Effect of Germanium Incorporation on the Electrochemical Performance of Electrospun Fe2O3 Nanofibers-Based Anodes in Sodium-Ion Batteries
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrospun NFs
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
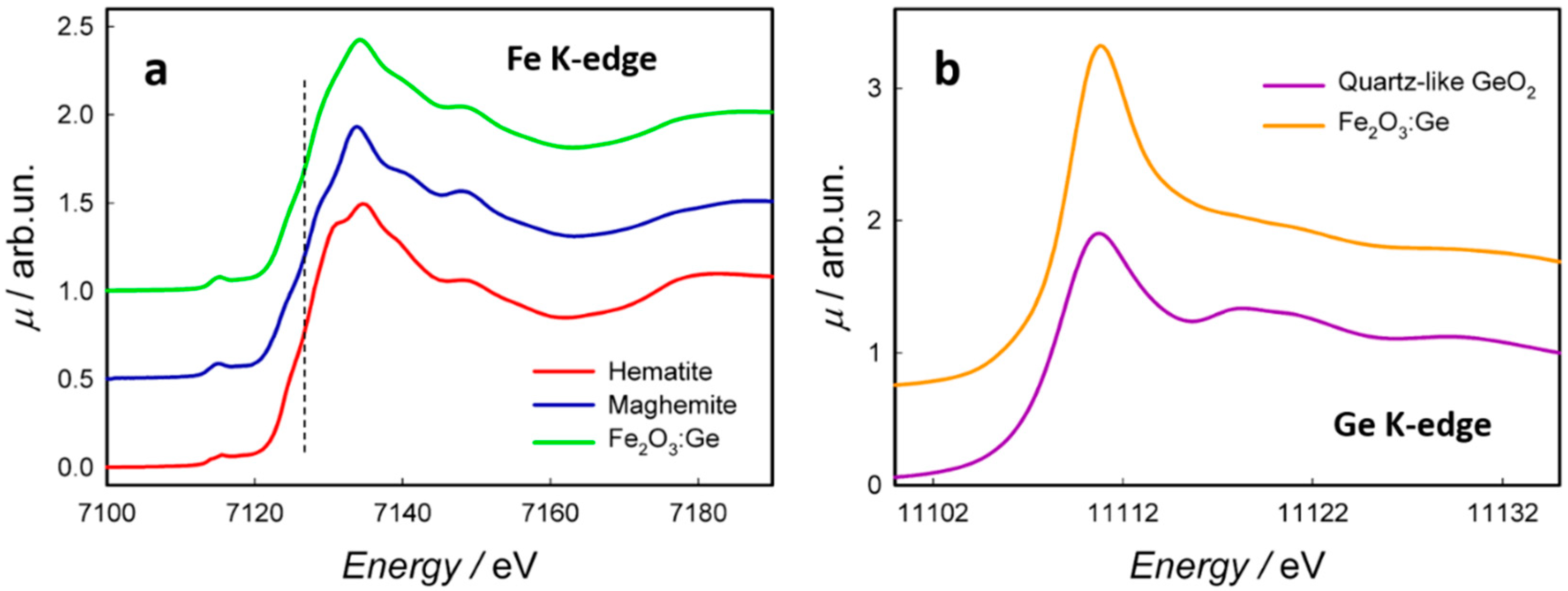
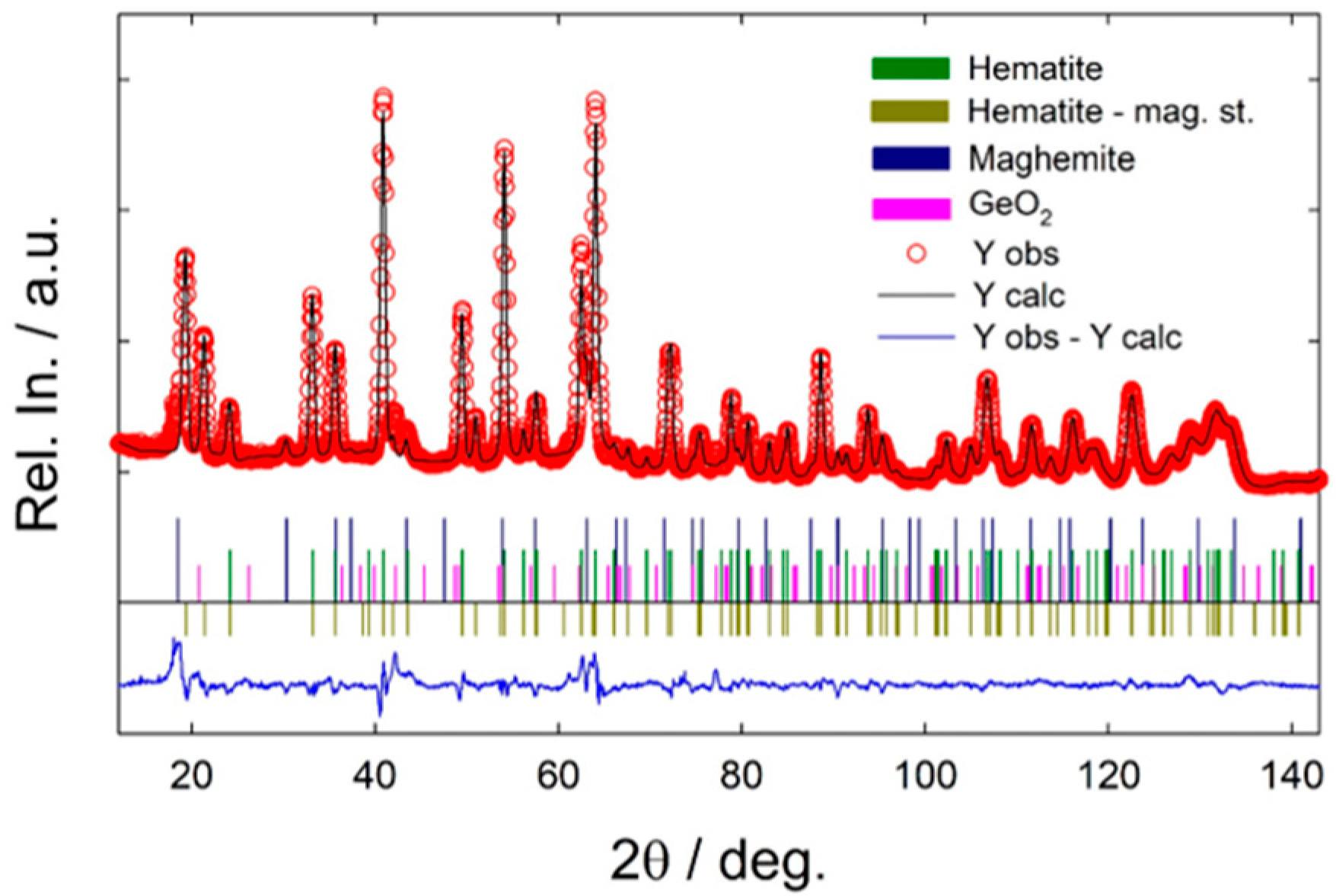
3.1. Physicochemical Properties of the NFs
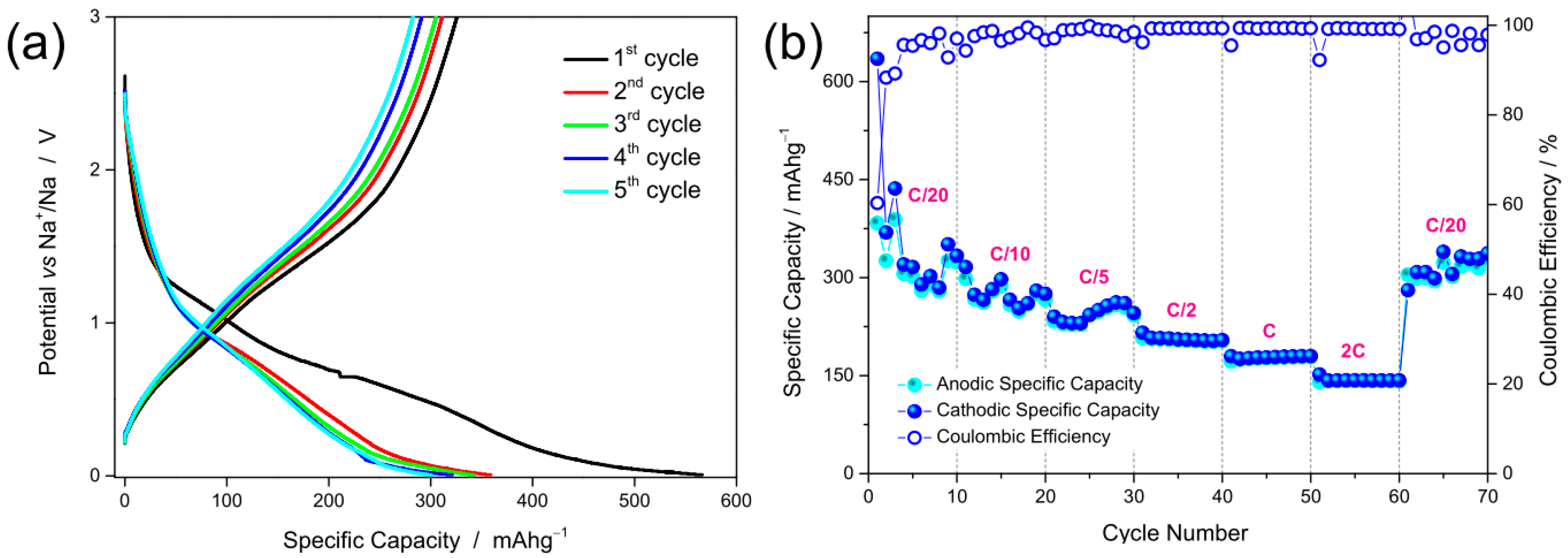
3.2. Electrochemical Properties of the NFs
4. Conclusions
- Reference Fe2O3 NFs, exhibiting grainy structure, consist exclusively of hematite.
- Fe2O3:Ge NFs have a more complex architecture and composition. They are formed by finer and elongated nanostructures developing mainly along the fiber axis; although α-Fe2O3 and γ-Fe2O3 are the dominant phases, an amorphous component is also present.
- Germanium, mostly dispersed as an ionized impurity (with 4+ oxidation state), occupies the tetrahedral sites of the maghemite lattice and probably the defective hematite surface sites.
- The synergy between nanostructured morphology and electronic transport properties of Fe2O3:Ge NFs, together with the pseudo-capacitive nature of the charge storage mechanism, are responsible for their excellent rate capability as anode for SIBs (140 mAh g−1 at 2C).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. Historic New Sustainable Development Agenda Unanimously Adopted by 193 UN Members. 2015. Available online: https://www.un.org/sustainabledevelopment/blog/2015/09/historic-new-sustainable-development-agenda-unanimously-adopted-by-193-un-members (accessed on 9 December 2020).
- World Bank. Tracking SDG7: The Energy Progress Report 2018; The World Bank: Washington, DC, USA, 2018. Available online: https://openknowledge.worldbank.org/handle/10986/29812 (accessed on 9 December 2020).
- Zekry, A.; Shaker, A.; Salem, M. Advances in Renewable Energies and Power Technologies: Solar and Wind Energies; Yahyaoui, I., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; Volume 1. [Google Scholar]
- Sui, Y.; Zhou, J.; Wang, X.; Wu, L.; Zhong, S.; Li, Y. Recent advances in black-phosphorus-based materials for electrochemical energy storage. Mater. Today 2020. [Google Scholar] [CrossRef]
- Zeng, X.X.; Xu, Y.T.; Yin, Y.X.; Wu, X.W.; Yue, J.; Guo, Y.G. Recent advances in nanostructured electrode-electrolyte design for safe and next-generation electrochemical energy storage. Mater. Today Nano 2019, 8, 100057. [Google Scholar] [CrossRef]
- Hou, R.; Liu, B.; Sun, Y.; Liu, L.; Meng, J.; Levi, M.D.; Yan, X. Recent advances in dual-carbon based electrochemical energy storage devices. Nano Energy 2020, 72, 104728. [Google Scholar] [CrossRef]
- Jin, W.; Maduraiveeran, G. Recent advances of porous transition metal-based nanomaterials for electrochemical energy conversion and storage applications. Mater. Today Energy 2019, 13, 64–84. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Stefanakos, E.K. Clean Energy and Fuel Storage. Appl. Sci. 2019, 9, 3270. [Google Scholar] [CrossRef] [Green Version]
- Bhalothia, D.; Krishnia, L.; Yang, S.S.; Yan, C.; Hsiung, W.H.; Wang, K.W.; Chen, T.Y. Recent advancements and future prospects of noble metal-based heterogeneous nanocatalysts for oxygen reduction and hydrogen evolution reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Duan, D.; Han, Y.; Wang, K.; Hao, X.; Wang, J.; Liu, S.; Wu, F. An Integrated Structural Air electrode based on parallel porous nitrogen-doped carbon nanotube arrays for rechargeable Li–air batteries. Nanomaterials 2019, 9, 1412. [Google Scholar] [CrossRef] [Green Version]
- Fedoseeva, Y.V.; Lobiak, E.V.; Shlyakhova, E.V.; Kovalenko, K.A.; Kuznetsova, V.R.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Nishchakova, A.D.; Makarova, A.A.; Bulusheva, L.G.; et al. Hydrothermal activation of porous nitrogen-doped carbon materials for electrochemical capacitors and sodium-ion batteries. Nanomaterials 2020, 10, 2163. [Google Scholar] [CrossRef]
- Amrouche, S.O.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q. Redox species of redox flow batteries: A review. Molecules 2015, 20, 20499–20517. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sust. Energ. Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Busacca, C.; Di Blasi, O.; Briguglio, N.; Ferraro, M.; Antonucci, V.; Di Blasi, A. Electrochemical performance investigation of electrospun urchin-like V2O3-CNF composite nanostructure for vanadium redox flow battery. Electrochim. Acta 2017, 230, 174–180. [Google Scholar] [CrossRef]
- Di Blasi, A.; Busacca, C.; Di Blasi, O.; Briguglio, N.; Antonucci, V. Synthesis and characterization of electrospun nickel-carbon nanofibers as electrodes for vanadium redox flow battery. J. Electrochem. Soc. 2018, 165, A1478. [Google Scholar] [CrossRef]
- Busacca, C.; Di Blasi, O.; Giacoppo, G.; Briguglio, N.; Antonucci, V.; Di Blasi, A. High performance electrospun nickel manganite on carbon nanofibers electrode for vanadium redox flow battery. Electrochim. Acta 2020, 355, 136755. [Google Scholar] [CrossRef]
- Santangelo, S. Electrospun nanomaterials for energy applications: Recent advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Sawicki, M.; Shaw, L.L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129–53154. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A Brief review of post-lithium-ion batteries. Int. J. Electrochem. Sci 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Wang, B.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Liu, Q.; Li, C.C. Post-lithium ion battery era: Recent advances in rechargeable potassium-ion batteries. Chem. Eur. 2021, 27, 512–536. [Google Scholar] [CrossRef]
- Liang, Y.; Lai, W.H.; Miao, Z.; Chou, S.L. Nanocomposite materials for the sodium–ion battery: A review. Small 2018, 14, 1702514. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Z. Micro/nanostructured materials for sodium ion batteries and capacitors. Small 2018, 14, 1702961. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Nanostructured electrode materials for advanced sodium-ion batteries. Matter 2019, 1, 90–114. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, G.; Peng, S.; Wang, J.; Yan, W.; Ramakrishna, S. One-dimensional nanomaterials toward electrochemical sodium-ion storage applications via electrospinning. Energy Storage Mater. 2020, 25, 443–476. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y.; Shen, Q.; Chen, C.; Qiu, F.; Li, P.; Jiao, L.; Qu, X. Recent advances in electrospun electrode materials for sodium-ion batteries. J. Energy Chem. 2021, 54, 225–241. [Google Scholar] [CrossRef]
- Janakiraman, S.; Khalifa, M.; Biswal, R.; Ghosh, S.; Anandhan, S.; Venimadhav, A. High performance electrospun nanofiber coated polypropylene membrane as a separator for sodium ion batteries. J. Power Sources 2020, 460, 228060. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, Q.; Cai, T.; Wang, Y.; Wang, D.; Kong, D.; Ren, H.; Zhou, J.; Xing, W. Graphitized electrospun carbon fibers with superior cyclability as a free-standing anode of potassium-ion batteries. J. Power Sources 2020, 474, 228479. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Yuan, F.; Liu, Q.; Sun, L.; Zhang, P.; Wang, Q.; Li, Z.; Wu, Y.A. A comprehensive review of carbons anode for potassium-ion battery: Fast kinetic, structure stability and electrochemical. J. Power Sources Sources 2021, 484, 229244. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-Ion Batteries Paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. Energy Chem 2019, 1, 100012. [Google Scholar] [CrossRef]
- Wang, L.; Światowska, J.; Dai, S.; Cao, M.; Zhong, Z.; Shen, Y.; Wang, M. Promises and challenges of alloy-type and conversion-type anode materials for sodium–ion batteries. Mater. Today Energy 2019, 11, 46–60. [Google Scholar] [CrossRef]
- Qi, S.; Xu, B.; Tiong, V.T.; Hu, J.; Ma, J. Progress on iron oxides and chalcogenides as anodes for sodium-ion batteries. Chem. Eng. J. 2020, 379, 122261. [Google Scholar] [CrossRef]
- Fiore, M.; Longoni, G.; Santangelo, S.; Pantò, F.; Stelitano, S.; Frontera, P.; Antonucci, P.L.; Ruffo, R. Electrochemical characterization of highly abundant, low cost iron(III) oxide as anode material for sodium-ion rechargeable batteries. Electrochim. Acta 2018, 269, 367–377. [Google Scholar] [CrossRef]
- Modafferi, V.; Triolo, C.; Fiore, M.; Palella, A.; Spadaro, L.; Pianta, N.; Ruffo, R.; Patanè, S.; Santangelo, S.; Musolino, M.G. Effect of hematite doping with aliovalent impurities on the electrochemical performance of α-Fe2O3@rGO-based anodes in sodium–ion batteries. Nanomaterials 2020, 10, 1588. [Google Scholar] [CrossRef]
- Wu, Z.G.; Zhong, Y.J.; Liu, J.; Wu, J.H.; Guo, X.D.; Zhong, B.H.; Zhang, Z.Y. Subunits controlled synthesis of α-Fe2O3 multi-shelled core–shell microspheres and their effects on lithium/sodium ion battery performances. J. Mater. Chem. A 2015, 3, 10092–10099. [Google Scholar] [CrossRef]
- Santangelo, S.; Frontera, P.; Pantò, F.; Stelitano, S.; Marelli, M.; Malara, F.; Patané, S.; Dal Santo, V.; Antonucci, P.L. Effect of Ti- or Si-doping on nanostructure and photo-electro-chemical activity of electro-spun iron oxide fibres. Int. J. Hydrogen Energy 2017, 42, 28070–28081. [Google Scholar] [CrossRef]
- Subramanian, A.; Gracia-Espino, E.; Annamalai, A.; Lee, H.H.; Lee, S.Y.; Choi, S.H.; Jang, J.S. Effect of tetravalent dopants on hematite nanostructure for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 427, 1203–1212. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, J.; Huang, H.; Huang, Q.; Zhao, Y.; Li, Y. Enhanced efficiency of hematite photoanode for water splitting with the doping of Ge. Int. J. Hydrogen Energy 2018, 43, 12646–12652. [Google Scholar] [CrossRef]
- Ponti, A.; Raza, M.H.; Pantò, F.; Ferretti, A.M.; Triolo, C.; Patanè, S.; Pinna, N.; Santangelo, S. Structure, defects and magnetism of electrospun hematite nanofibers silica-coated by atomic layer deposition. Langmuir 2020, 36, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, A.; Aquilanti, G.; Minicucci, M.; Principi, E.; Novello, N.; Cognigni, A.; Olivi, L. Novel XAFS capabilities at ELETTRA synchrotron light source. J. Phys. Conf. Ser. 2009, 190, 012043. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Rad. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Binitha, G.; Soumya, M.S.; Madhavan, A.A.; Praveen, P.; Balakrishnan, A.; Subramanian, K.R.V.; Reddy, M.V.; Shantikumar, V.N.; Sreekumaran, A.N.; Sivakumar, N. Electrospun α-Fe2O3 nanostructures for supercapacitor applications. J. Mater. Chem. A 2013, 38, 11698–11704. [Google Scholar] [CrossRef]
- Chaudhari, S.; Srinivasan, M. 1D hollow α-Fe2O3 electrospun nanofibers as high performance anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 23049–23056. [Google Scholar] [CrossRef]
- Li, N.; Jayaraman, S.; Tee, S.Y.; Kumar, P.S.; Lee, C.J.J.; Liew, S.L.; Chi, D.; Hor, T.S.A.; Ramakrishna, S.; Luo, H.K. Effect of La-Doping on optical bandgap and photoelectrochemical performance of hematite nanostructures. J. Mater. Chem. A 2014, 45, 19290–19297. [Google Scholar] [CrossRef]
- Saveh-Shemshaki, N.; Latifi, M.; Bagherzadeh, R.; Malekshahi Byranvand, M.; Naseri, N.; Dabirian, A. Synthesis of mesoporous functional hematite nanofibrous photoanodes by electrospinning. Polym. Adv. Technol. 2016, 27, 358–365. [Google Scholar] [CrossRef]
- Wu, R.A.; Lin, C.W.; Tseng, W.J. Preparation of electrospun Cu-doped α-Fe2O3 semiconductor nanofibers for NO2 gas sensor. Ceram. Int. 2017, 43, S535–S540. [Google Scholar] [CrossRef]
- Balbuena, J.; Cruz-Yusta, M.; Cuevas, A.L.; Martín, F.; Pastor, A.; Romero, R.; Sánchez, L. Hematite porous architectures as enhanced air purification photocatalyst. J. Alloys Compd. 2019, 797, 166–173. [Google Scholar] [CrossRef]
- Wang, H.G.; Zhou, Y.; Shen, Y.; Li, Y.; Zuo, Q.; Duan, Q. Fabrication, formation mechanism and the application in lithium-ion battery of porous Fe2O3 nanotubes via single-spinneret electrospinning. Electrochim. Acta 2015, 158, 105–112. [Google Scholar] [CrossRef]
- Ristic, M.; Kremenovic, A.; Reissner, M.; Petrovic, Z.; Music, S. Microstructural and magnetic properties of electrospun hematite/cuprospinel composites. J. Mater. Sci. Mater. Electron. 2020, 31, 9812–9825. [Google Scholar] [CrossRef]
- Mou, F.; Guan, J.G.; Shi, W.; Sun, Z.; Wang, S. Oriented contraction: A facile nonequilibrium heat-treatment approach for fabrication of maghemite fiber-in-tube and tube-in-tube nanostructures. Langmuir 2010, 26, 15580–15585. [Google Scholar] [CrossRef]
- Allieta, M.; Marelli, M.; Malara, F.; Bianchi, C.L.; Santangelo, S.; Triolo, C.; Patane, S.; Ferretti, A.M.; Kmente, Š.; Pontia, A.; et al. Shaped-controlled silicon-doped hematite nanostructures for enhanced PEC water splitting. Catal. Today 2019, 328, 43–49. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.Y.; Tian, Z.F.; Ruan, G.S.; Ye, Y.X.; Liang, C.H.; Shao, G.S. Highly oriented Ge-doped hematite nanosheet arrays for photoelectrochemical water oxidation. Nano Energy 2014, 9, 282–290. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.C.; Zhai, J.; Jiang, L. Preparation of superhydrophilic α-Fe2O3 nanofibers with tunable magnetic properties. Thin Solid Films 2006, 510, 271–274. [Google Scholar] [CrossRef]
- Singh, B.P.; Kumar, A.; Areizaga-Martinez, H.I.; Vega-Olivencia, C.A.; Tomar, M.S. Synthesis, characterization, and electrocatalytic ability of γ-Fe2O3 nanoparticles for sensing acetaminophen. Indian J. Pure Appl. Phys. 2017, 55, 722–728. [Google Scholar]
- Wang, J.; Zhou, H.; Zhuang, J.; Liu, Q. Magnetic γ-Fe2O3, Fe3O4, and Fe nanoparticles confined within ordered mesoporous carbons as efficient microwave absorbers. Phys. Chem. Chem. Phys 2015, 17, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.N.; Haram, S.K. Synthesis and Characterization of Uncapped γ-Fe2O3 Nanoparticles Prepared by Flame Pyrolysis of Ferrocene in Ethanol. J. Nanosci. Nanotechnol. 2006, 6, 2155–2158. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Kumar, P. Zn Doped α-Fe2O3: An efficient material for UV driven photocatalysis and electrical conductivity. Crystals 2020, 10, 273. [Google Scholar]
- Bouhjar, F.; Mollar, M.; Chourou, M.L.; Marí, B.; Bessais, B. Hydrothermal synthesis of nanostructured Cr-doped hematite with enhanced photoelectrochemical activity. Electrochim. Acta 2018, 260, 838–846. [Google Scholar] [CrossRef]
- Cao, Z.; Qin, M.; Gu, Y.; Jia, B.; Chen, P.; Qu, X. Synthesis and characterization of Sn-doped hematite as visible light photocatalyst. Mater. Res. Bull. 2016, 77, 41–47. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F., Jr.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl. Mater. Interf. 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Serrano, A.; Fernandez, J.F.; de la Fuente, O.R.; García, M.A. A novel route to obtain metal and oxide nanoparticles co-existing on a substrate. Mater. Today Chem. 2017, 4, 64–72. [Google Scholar] [CrossRef]
- Massey, M.J.; Baier, U.; Merlin, R.; Weber, W.H. Effects of pressure and isotopic substitution on the Raman spectrum of α-Fe2O3: Identification of two-magnon scattering. Phys. Rev. B 1990, 41, 7822. [Google Scholar] [CrossRef]
- Ahmmad, B.; Leonard, K.; Islam, M.S.; Kurawaki, J.; Muruganandham, M.; Ohkubo, T.; Kuroda, Y. Green synthesis of mesoporous hematite (α-Fe2O3) nanoparticles and their photocatalytic activity. Adv. Powder Technol. 2013, 24, 160–167. [Google Scholar] [CrossRef]
- Marshall, C.P.; Dufresne, W.J.; Rufledt, C.J. Polarized Raman spectra of hematite and assignment of external modes. J. Raman Spectrosc. 2020, 51, 1522–1529. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.M.; Sciau, P. Al-for-Fe substitution in hematite: The effect of low Al concentrations in the Raman spectrum of Fe2O3. J. Raman Spectrosc. 2008, 39, 40–46. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films and powders produced by sol–gel syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.S.; Warule, S.S.; Muduli, S.; Kale, B.B.; Jouen, S.; Lefez, B.; Hannoyer, B.; Ogale, S.B. Maghemite (hematite) core (shell) nanorods via thermolysis of a molecular solid of Fe-complex. Dalton Trans. 2011, 40, 8003–8011. [Google Scholar] [CrossRef]
- El Mendili, Y.; Grasset, F.; Randrianantoandro, N.; Nerambourg, N.; Greneche, J.M.; Bardeau, J.F. Improvement of thermal stability of maghemite nanoparticles coated with oleic acid and oleylamine molecules: Investigations under laser irradiation. J. Phys. Chem. C 2015, 119, 10662–10668. [Google Scholar] [CrossRef]
- Yan, W.; Fan, H.; Zhai, Y.; Yang, C.; Ren, P.; Huang, L. Low temperature solution-based synthesis of porous flower-like-Fe2O3 superstructures and their excellent gas-sensing properties. Sens. Actuators B 2011, 160, 1372–1379. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, J.; Ren, X.; Zhuang, J.; Roy, V.A.; Burkhartsmeyer, J.M.; Wong, K.S.; Choy, W.C. All-room-temperature solution-processed new nanocomposites based hole transport layer from synthesis to film formation for high-performance organic solar cells towards ultimate energy-efficient fabrication. Nano Energy 2018, 47, 26–34. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, T.; Chen, J.; Liu, H.; Su, D.; Tang, Z.; Xie, J.; Chen, L.; Yuan, A.; Kong, Q. Germanium-based complex derived porous GeO2 nanoparticles for building high performance Li-ion batteries. Ceram. Int. 2018, 44, 1127–1133. [Google Scholar] [CrossRef]
- Phani, A.R.; Di Claudio, D.; Passacantando, M.; Santucci, S. GeO2 based high k dielectric material synthesized by sol–gel process. J. Non-Cryst Solids 2007, 353, 692–696. [Google Scholar] [CrossRef]
- Mura, A.; Hideshima, I.; Liu, Z.; Hosoi, T.; Watanabe, H.; Arima, K. Water growth on GeO2/Ge (100) stack and its effect on the electronic properties of GeO2. J. Phys. Chem. C 2013, 117, 165–171. [Google Scholar] [CrossRef]
- Jain, S.; Shah, J.; Negi, N.S.; Sharma, C.; Kotnala, R.K. Significance of interface barrier at electrode of hematite hydroelectric cell for generating ecopower by water splitting. Int. J. Energy Res. 2019, 43, 4743–4755. [Google Scholar] [CrossRef]
- Spadaro, L.; Palella, A.; Arena, F. Copper-Iron-Zinc-Cerium oxide compositions as most suitable catalytic materials for the synthesis of green fuels via CO2 hydrogenation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Palella, A.; Spadaro, L.; Di Chio, R.; Arena, F. Effective low-temperature catalytic methane oxidation over MnCeOx catalytic compositions. Catal. Today 2020. [Google Scholar] [CrossRef]
- Patra, D.; Gopalan, B.; Ganesan, R. Direct solid-state synthesis of maghemite as a magnetically recoverable adsorbent for the abatement of methylene blue. J. Environ. Chem. Eng. 2019, 7, 103384. [Google Scholar] [CrossRef]
- Magro, M.; Molinari, S.; Venerando, A.; Baratella, D.; Zoppellaro, G.; Salviulo, G.; Zboril, R.; Vianello, F. Colloidal maghemite nanoparticles with oxyhydroxide-like interface and chiroptical properties. Appl. Surf. Sci. 2020, 534, 147567. [Google Scholar] [CrossRef]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef]
- Fracchia, M.; Visibile, A.; Ahlberg, A.; Vertova, A.; Minguzzi, A.; Ghigna, P.; Rondinini, S. α- and γ-FeOOH: Stability, Reversibility, and Nature of the Active Phase under Hydrogen Evolution. ACS Appl. Energy Mater. 2018, 1, 1716–1725. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E., Jr.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Bertini, L.; Ghigna, P.; Scavini, M.; Cargnonia, F. Germanium K edge in GeO2 polymorphs. Correlation between local coordination and electronic structure of germanium. Phys. Chem. Chem. Phys. 2003, 5, 1451–1456. [Google Scholar] [CrossRef]
- Mastelaro, V.R.; Zanotto, E.D. X-ray Absorption Fine Structure (XAFS) Studies of Oxide Glasses—A 45-Year Overview. Materials 2018, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.H.; Jiao, F.; Bruce, P.G.; Harrison, A.; Kockelmann, W.; Ritter, C. Neutron diffraction study of mesoporous and bulk hematite, α-Fe2O3. Chem. Mater. 2008, 20, 4891–4899. [Google Scholar] [CrossRef]
- Ritter, C. Neutrons not entitled to retire at the age of 60: More than ever needed to reveal magnetic structures. Solid State Phenom. 2011, 170, 263–269. [Google Scholar] [CrossRef]
- Graves, J. A powder neutron diffraction investigation of vacancy ordering and covalence in γ-Fe2O3. Sol. St. Chem. 1983, 49, 325–333. [Google Scholar] [CrossRef]
- Yu, L.; Hu, R.; Sang, X.; Liu, J.; Thomas, M.P.; Hudak, B.M.; Patel, A.; Page, K.; Guilton, B.S. Shell-induced Ostwald ripening: Simultaneous structure, composition, and morphology transformations during the creation of hollow iron oxide nanocapsules. ACS Nano 2018, 12, 9051–9059. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovičovà, B.; Ferrara, C.; Brugnetti, G.; Ritter, C.; Fracchia, M.; Ghigna, P.; Pollastri, S.; Triolo, C.; Spadaro, L.; Ruffo, R.; et al. Effect of Germanium Incorporation on the Electrochemical Performance of Electrospun Fe2O3 Nanofibers-Based Anodes in Sodium-Ion Batteries. Appl. Sci. 2021, 11, 1483. https://doi.org/10.3390/app11041483
Petrovičovà B, Ferrara C, Brugnetti G, Ritter C, Fracchia M, Ghigna P, Pollastri S, Triolo C, Spadaro L, Ruffo R, et al. Effect of Germanium Incorporation on the Electrochemical Performance of Electrospun Fe2O3 Nanofibers-Based Anodes in Sodium-Ion Batteries. Applied Sciences. 2021; 11(4):1483. https://doi.org/10.3390/app11041483
Chicago/Turabian StylePetrovičovà, Beatrix, Chiara Ferrara, Gabriele Brugnetti, Clemens Ritter, Martina Fracchia, Paolo Ghigna, Simone Pollastri, Claudia Triolo, Lorenzo Spadaro, Riccardo Ruffo, and et al. 2021. "Effect of Germanium Incorporation on the Electrochemical Performance of Electrospun Fe2O3 Nanofibers-Based Anodes in Sodium-Ion Batteries" Applied Sciences 11, no. 4: 1483. https://doi.org/10.3390/app11041483
APA StylePetrovičovà, B., Ferrara, C., Brugnetti, G., Ritter, C., Fracchia, M., Ghigna, P., Pollastri, S., Triolo, C., Spadaro, L., Ruffo, R., & Santangelo, S. (2021). Effect of Germanium Incorporation on the Electrochemical Performance of Electrospun Fe2O3 Nanofibers-Based Anodes in Sodium-Ion Batteries. Applied Sciences, 11(4), 1483. https://doi.org/10.3390/app11041483












