Technical Feasibility and Histological Analysis of Balloon-Expandable Metallic Stent Placement in a Porcine Eustachian Tube
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
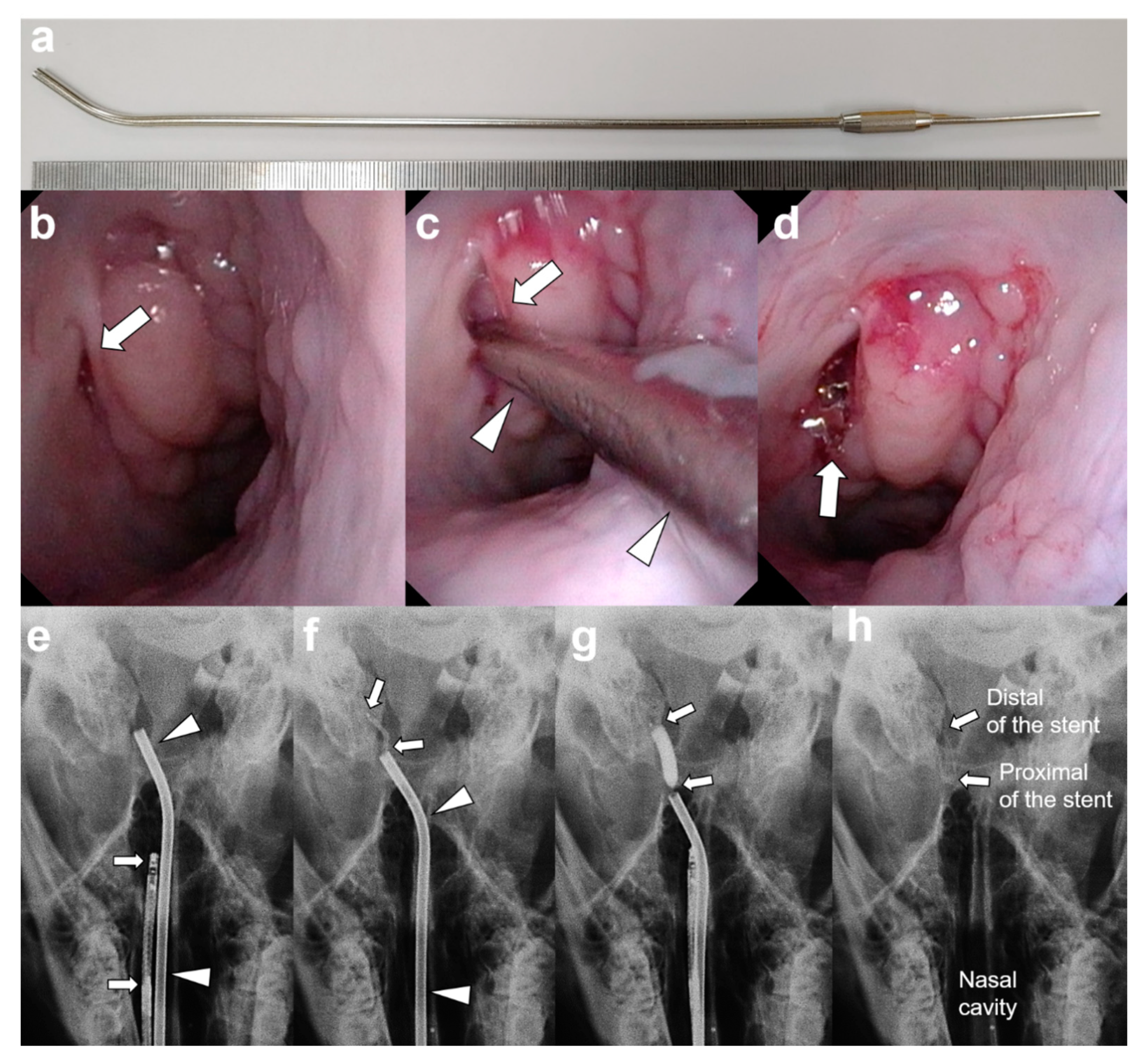
2.2. Metallic Guiding Sheath
2.3. Stent Placement under Endoscopic and Fluoroscopic Guidance
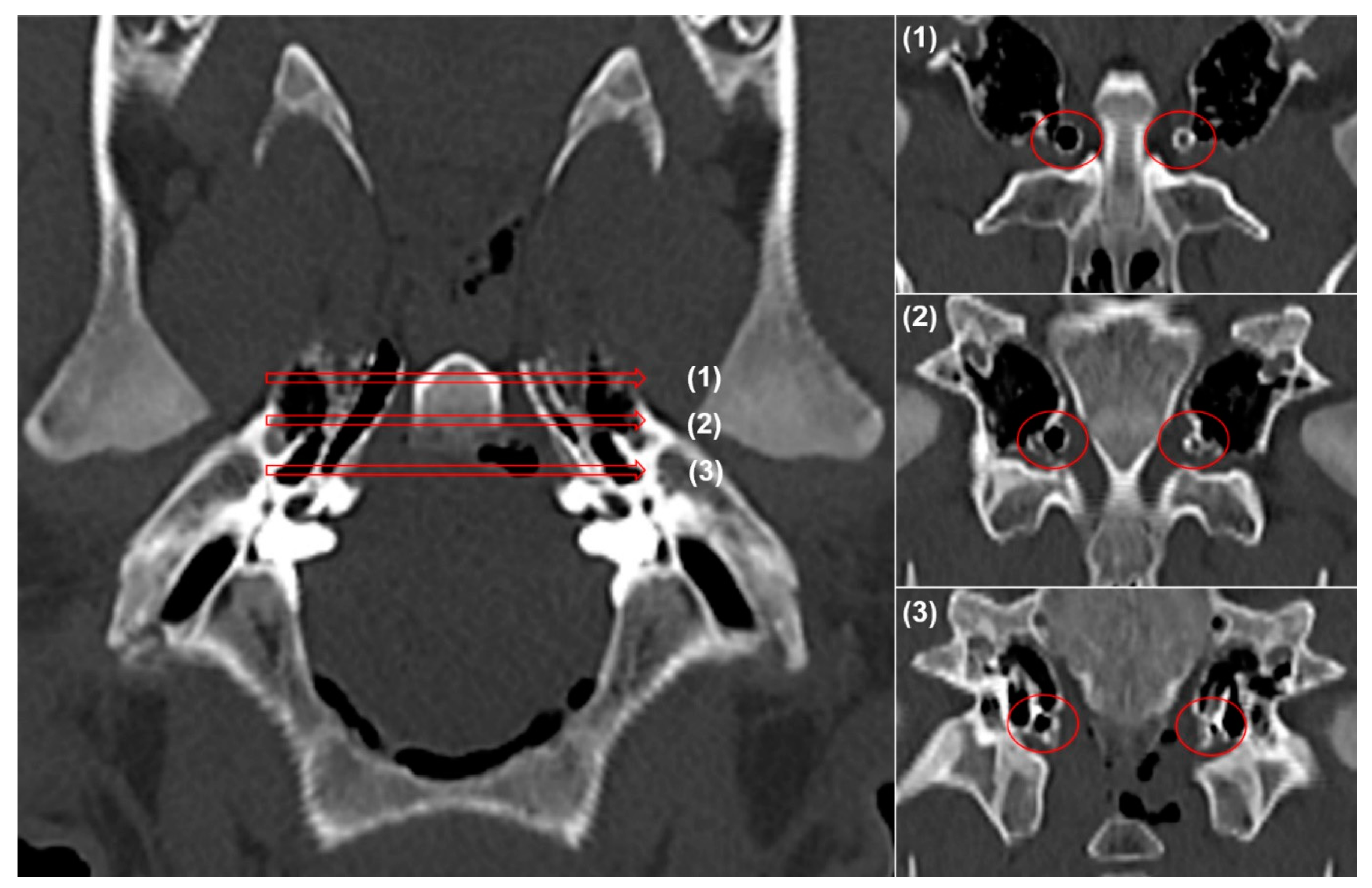
2.4. Computed Tomography
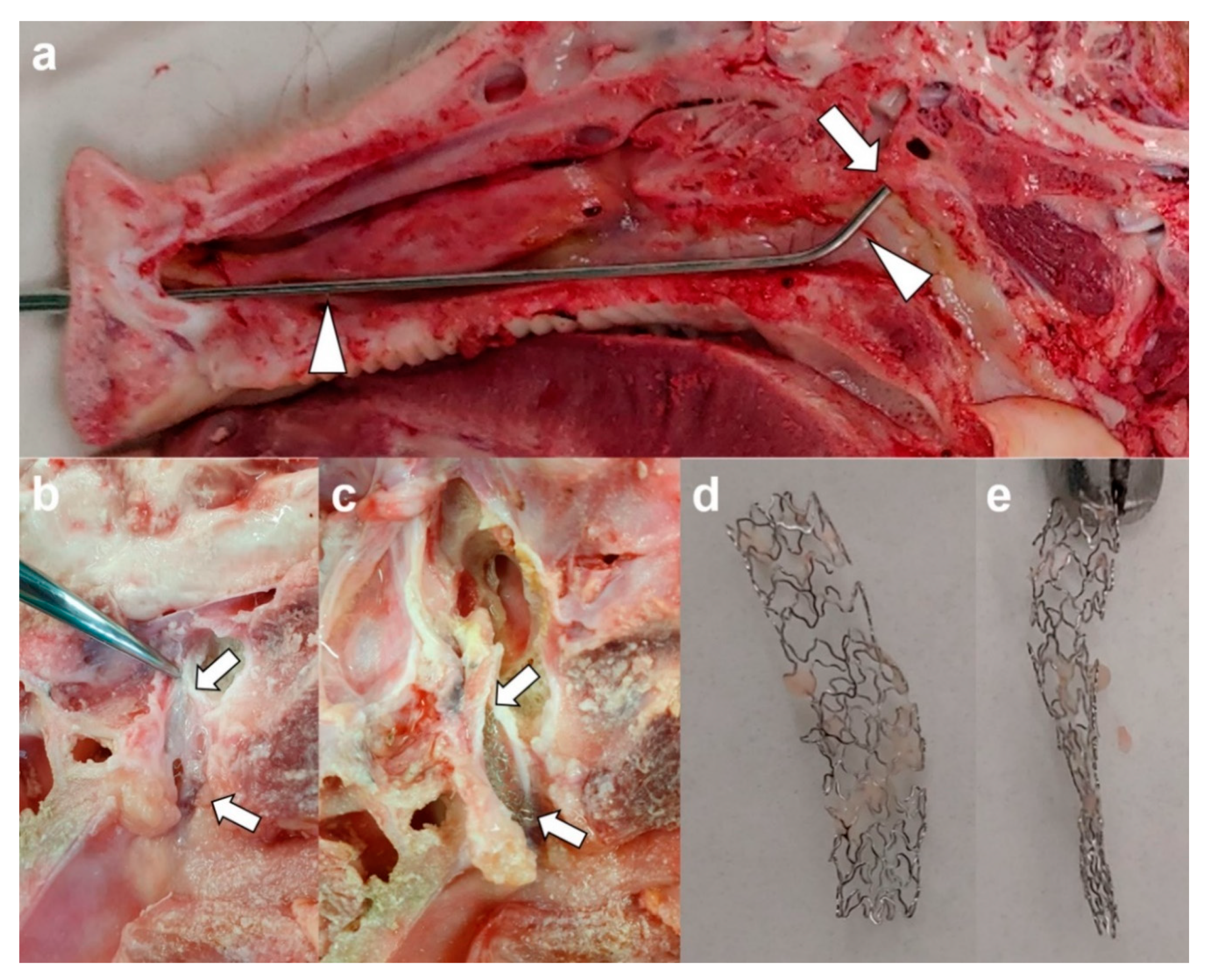
2.5. Gross and Histological Examination
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, M.E.; Scoffings, D.J.; Tysome, J.R. Imaging of the Eustachian tube and its function: A systematic review. Neuroradiology 2016, 58, 543–556. [Google Scholar] [CrossRef] [Green Version]
- Adil, E.; Poe, D. What is the full range of medical and surgical treatments available for patients with Eustachian tube dysfunction? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, C.D. Pathogenesis of otitis media: Role of eustachian tube. Pediatr. Infect. Dis. J. 1996, 15, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W., Jr.; Wright, J.W., 3rd. Preliminary results with use of an eustachian tube prosthesis. Laryngoscope 1977, 87, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lesinski, S.G.; Fox, J.M.; Seid, A.B.; Bratcher, G.O.; Cotton, R. Does the Silastic Eustachian Tube prosthesis improve eustachian tube function? Laryngoscope 1980, 90, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Norman, G.; Harden, M.; Coatesworth, A.; Kimberling, D.; Schilder, A.; McDaid, C. Interventions for adult Eustachian tube dysfunction: A systematic review. Health Technol. Assess. 2014, 18, 1–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Tsauo, J.; Song, H.Y.; Park, H.J.; Kang, W.S.; Park, J.H.; Wang, Z. Fluoroscopy-guided balloon dilation in patients with Eustachian tube dysfunction. Eur. Radiol. 2018, 28, 910–919. [Google Scholar] [CrossRef]
- Song, H.Y.; Park, H.J.; Kang, W.S.; Kim, K.Y.; Park, J.H.; Yoon, S.H.; Jeon, J.Y. Fluoroscopic Balloon Dilation Using a Flexible Guide Wire to Treat Obstructive Eustachian Tube Dysfunction. J. Vasc. Interv. Radiol. 2019, 30, 1562–1566. [Google Scholar] [CrossRef]
- Pohl, F.; Schuon, R.A.; Miller, F.; Kampmann, A.; Bultmann, E.; Hartmann, C.; Lenarz, T.; Paasche, G. Stenting the Eustachian tube to treat chronic otitis media—A feasibility study in sheep. Head Face Med. 2018, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Ockermann, T.; Reineke, U.; Upile, T.; Ebmeyer, J.; Sudhoff, H.H. Balloon dilation eustachian tuboplasty: A feasibility study. Otol. Neurotol. 2010, 31, 1100–1103. [Google Scholar] [CrossRef]
- Kivekäs, I.; Chao, W.C.; Faquin, W.; Hollowell, M.; Silvola, J.; Rasooly, T.; Poe, D. Histopathology of balloon-dilation Eustachian tuboplasty. Laryngoscope 2015, 125, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Silvola, J.; Kivekäs, I.; Poe, D.S. Balloon Dilation of the Cartilaginous Portion of the Eustachian Tube. Otolaryngol. Head Neck Surg. 2014, 151, 125–130. [Google Scholar] [CrossRef]
- Randrup, T.S.; Ovesen, T. Balloon eustachian tuboplasty: A systematic review. Otolaryngol. Head Neck Surg. 2015, 152, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ockermann, T.; Reineke, U.; Upile, T.; Ebmeyer, J.; Sudhoff, H.H. Balloon dilatation eustachian tuboplasty: A clinical study. Laryngoscope 2010, 120, 1411–1416. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, W.S.; Kim, K.Y.; Kang, B.C.; Park, J.W.; Kim, M.T.; Bekheet, N.G.; Hwang, S.J.; Choi, J.; Cho, K.J.; et al. Transnasal Placement of a Balloon-Expandable Metallic Stent: Human Cadaver Study of the Eustachian Tube. J. Vasc. Interv. Radiol. 2018, 29, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- An, F.-W.; Yuan, H.; Guo, W.; Hou, Z.-H.; Cai, J.-M.; Luo, C.-C.; Yu, N.; Jiang, Q.-Q.; Cheng, W.; Liu, W.; et al. Establishment of a Large Animal Model for Eustachian Tube Functional Study in Miniature Pigs. Anat. Rec. (Hoboken) 2019, 302, 1024–1038. [Google Scholar] [CrossRef]
- Williams, B.; Taylor, B.A.; Clifton, N.; Bance, M. Balloon dilation of the Eustachian tube: A tympanometric outcomes analysis. J. Otolaryngol. Head Neck Surg. 2016, 45, 13. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, J.H.; Song, H.Y.; Choi, K.D.; Ryu, M.H.; Yun, S.C.; Kim, J.H.; Kim, D.H.; Yoo, M.W.; Hwang, D.W.; et al. Over-the-wire versus through-the-scope stents for the palliation of malignant gastric outlet obstruction: A retrospective comparison study. Eur. Radiol. 2016, 26, 4249–4258. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, P.H.; Shin, J.H.; Tsauo, J.; Kim, M.T.; Cho, Y.C.; Kim, J.H.; Song, H.Y. Removal of Retrievable Self-Expandable Metallic Tracheobronchial Stents: An 18-Year Experience in a Single Center. Cardiovasc. Intervent. Radiol. 2016, 39, 1611–1619. [Google Scholar] [CrossRef]
- Chew, B.H.; Denstedt, J.D. Technology insight: Novel ureteral stent materials and designs. Nat. Clin. Pract. Urol. 2004, 1, 44–48. [Google Scholar] [CrossRef]
- Jun, E.J.; Park, J.H.; Tsauo, J.; Yang, S.G.; Kim, D.K.; Kim, K.Y.; Kim, M.T.; Yoon, S.H.; Lim, Y.J.; Song, H.Y. EW-7197, an activin-like kinase 5 inhibitor, suppresses granulation tissue after stent placement in rat esophagus. Gastrointest. Endosc. 2017, 86, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Familiari, P.; Bulajic, M.; Mutignani, M.; Lee, L.S.; Spera, G.; Spada, C.; Tringali, A.; Costamagna, G. Endoscopic removal of malfunctioning biliary self-expandable metallic stents. Gastrointest. Endosc. 2005, 62, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Faigel, D.O. Preventing biliary stent occlusion. Gastrointest. Endosc. 2000, 51, 104–107. [Google Scholar] [CrossRef]
- Lee, H.J.; Labaki, W.; Yu, D.H.; Salwen, B.; Gilbert, C.; Schneider, A.L.; Ortiz, R.; Feller-Kopman, D.; Arias, S.; Yarmus, L. Airway stent complications: The role of follow-up bronchoscopy as a surveillance method. J. Thorac. Dis. 2017, 9, 4651–4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.Y.; Park, J.H.; Kim, D.H.; Tsauo, J.; Kim, M.T.; Son, W.C.; Kang, S.G.; Kim, D.H.; Song, H.Y. Sirolimus-eluting Biodegradable Poly-l-Lactic Acid Stent to Suppress Granulation Tissue Formation in the Rat Urethra. Radiology 2018, 286, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, M.T.; Kim, K.Y.; Bakheet, N.; Kim, T.H.; Jeon, J.Y.; Jeon, J.Y.; Park, W.; Lopera, J.E.; Kim, D.H.; et al. Local Heat Treatment for Suppressing Gastroduodenal Stent-Induced Tissue Hyperplasia Using Nanofunctionalized Self-Expandable Metallic Stent in Rat Gastric Outlet Model. ACS Biomater. Sci. Eng. 2020, 6, 2450–2458. [Google Scholar] [CrossRef]
- Park, J.H.; Park, W.; Cho, S.; Kim, K.Y.; Tsauo, J.; Yoon, S.H.; Son, W.C.; Kim, D.H.; Song, H.Y. Nanofunctionalized stent-mediated local heat treatment for the suppression of stent-induced tissue hyperplasia. ACS Appl. Mater. Interfaces 2018, 10, 29357–29366. [Google Scholar] [CrossRef]
- Park, W.; Kim, K.Y.; Kang, J.M.; Ryu, D.S.; Kim, D.-H.; Song, H.-Y.; Kim, S.-H.; Lee, S.O.; Park, J.-H. Metallic stent mesh coated with silver nanoparticles suppresses stent-induced tissue hyperplasia and biliary sludge in the rabbit extrahepatic bile duct. Pharmaceutics 2020, 12, 563. [Google Scholar] [CrossRef]
- Han, K.; Park, J.H.; Yang, S.G.; Lee, D.H.; Tsauo, J.; Kim, K.Y.; Kim, M.T.; Gang, S.G.; Kim, D.K.; Kim, D.H.; et al. EW-7197 eluting nano-fiber covered self-expandable metallic stent to prevent granulation tissue formation in a canine urethral model. PLoS ONE 2018, 13, e0192430. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, T.H.; Cho, Y.C.; Bakheet, N.; Lee, S.O.; Kim, S.H.; Kim, K.Y. Balloon-Expandable Biodegradable Stents Versus Self-Expandable Metallic Stents: A Comparison Study of Stent-Induced Tissue Hyperplasia in the Rat Urethra. Cardiovasc. Interv. Radiol. 2019, 42, 1343–1351. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Banerjee, S.; Barth, B.; Desilets, D.; Kaul, V.; Kethu, S.; Pedrosa, M.; Pfau, P.; Tokar, J.; Wang, A.; et al. Enteral stents. Gastrointest. Endosc. 2011, 74, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Chen, C.C.; Wang, C.Y.; Chang, S.H.; Hsieh, M.J.; Lee, C.H.; Wu, V.C.C.; Hsieh, I.C. The development of coronary artery stents: From bare-metal to bio-resorbable types. Metals 2016, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., 2nd; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, H.; Atmakuri, D.R.; Torii, S.; Braumann, R.; Smith, S.; Jinnouchi, H.; Gupta, A.; Harari, E.; Shkullaku, M.; Kutys, R.; et al. Very Late Pathological Responses to Cobalt-Chromium Everolimus-Eluting, Stainless Steel Sirolimus-Eluting, and Cobalt-Chromium Bare Metal Stents in Humans. J. Am. Heart Assoc. 2017, 17, e007244. [Google Scholar] [CrossRef] [Green Version]
- Kereiakes, D.J.; Cox, D.A.; Hermiller, J.B.; Midei, M.G.; Bachinsky, W.B.; Nukta, E.D.; Leon, M.B.; Fink, S.; Marin, L.; Lansky, A.J. Usefulness of a cobalt chromium coronary stent alloy. Am. J. Cardiol. 2003, 92, 463–466. [Google Scholar] [CrossRef]
- Gurr, A.; Stark, T.; Probst, G.; Dazert, S. The temporal bone of lamb and pig as an alternative in ENT-education. Laryngorhinootologie 2010, 89, 17–24. [Google Scholar] [CrossRef]
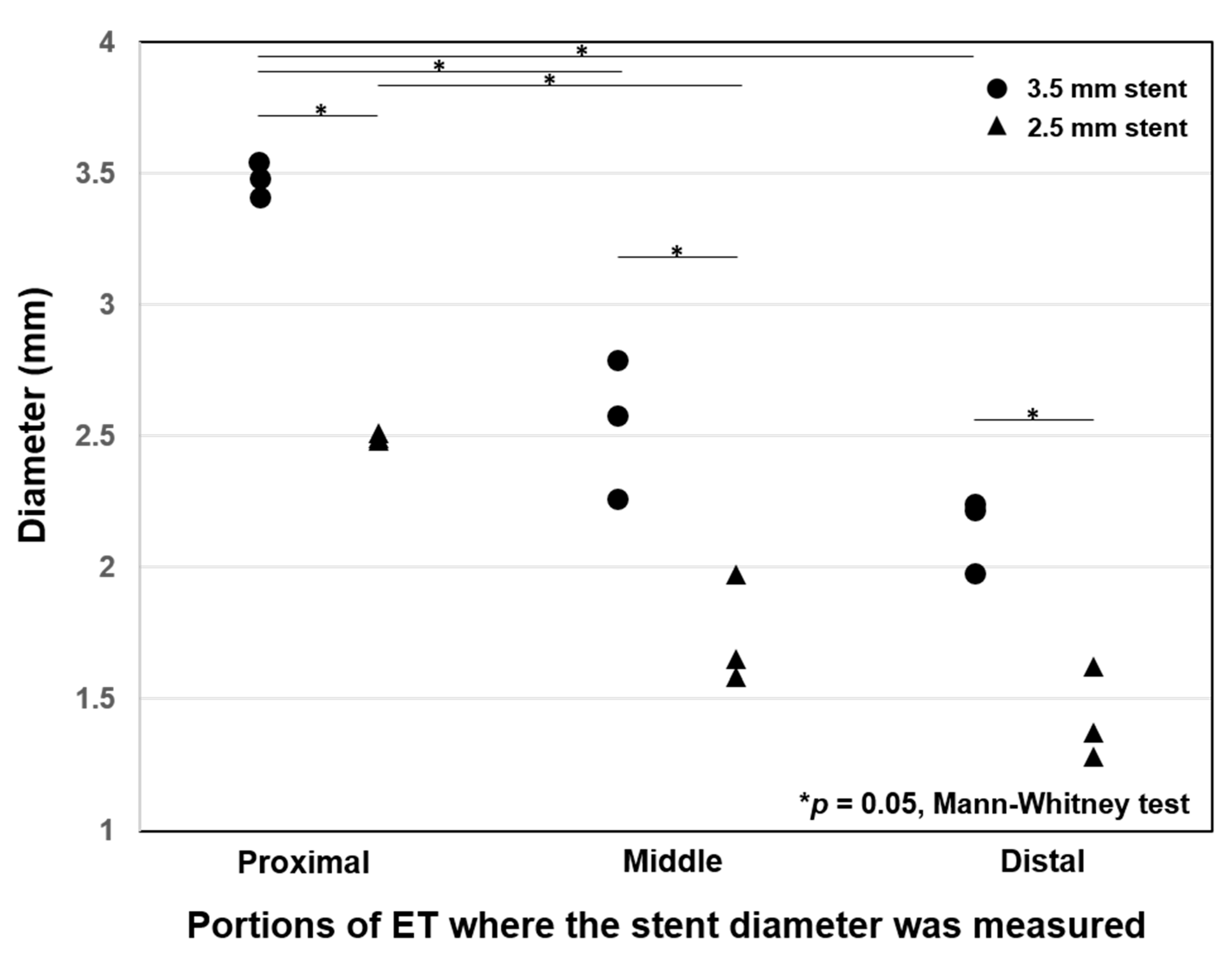

| Stent Diameter/Site | Location | Luminal Diameter (mm) | Decrease Rate (%) |
|---|---|---|---|
| 3.5 mm/Right ET | Proximal | 3.48 ± 0.06 | 0.9 ± 0.8 |
| Middle | 2.54 ± 0.17 | 27.4 ± 7.6 | |
| Distal | 2.15 ± 0.14 | 38.6 ± 4.1 | |
| 2.5 mm/Left ET | Proximal | 2.49 ± 0.04 | 0.4 ± 0.6 |
| Middle | 1.73 ± 0.21 | 30.8 ± 8.3 | |
| Distal | 1.42 ± 0.18 | 43.2 ± 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kang, W.S.; Kang, J.M.; Ryu, D.S.; Kwak, M.Y.; Song, H.-Y.; Park, J.-H.; Park, H.J. Technical Feasibility and Histological Analysis of Balloon-Expandable Metallic Stent Placement in a Porcine Eustachian Tube. Appl. Sci. 2021, 11, 1359. https://doi.org/10.3390/app11041359
Kim Y, Kang WS, Kang JM, Ryu DS, Kwak MY, Song H-Y, Park J-H, Park HJ. Technical Feasibility and Histological Analysis of Balloon-Expandable Metallic Stent Placement in a Porcine Eustachian Tube. Applied Sciences. 2021; 11(4):1359. https://doi.org/10.3390/app11041359
Chicago/Turabian StyleKim, Yehree, Woo Seok Kang, Jeon Min Kang, Dae Sung Ryu, Min Young Kwak, Ho-Young Song, Jung-Hoon Park, and Hong Ju Park. 2021. "Technical Feasibility and Histological Analysis of Balloon-Expandable Metallic Stent Placement in a Porcine Eustachian Tube" Applied Sciences 11, no. 4: 1359. https://doi.org/10.3390/app11041359
APA StyleKim, Y., Kang, W. S., Kang, J. M., Ryu, D. S., Kwak, M. Y., Song, H.-Y., Park, J.-H., & Park, H. J. (2021). Technical Feasibility and Histological Analysis of Balloon-Expandable Metallic Stent Placement in a Porcine Eustachian Tube. Applied Sciences, 11(4), 1359. https://doi.org/10.3390/app11041359







