Effect of Pd Ions on the Generation of Ag and Au Heterogeneous Nanoparticles Using Laser Ablation in Liquid
Abstract
:Featured Application
Abstract
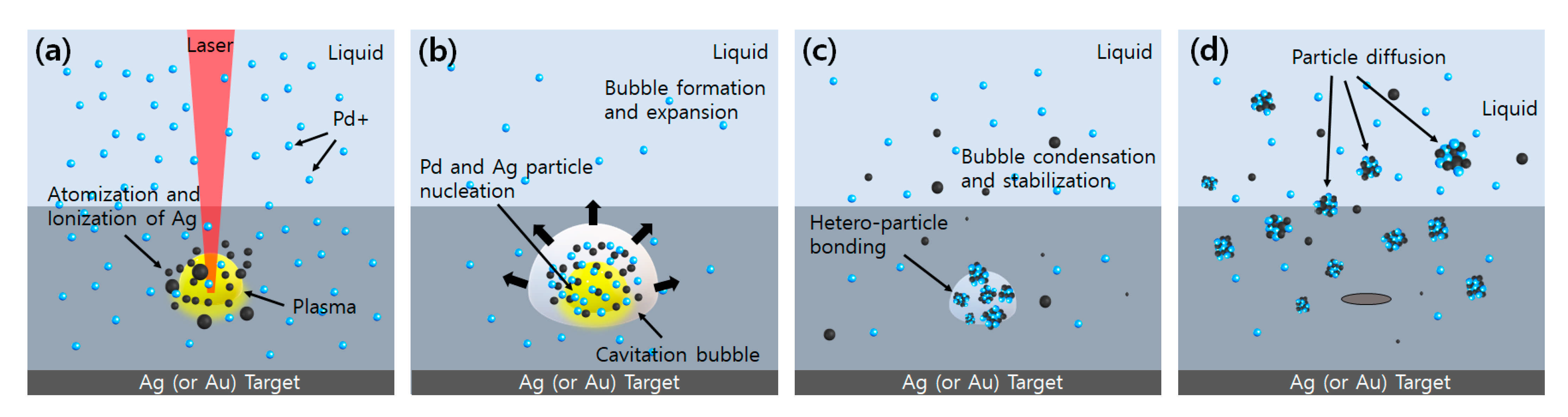
1. Introduction
2. Modeling of Electric Field Concentration by Conducting Nanoparticles
3. Experimental
4. Experimental Results and Discussion
4.1. Electric Field Concentration Simulation Analysis between Nanoparticles
4.2. TEM Images of Heterogeneous Nanoparticles
4.3. Size Distribution of Heterogeneous Nanoparticles
4.4. Composition of Nanoparticles Using TEM-EDS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Worley, J.; Dénommée, S.; Kingston, C.; Jakubek, Z.J.; Deslandes, Y.; Botton, G.A. Synthesis of metal alloy nanoparticles in solution by laser irradiation of a metal powder suspension. J. Phys. Chem. B 2003, 107, 6920–6923. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ma, Z.; Spasova, M.; Yelsukova, A.E.; Lu, S.; Farle, M.; Wiedwald, U.; Gökce, B. Formation Mechanism of Laser-Synthesized Iron-Manganese Alloy Nanoparticles, Manganese Oxide Nanosheets and Nanofibers. Part. Part. Syst. Charact. 2017, 34, 1600225. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic nanoparticles: Enhanced magnetic and optical properties for emerging biological applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.J.; Maye, M.M. Core-Shell Assembled Nanoparticles as Catalysts. Adv. Mater. 2001, 13, 1507–1511. [Google Scholar] [CrossRef]
- Zhu, J. Surface plasmon resonance from bimetallic interface in Au–Ag core–shell structure nanowires. Nanoscale Res. Lett. 2009, 4, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Langhammer, C.; Yuan, Z.; Zorić, A.I.; Kasemo, B. Plasmonic Properties of Supported Pt and Pd Nanostructures. Nano Lett. 2006, 6, 833–838. [Google Scholar] [CrossRef]
- Quintanilla, M.; Kuttner, C.; Smith, J.D.; Seifert, A.; Skrabalak, S.E.; Liz-Marzán, L.M. Heat generation by branched Au/Pd nanocrystals: Influence of morphology and composition. Nanoscale 2019, 11, 19561–19570. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, W.; Bladt, E.; Vanrompay, H.; Smith, J.D.; Skrabalak, S.E.; Bals, S. Thermal Stability of Gold/Palladium Octopods Studied in Situ in 3D: Understanding Design Rules for Thermally Stable Metal Nanoparticles. ACS Nano 2019, 13, 6522–6530. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Cheng, Y.; Schiffrin, D.J. Electrodeposition of metallic gold clusters at the water/1,2-dichloroethane interface. J. Chem. Soc. Faraday Trans. 1996, 92, 3865–3871. [Google Scholar] [CrossRef]
- Platt, M.; Dryfe, R.A.W.; Roberts, E.P. Controlled deposition of nanoparticles at the liquid-liquid interface. Chem. Commun. 2002, 20, 2324–2325. [Google Scholar] [CrossRef]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.L.; Delcourt, M.O. Radiation-induced synthesis of mono-and multi-metallic clusters and nanocolloids. New. J. Chem 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Gaddy, G.A.; Korchev, A.S.; McLain, J.L.; Slaten, B.L.; Steigerwalt, E.S.; Mills, G. Light-induced formation of silver particles and clusters in crosslinked PVA/PAA films. J. Phys. Chem. B 2004, 108, 14850–14857. [Google Scholar] [CrossRef]
- Bradley, J.S.; Via, G.H.; Bonneviot, L.; Hill, E.W. Infrared and EXAFS Study of Compositional Effects in Nanoscale Colloidal Palladium−Copper Alloys. Chem. Mater. 1996, 8, 1895–1903. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Menazea, A.A.; Elashmawi, I.; El-Kader, F.H.A.; Hakeem, N.A. Nanosecond Pulsed Laser Ablation in Liquids as New Route for Preparing Polyvinyl Carbazole/Silver Nanoparticles Composite: Spectroscopic and Thermal Studies. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2564–2571. [Google Scholar] [CrossRef]
- Rodrigues, C.J.; Bobb, J.A.; John, M.G.; Fisenko, S.P.; El-Shall, M.S.; Tibbetts, K.M. Nucleation and growth of gold nanoparticles initiated by nanosecond and femtosecond laser irradiation of aqueous [AuCl4]. Phys. Chem. Chem. Phys. 2018, 20, 28465–28475. [Google Scholar] [CrossRef] [PubMed]
- John, M.G.; Tibbetts, K.M. One-step femtosecond laser ablation synthesis of sub-3 nm gold nanoparticles stabilized by silica. Appl. Surf. Sci. 2019, 475, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Hayasaka, Y.; Nakamura, T.; Morikawa, T.; Sato, S. Core-shell like Au-Ir nanoparticles with spatially variant elec-tronic state of Au synthesized by femtosecond laser irradiation of solution. Appl. Surf. Sci. 2018, 457, 1044–1049. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Heinz, M.; Srabionyan, V.V.; Avakyan, L.; Bugaev, A.L.; Skidanenko, A.V.; Kaptelinin, S.Y.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; et al. Formation of bimetallic gold-silver nanoparticles in glass by UV laser irradiation. J. Alloys Compd. 2018, 767, 1253–1263. [Google Scholar] [CrossRef]
- Zhang, D.; Choi, W.; Jakobi, J.; Kalus, M.-R.; Barcikowski, S.; Cho, S.-H.; Sugioka, K. Spontaneous Shape Alteration and Size Separation of Surfactant-Free Silver Particles Synthesized by Laser Ablation in Acetone during Long-Period Storage. Nanomaterials 2018, 8, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Choi, W.; Oshima, Y.; Wiedwald, U.; Cho, S.H.; Lin, H.P.; Sugioka, K. Magnetic Fe@ FeOx, Fe@ C and α-Fe2O3 single-crystal nanoblends synthesized by femtosecond laser ablation of fe in acetone. Nanomaterials 2018, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gökce, B.; Sommer, S.; Streubel, R.; Barcikowski, S. Debris-free rear-side picosecond laser ablation of thin ger-manium wafers in water with ethanol. Appl. Surf. Sci. 2016, 367, 222–230. [Google Scholar] [CrossRef]
- Kruusing, A. Underwater and water-assisted laser processing: Part 1—General features, steam cleaning and shock processing. Opt. Lasers. Eng. 2004, 41, 307–327. [Google Scholar] [CrossRef]
- Kruusing, A. Underwater and water-assisted laser processing: Part 2—Etching, cutting and rarely used methods. Opt. Lasers Eng. 2004, 41, 329–352. [Google Scholar] [CrossRef]
- Shih, C.Y.; Shugaev, M.V.; Wu, C.; Zhigilei, L.V. The effect of pulse duration on nanoparticle generation in pulsed laser ablation in liquids: Insights from large-scale atomistic simulations. Phys. Chem. Chem. Phys. 2020, 22, 7077–7099. [Google Scholar] [CrossRef]
- Kanitz, A.; Kalus, M.R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma. Sources Sci. Technol. 2019, 28, 103001. [Google Scholar] [CrossRef]
- Lau, M.; Barcikowski, S. Quantification of mass-specific laser energy input converted into particle properties during pico-second pulsed laser fragmentation of zinc oxide and boron carbide in liquids. Appl. Surf. Sci. 2015, 348, 22–29. [Google Scholar] [CrossRef]
- Rehbock, C.; Zwartscholten, J.; Barcikowski, S. Biocompatible Gold Submicrometer Spheres with Variable Surface Texture Fabricated by Pulsed Laser Melting in Liquid. Chem. Lett. 2014, 43, 1502–1504. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Pyatenko, A.; Kawaguchi, K.; Li, X.; Swiatkowska-Warkocka, Z.; Koshizaki, N. Selective Pulsed Heating for the Synthesis of Semiconductor and Metal Submicrometer Spheres. Angew. Chem. 2010, 122, 6505–6508. [Google Scholar] [CrossRef]
- Werner, D.; Furube, A.; Okamoto, T.; Hashimoto, S. Femtosecond laser-induced size reduction of aqueous gold nanoparti-cles: In situ and pump− probe spectroscopy investigations revealing Coulomb explosion. J. Phys. Chem. C 2011, 115, 8503–8512. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond Dynamics of Silver Nanoclusters. Photoejection of Electrons and Fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Femtosecond transient-absorption dynamics of colloi-dal gold nanorods: Shape independence of the electron-phonon relaxation time. Phys. Rev. B 2000, 61, 6086. [Google Scholar] [CrossRef]
- Besner, S.; Kabashin, A.V.; Meunier, M. Fragmentation of colloidal nanoparticles by femtosecond laser-induced supercon-tinuum generation. Appl. Phys. Lett. 2006, 89, 233122. [Google Scholar] [CrossRef]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Hennig, G. Surface Structuring with Ultra-short Laser Pulses: Basics, Limitations and Needs for High Throughput. Phys. Procedia 2014, 56, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, M.; Masuda, K. Laser fragmentation of water-suspended gold flakes via spherical submicroparticles to fine na-noparticles. J. Phys. Chem. B 2005, 109, 9379–9388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Yoon, S.; Choi, H.W.; Kim, J.; Farson, D.F.; Cho, S.-H. The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers. Appl. Sci. 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- El-Hussein, A.; Mfouo-Tynga, I.; Abdel-Harith, M.; Abrahamse, M.H.A.H. Comparative study between the photodynamic ability of gold and silver nanoparticles in mediating cell death in breast and lung cancer cell lines. J. Photochem. Photobiol. B Biol. 2015, 153, 67–75. [Google Scholar] [CrossRef]
- Torrisi, L.; Restuccia, N. Laser-Generated Au Nanoparticles for Bio-Medical Applications. IRBM 2018, 39, 307–312. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Chassagnon, R.; Moutarlier, V.; Blondeau-Patissier, V.; Assoul, M.; Monteil, G. Nanoparticles alloying in liquids: Laser-ablation-generated Ag or Pd nanoparticles and laser irradiation-induced AgPd nanoparticle alloying. Nanotechnology 2017, 28, 155703. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Naqvi, Q.A.; Baqir, M.A. Investigation of the plasmon resonance of core-shell nanoparticle in the near-infrared region. J. Electromagn. Waves Appl. 2019, 33, 2462–2475. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chen, S.; Meng, L.Y.; Shan, H.Y.; Li, J.F.; Qian, L.; Williams, C.T.; Tian, Z.Q. How to light special hot spots in multiparti-cle–film configurations. ACS Nano 2016, 10, 581–587. [Google Scholar] [CrossRef]
- Schäfer, C.; Perera, P.N.; Laible, F.; Olynick, D.L.; Schwartzberg, A.M.; Weber-Bargioni, A.; Fleischer, M. Selectively ac-cessing the hotspots of optical nanoantennas by self-aligned dry laser ablation. Nanoscale 2020, 12, 19170–19177. [Google Scholar] [CrossRef]
- Kuttner, C. Plasmonics in Sensing: From Colorimetry to SERS Analytics. In Plasmonics; Gric., T., Ed.; Books on Demand: Norderstedt, Germany, 2018; pp. 151–180. [Google Scholar]





| Parameters | Values | Classification |
|---|---|---|
| Particle Diameter (nm) | 40~200 | Case 1 |
| Distance between particles (nm) | 4~160 | Case 2 |
| The ratio of diameters between particles | 2d~4d, 1/2d~1/4d | Case 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.; Yoo, K.S.; Kim, J. Effect of Pd Ions on the Generation of Ag and Au Heterogeneous Nanoparticles Using Laser Ablation in Liquid. Appl. Sci. 2021, 11, 1394. https://doi.org/10.3390/app11041394
Yoon S, Yoo KS, Kim J. Effect of Pd Ions on the Generation of Ag and Au Heterogeneous Nanoparticles Using Laser Ablation in Liquid. Applied Sciences. 2021; 11(4):1394. https://doi.org/10.3390/app11041394
Chicago/Turabian StyleYoon, Sangwoo, Kye Sang Yoo, and Joohan Kim. 2021. "Effect of Pd Ions on the Generation of Ag and Au Heterogeneous Nanoparticles Using Laser Ablation in Liquid" Applied Sciences 11, no. 4: 1394. https://doi.org/10.3390/app11041394





