, 167, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Huang, X.; Tang, T.-Y.D.; Mann, S. Synthetic cellularity based on non-lipid micro-compartments and protocell models. Curr. Opin. Chem. Biol. 2014, 22, 1–11. [Google Scholar] [CrossRef]
- Pir Cakmak, F.; Grigas, A.T.; Keating, C.D. Lipid Vesicle-Coated Complex Coacervates. Langmuir 2019, 35, 7830–7840. [Google Scholar] [CrossRef]
- Lin, Y.; Jing, H.; Liu, Z.; Chen, J.; Liang, D. Dynamic Behavior of Complex Coacervates with Internal Lipid Vesicles under Nonequilibrium Conditions. Langmuir 2020, 36, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, F.; Greengard, P.; Brunner, J.; Bahler, M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J. Cell Biol. 1989, 108, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ganzella, M.; Zhou, J.; Zhu, S.; Jahn, R.; Zhang, M. Vesicle Tethering on the Surface of Phase-Separated Active Zone Condensates. Mol. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.E.; Bhatt, A.; Erdmann, P.S.; Hou, Z.; Maier, J.; Pirkuliyeva, S.; Engelke, M.; Becker, S.; Plitzko, J.; Wienands, J.; et al. Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness. Nat. Commun. 2020, 11, 848. [Google Scholar] [CrossRef] [PubMed]
- Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. Chem. 1897, 22U, 289–330. [Google Scholar] [CrossRef]
- Voorhees, P.W. Ostwald Ripening of Two-Phase Mixtures. Annu. Rev. Mater. Sci. 1992, 22, 197–215. [Google Scholar] [CrossRef]
- Zwicker, D.; Hyman, A.A.; Jülicher, F. Suppression of Ostwald ripening in active emulsions. Phys. Rev. E 2015, 92, 012317. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.; Brangwynne, C.P.; Haataja, M. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys. 2018, 81, 046601. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.F.; Wurtz, J.D. Novel physics arising from phase transitions in biology. J. Phys. D Appl. Phys. 2018, 52, 023001. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Niu, L.; Zhu, X.; Zhao, M.; Zhang, Z.; Mann, S.; Liang, D. Non-equilibrium behaviour in coacervate-based protocells under electric-field-induced excitation. Nat. Commun. 2016, 7, 10658. [Google Scholar] [CrossRef] [Green Version]
- Dine, E.; Gil, A.A.; Uribe, G.; Brangwynne, C.P.; Toettcher, J.E. Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst. 2018, 6, 655–663.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, K.J.; Kago, G.; Wang, L.; Richter, J.B.; Hayden, C.C.; Lafer, E.M.; Stachowiak, J.C. Liquid-like protein interactions catalyze assembly of endocytic vesicles. bioRxiv 2019, 860684. [Google Scholar] [CrossRef] [Green Version]
- Söding, J.; Zwicker, D.; Sohrabi-Jahromi, S.; Boehning, M.; Kirschbaum, J. Mechanisms for Active Regulation of Biomolecular Condensates. Trends Cell Biol. 2020, 30, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, J.D.; Lee, C.F. Chemical-Reaction-Controlled Phase Separated Drops: Formation, Size Selection, and Coarsening. Phys. Rev. Lett. 2018, 120, 078102. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Kawaguchi, K. Surface wetting by kinetic control of liquid-liquid phase separation. arXiv 2020, arXiv:2003.13666. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botterbusch, S.; Baumgart, T. Interactions between Phase-Separated Liquids and Membrane Surfaces. Appl. Sci. 2021, 11, 1288. https://doi.org/10.3390/app11031288
Botterbusch S, Baumgart T. Interactions between Phase-Separated Liquids and Membrane Surfaces. Applied Sciences. 2021; 11(3):1288. https://doi.org/10.3390/app11031288
Chicago/Turabian StyleBotterbusch, Samuel, and Tobias Baumgart. 2021. "Interactions between Phase-Separated Liquids and Membrane Surfaces" Applied Sciences 11, no. 3: 1288. https://doi.org/10.3390/app11031288
APA StyleBotterbusch, S., & Baumgart, T. (2021). Interactions between Phase-Separated Liquids and Membrane Surfaces. Applied Sciences, 11(3), 1288. https://doi.org/10.3390/app11031288





