Abstract
The management of energy consumption in the building sector is of crucial concern for modern societies. Fossil fuels’ reduced availability, along with the environmental implications they cause, emphasize the necessity for the development of new technologies using renewable energy resources. Taking into account the growing resource shortages, as well as the ongoing deterioration of the environment, the building energy performance improvement using phase change materials (PCMs) is considered as a solution that could balance the energy supply together with the corresponding demand. Thermal energy storage systems with PCMs have been investigated for several building applications as they constitute a promising and sustainable method for reduction of fuel and electrical energy consumption, while maintaining a comfortable environment in the building envelope. These compounds can be incorporated into building construction materials and provide passive thermal sufficiency, or they can be used in heating, ventilation, and air conditioning systems, domestic hot water applications, etc. This study presents the principles of latent heat thermal energy storage systems with PCMs. Furthermore, the materials that can be used as PCMs, together with the most effective methods for improving their thermal performance, as well as various passive applications in the building sector, are also highlighted. Finally, special attention is given to the encapsulated PCMs that are composed of the core material, which is the PCM, and the shell material, which can be inorganic or organic, and their utilization inside constructional materials.
1. Introduction
The contemporary societies have enhanced energy needs, leading to an increasingly intensive research for the development of energy storage technologies. Global energy consumption, along with CO2 and greenhouse gasses emissions, is accelerating at a very fast pace due to global population growth, rapid global economic growth, and the ever-increasing human dependence on energy-consuming appliances. This rapid increase in global energy consumption has particular environmental implications that pose serious challenges to human health and the environment. Another consequence of the increment of global energy consumption is the reduction in the availability of traditional energy resources, such as oil, coal, and natural gas. This outcome has created a growing need for the development of new systems for the conversion and storage of clean and sustainable energy [1,2].
The building sector has a very large impact on the overall global energy consumption, and, furthermore, to the environment, by high emissions of greenhouse gasses, CO2, and the acidification of the oceans. The increased energy consumption does not occur only during the production of structural materials and the building construction, but mostly for the operation of the premises [3]. It has been proven that buildings’ operation energy consumption for providing indoor comfort accounts for 30% of total energy consumption [4]. Thermal energy storage (TES) is a promising and sustainable method for decreasing the energy consumptions in the building sector. Systems of TES using phase change materials (PCMs) find numerous applications for providing and maintaining a comfortable environment of the building envelope, without consumption of electrical energy or fuel [5].
Phase change materials are substances that are able to absorb and store large amounts of thermal energy. The mechanism of PCMs for energy storage relies on the increased energy need of some materials to undergo phase transition. They are able to absorb sensible heat as their temperature rise, and, at the phase change temperature, absorb a large amount of heat, which is called latent heat of fusion, in order to change phase. The energy stays stored in the PCM until the temperature decreases and the material undergoes phase transition again, which also signifies the energy release [1].
The materials used as PCMs can be classified based on the type of phase change to solid-liquid, liquid-gas, and solid-solid compounds. The latent heat in solid-solid PCMs, such as polyurethanes, cross-linked polyethylene, and other polymers, is relatively low compared to the latent heat of those that are in the solid-liquid form [6]. As an outcome, the PCMs used for building applications are mostly solid-liquid type. These can be organic, such as paraffin, fatty acids, etc., inorganic, such as hydrate salts and metals, or their eutectic mixtures. Organic PCMs are the best candidates for multiple building applications, though their low thermal conductivity requires the fabrication of composite materials with enhanced thermal conductivity. The most investigated approach is the encapsulation of PCMs with thermal conductive materials. The encapsulation method presents many advantages, such as the leakage of liquid phase management and the protection of the PCM from corrosion. Another approach is the formation of form-stable composite PCMs, which consist of the PCM dispersed inside a cross-linked polymer matrix, a porous mineral material or expanded graphite or perlite [3,5].
There has been a lot of research for the utilization of these materials in active and passive building applications for the reduction of electrical energy and fuel consumption. Phase change materials are used in active applications for heating, ventilation, and air conditioning systems, domestic hot water applications, suspended ceilings, external solar facades, etc. [4]. Furthermore, PCMs are used in passive applications in buildings that aim for the improvement of the thermal performance of the construction systems such as the ceiling, the walls, and the floor. These applications include the incorporation of the PCMs inside constructional materials [7].
2. Thermal Energy Storage (TES)
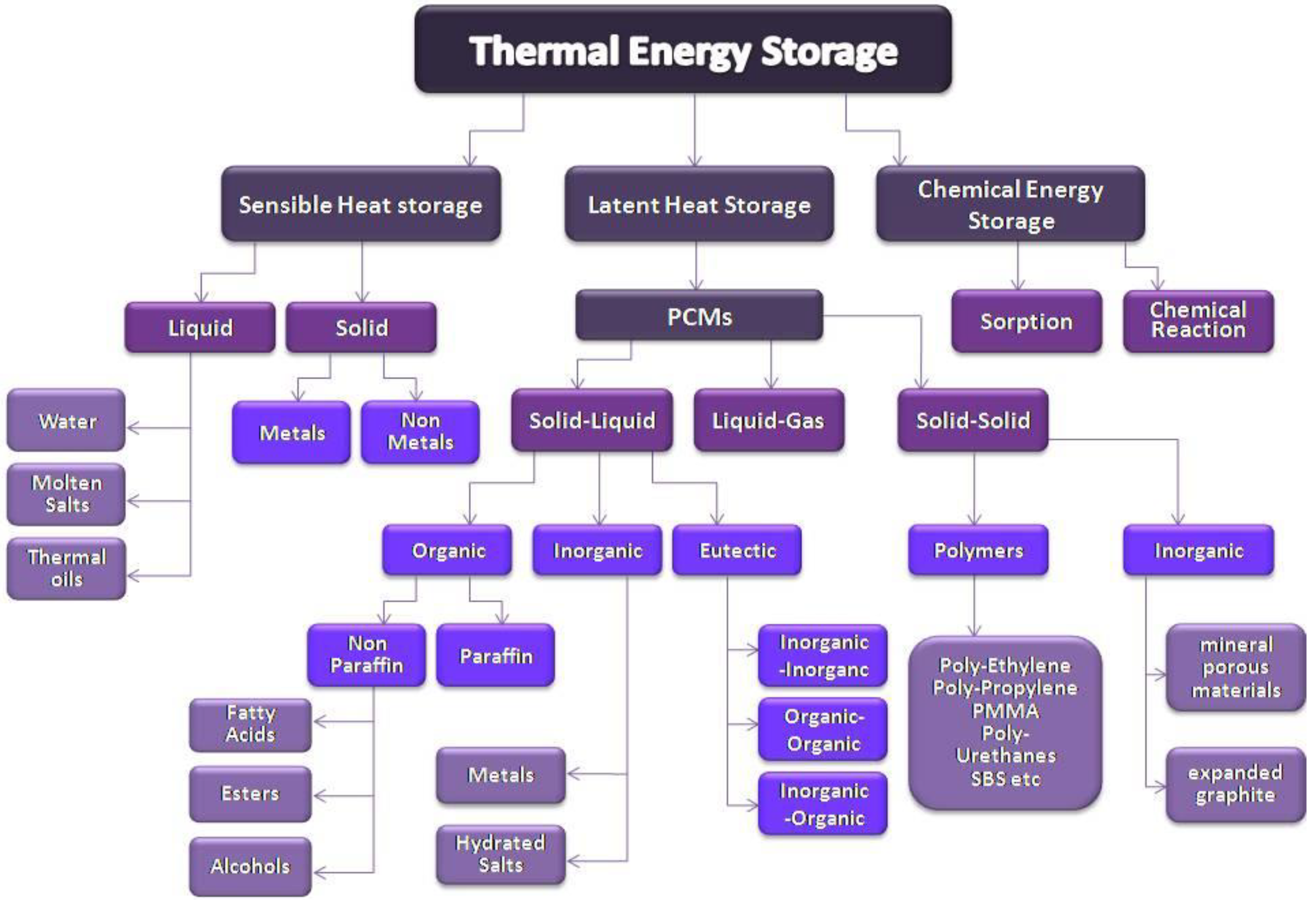
Energy storage systems aim for the conversion of energy into a form that can be stored in order to be used when there is necessity. Thermal energy storage system is a type of a sustainable energy storage system that is based on the utilization of materials that can store thermal energy when increasing their temperature and release it when the temperature is reduced. There are three types of TES systems: sensible heat, latent heat, and chemical storage system (Figure 1). The present work presents latent heat storage systems using PCMs [8,9].
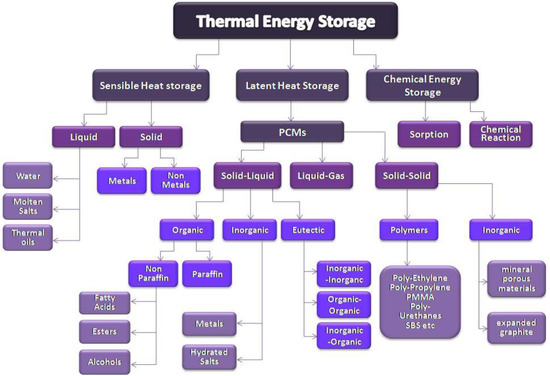
Figure 1.
Classification of thermal energy storage types and materials.
2.1. Sensible Heat Storage (SHS)
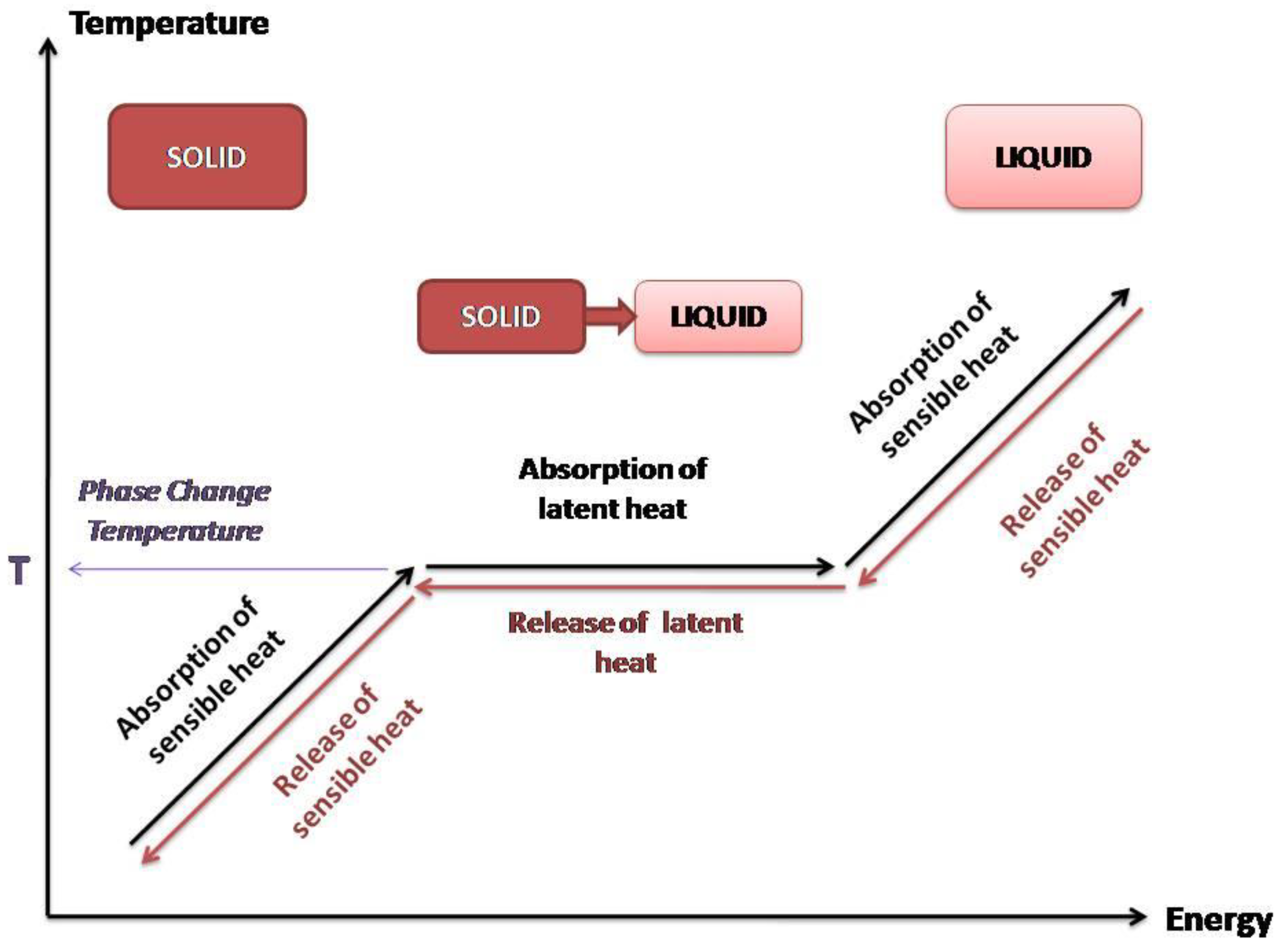
In TES systems, thermal energy can be stored either as sensible heat or as latent heat (Figure 2). In case of sensible heat storage (SHS) systems, storing of energy is induced by utilization of the heat capacity gained by temperature increment of the material. The energy storage capacity of SHS systems depends on the specific heat capacity of the material, the quantity of the material and the temperature change gradient. Radiation, convection, or conduction are the parameters that affect and can raise the corresponding temperature [9,10].
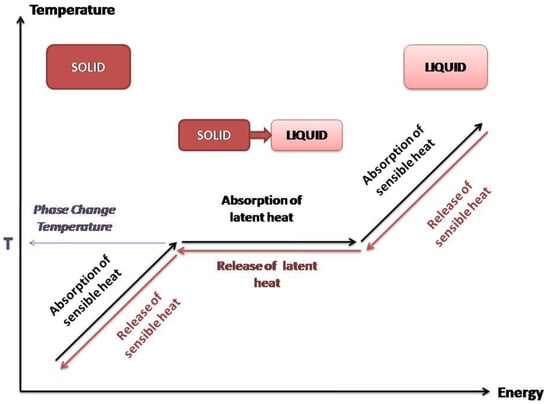
Figure 2.
Sensible and latent heat.
The materials used for SHS are either in the liquid phase or the solid phase. The utilized liquid phase materials are water, molten salts, and oils. Water as an SHS material is very efficient for applications in temperatures below 100 °C, due to its high specific heat capacity, abundance, and low cost. Molten salts also have desired properties, and they are suitable for application above 100 °C. On the other hand, oils have low specific heat capacity and thermal conductivity, thus their performance is limited. Solid-phase materials used for SHS can be metals or nonmetals. Metals such as aluminum, copper, iron, and metal alloys have high thermal conductivity and specific heat capacity, though their high cost limits their application. Nonmetallic materials that can be used for SHS are rocks, concrete, marbles, bricks, granite, etc., but the disadvantage of all of these materials lies in their low thermal conductivity and specific heat capacity [6,8].
2.2. Latent Heat Storage
Latent heat storage (LHS) systems are based on the absorption or release of heat that takes place during the phase transition of a material (gas to liquid, liquid to solid, and vice versa). The amount of energy being stored depends on the mass of the material used and the latent heat of fusion, which is characteristic for every material, and it is related to its molecular structure. The materials used for LHS are also PCMs due to the fact that they store energy through phase transition. The energy storage consists of both sensible and latent heat, as presented in Figure 2. The increase of temperature to the phase change temperature results in the absorption of sensible heat from the PCM. At phase change temperature, the PCM absorbs latent heat at a molecular level for the phase transition. This amount of heat is called latent heat of fusion or evaporation, depending on the kind of phase change. The systems of LHS that use PCMs are considered as a promising thermal storage technology and they have been investigated in a large range for many applications [6,8,9].
2.3. Thermal Energy Storage in Buildings
There is urgent necessity for the development of new technologies for energy conservation in the building sector. The application of TES systems is a promising technology with many advantages, including the increasing of energy and economic efficiency in buildings, as well as the reduction of electrical energy consumption and, furthermore, the reduction of environmental pollution and CO2 emissions [11].
The systems of TES that are applied in buildings can be either active or passive. Active TES systems are characterized by forced convection heat transfer and, in some cases, also mass transfer, such as a heat exchanger. Active systems mainly aim to control the indoor conditions of a building, for example providing free cooling. Additionally, an active TES system can be applied for peak shaving purposes in heating, ventilation, and air conditioning systems [7].
On the other hand, passive TES systems aim to create or maintain comfort conditions in the building, exploiting naturally available energy sources such as the solar energy or the wind, along with architectural design of building components, such as shading effect using blinds, etc. Passive TES systems minimize the use of mechanically-assisted heating or cooling systems. The main idea is to maximize the comfort conditions of the building envelope without using external energy sources. Some examples of passive TES systems are the use of ventilation facades and the PCM wallboards [12,13].
3. Phase Change Materials
3.1. Classification of PCMs
There is a wide variety of compounds that are used as phase change materials for numerous different applications due to their different physical and chemical properties. For building applications, solid-liquid PCMs are preferred because of their ability to absorb and release large amounts of energy within a narrow range of temperature. Solid-liquid PCMs used in buildings can be classified into three major groups: organic compounds, inorganic compounds, and eutectics of organic and/or inorganic compounds.
3.1.1. Organic Materials
Organic PCMs can be paraffinic, such as paraffin wax, which is the most commercially used organic PCM for building applications, or they can be nonparaffinic, such as fatty acids, fatty acid esters, glycolic acids, alcohols, etc. Paraffinic PCMs consist of hydrocarbon chains, while nonparaffinic also contain electronegative atoms in their chains [8,9]. Paraffin waxes consist of hydrocarbon chains ranging from 8 to 15 carbon atoms, and pure paraffin of hydrocarbon chains range from 14 to 40 carbon atoms. Thermal properties of some paraffin waxes and pure paraffins are presented in Table 1.

Table 1.
Thermal properties of some paraffins.
Nonparaffinic PCMs can be fabricated from biosourced raw materials, such as animal fats, and vegetable oils, such as beef tallow, margarine, coconut oil, castor oil, etc. These materials are sustainable, biodegradable, nontoxic, and less flammable. On the other hand, their major drawbacks are that they are more corrosive than paraffin and more expensive [14,15]. Some nonparaffinic PCMs are presented in Table 2.

Table 2.
Thermal properties of nonparaffins.
Organic PCMs exhibit a great number of advantages. First of all, the phase change temperature rises in proportion to the number of carbon atoms in the chain, providing availability in a broad range of temperatures for different applications. Another advantage of this type of PCMs is that they are noncorrosive, chemically inert, and thermally stable in the temperature range needed, therefore, no degradation problems occur during their use. Additionally, they demonstrate a congruent melting process which means that the phase change happens repeatedly without phase segregation. Furthermore, they have high latent heat, good nucleation properties, low liquid phase sub-cooling capability, and minimal volume variation. Finally, they are compatible with most of the construction materials, they can be recycled, and their cost is relatively low. On the other hand, organic PCMs also exhibit some disadvantages based on their low energy storage capacity, low density, low thermal conductivity, and high flammability [8,9,14,15].
3.1.2. Inorganic Materials
There are two kinds of inorganic compounds that can be used as PCMs: hydrated salts and metals. Hydrated salts have been immensely studied for their use as PCMs. Their general formula is AB·nH2O, and the phase change is in fact the dehydration reaction of the hydrated salt. Regarding metal category, they include low melting metals (eutectics) but these materials have not yet been seriously studied as PCMs [8]. The greatest properties of inorganic PCMs are the high thermal conductivity and the high energy efficiency (high enthalpy). In addition, other advantages are the low volume change during phase transition and that they are inflammable [14].
The main drawback of inorganic PCMs is the incongruent melting which leads to phase segregation and, furthermore, to decreased performance after every charge-discharge circle. Another disadvantage is the poor nucleating properties that resulting in supercooling of the liquid phase before crystallization. Additionally, inorganic PCMs are widely corrosive, in some cases toxic, show limited compatibility with construction materials, and they are rather expensive [14,15]. The advantages and disadvantages for both organic and inorganic PCMs are summarized in Table 3.

Table 3.
Advantages and disadvantages of organic and inorganic phase change materials (PCMs).
3.1.3. Eutectic Mixtures
The eutectic accrues from the mixture of two or more compounds of organic, inorganic, or both PCMs. In this way, an immeasurable number of eutectic mixtures can be fabricated. This gives the opportunity to produce a material with the desiring thermal properties, such as phase change temperature and latent heat. So, the most important advantage of eutectic mixtures is the ability of producing a material with the suitable phase change temperature for an application. The organic eutectic mixtures show some extra advantages in comparison with inorganic or inorganic/organic mixtures. These are the long-term stability, high phase change enthalpy, and facility of impregnating into porous support material due to their high chemical compatibility and surface tension. The main disadvantage is their high cost [14,18]. Thermal properties of some hydrated salts and eutectic mixtures are presented in Table 4.

Table 4.
Thermal properties of inorganic materials and eutectic mixtures used as PCMs.
3.2. PCM Properties
For a material to be used as PCM, there are some thermal, physical, chemical, and kinetic properties that have to be satisfied. In addition, the final decision for a PCM to be manufactured for thermal storage also takes into account the environmental impact and the economics. Regarding the thermal properties, firstly, the phase transition temperature has to be in the desired operating temperature range in order to aver that the absorbing and releasing of heat happens in the desired range for the application temperature. Another very important characteristic for PCMs is high thermal conductivity of both liquid and solid phases, which is required in order to support the absorbing and releasing process of thermal energy. The latent heat of fusion per unit volume also has to be high, so that the storage density is higher and sensible storage can be achieved. In addition, high specific heat offers extra sensible heat storage. Finally, and also very important, is for the melting to happen congruently, in order for the phase change to be reproducible with stable cycling and long life [11,14,19].
On the physical properties needed, an important requirement is the minimum volume change during phase transition as well as small vapor pressure at the temperature of operation. Also, it is desired that the material has high density [14,19]. The kinetics of the charging/discharging cycle are also considerable because the insufficient nucleation rate leads to supercooling of the liquid phase, which is undesirable because the melting and solidification process take place in different temperatures. Therefore, high nucleation as well as enhanced crystal growth are required [8,14]. Apart from that, several chemical properties have to be satisfied by a PCM. Firstly, the freezing and melting cycle has to be completely reversible and the material should not degrade after a big number of cycles. Additionally, the material has to be noncorrosive to the environment used [11,14]. Finally, the materials used as PCMs have to be nontoxic, inflammable, and nonexplosive, both for safety and environmental reasons. From an economical point of view, they have to be available and abundant in nature, and cost-effective [19].
4. Improvement of Thermal Performance
4.1. Encapsulation
The practical use of solid-liquid PCMs impedes, due to some deterrents, characteristics, such as leakage of the liquid phase. To overcome these problems, new types of PCMs forms have been investigated, such as form-stable (or shape-stabilized) PCMs and encapsulated PCMs [20]. Encapsulation methods are used in order to overcome the leakage problems by keeping the material suitably contained inside a coating, a shell, or a matrix (form-stable PCMs). Encapsulation of PCMs can bring great improvement in enhancing the stability and heat transfer efficiency of the PCM, as well as facilitating its utilization, storage, and transportation.
The obstruction of material interaction with the environment not only prevents the leaking of the material, but also protects it from interacting with the environment, which could provoke degradation or corrosion during the melting-freezing cycles [2,21]. Furthermore, encapsulation increases the surface area of the PCM, which enhances the heat transfer efficiency. Moreover, the capsule form gives the capability of controlling the volume changes during melting, as well as the possibility of increasing thermal conductivity by inserting additives such as nanoparticles or C-based materials. Another great capability of manufacturing composite PCMs is that they can be applied directly in applications without any extra devices. For example, the fabrication of a PCM can be in forms such as powders or pastes, and they can be used directly as additives in constructional materials [21,22].
The encapsulated PCMs are composed of the core material, which is the PCM, and the shell material, which can be inorganic or organic. Their shape can be spherical, tubular, oval, or irregular, depending on the materials and the fabrication process. Additionally, a core-shell composite can have single or several cores within the capsule and multiple shells. A classification of encapsulated PCMs is related to their size. The PCMs can be characterized as macroencapsulated when their size is bigger than 1000 μm, microencapsulated when their size is between 1 and 1000 μm, and, finally, nanoencapsulated when their size is less than 1 μm [21]. The most interesting encapsulated PCMs to investigate are microencapsulated and nanoencapsulated PCMs because of the higher surface area per volume unit, which seems to facilitate thermal transfer and lead to better thermal performance. Another reason is that small particle sizes are more durable to mechanical stress [22].
4.1.1. Microencapsulation
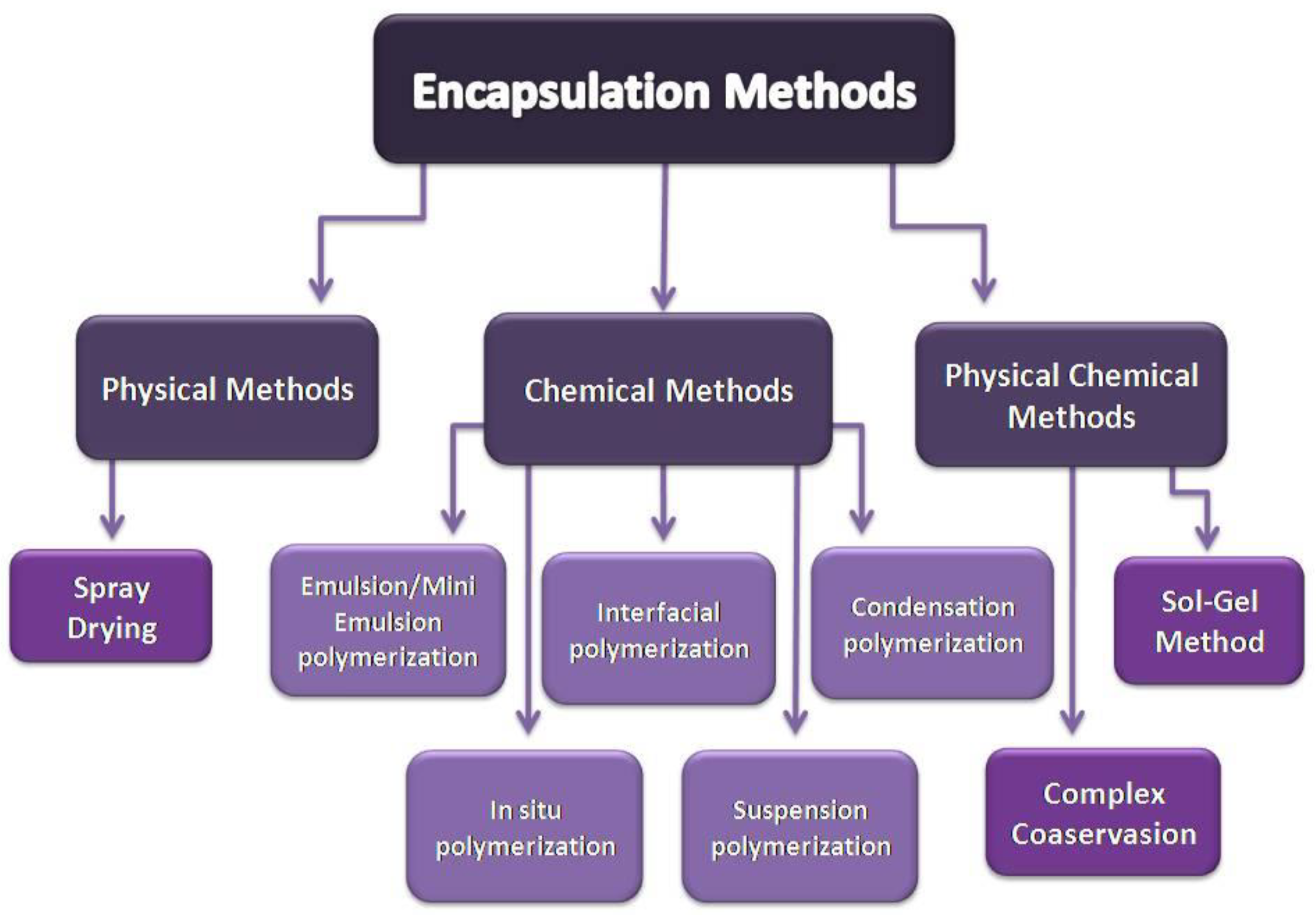
Microencapsulated PCMs emerge from a coating process of the pure PCM. Droplets or individual particles of the PCM are coated with another material to form capsules of 1–1000 μm size. The core material can be any solid-liquid PCM, and the shell (container) material can be an inorganic or a polymer material. The most studied inorganic shells are SiO2 and TiO2 [23], while the organic shells used are poly(methyl methacrylate) (PMMA) [24], polystyrene [25], melamine-formaldehyde [26], and other, more complicated, structures [27]. There are numerous microencapsulation methods that can be used (Figure 3). The selection of the suitable microencapsulation method depends on the physical and chemical properties of both core and shell materials [22] and on the chemical environment for which they are intended to be used, for example in building [28].
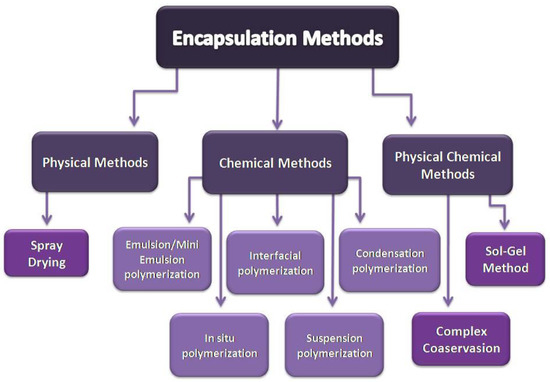
Figure 3.
Physical, chemical, and physicochemical methods of encapsulation.
4.1.2. Methods of Microencapsulation
In physical methods, the material that creates the shell does not take part in any chemical reactions. The shell is mechanically applied on the core material by physical processes such as binding due to adhesion, drying, etc. There are a lot of physical methods, such as spray drying, vibrational nozzle, centrifugal extrusion, solvent evaporation, etc., although the method that is most used for microencapsulating PCMs is spray drying. The equipment and knowledge in this method is widely available. Additionally, the method can be easily scaled up, with average microcapsule size around some micrometers and efficient thermal performance; although, the high operating temperature, the agglomeration of microcapsules in the drying chamber, and the remaining uncoated particles are some its disadvantages [22,29].
In physicochemical synthesis methods, the capsule emerges through a physicochemical process. A physicochemical process is a combination of physical and chemical methods. The physical methods include processes such as cooling, heating, phase separation, etc., while the chemical methods include chemical processes such as condensation, hydrolysis, cross-linking, etc. Those methods combined result in microencapsulation. The most widely-used physicochemical methods are complex coacervation and sol-gel technique.
The complex coacervation method is suitable for fabrication of microcapsules with shell of organic materials such as chitosan, gum Arabic, gelatin, etc. This method is based on phase separation. The shell polymer is dissolved in an emulsion with the PCM (core material). The shell is formed around the droplet of the core material by evaporation of the solvent. In another way, the formation of the microcapsule can be managed by electrostatic coalescing of two different organic polymers around the droplet, and then decreasing the temperature and pH of the emulsion to initiate phase separation [30]. On the other hand, the sol-gel technique is used for the fabrication of inorganic shell microcapsules, such as SiO2, TiO2, etc. The sol-gel technique includes a sol solution, which is a solution of the hydrolyzed precursor compound, and an emulsion of the PCM. When the sol is added to the emulsion, the synthesis of the shell is happening on the PCMs droplet’s surface, producing a gel of the microcapsules [31].
Finally, in chemical methods, the shell results from polymerization on the surface of a droplet or a particle of the core material. The most important methods are emulsion polymerization, suspension polymerization, interfacial polymerization, condensation polymerization, and in situ polymerization [22,31]. Suspension and emulsion polymerizations are widely used for PMMA and polystyrene shells, while the interfacial polymerization is preferred when the final shell consists of two or more monomers. In in situ polymerization, no monomers are used. The precursor compound hydrolyses in situ, and it initiates the polymerization. It is used for the synthesis of organic shell materials such as polyurea-formaldehyde and melamine-formaldehyde. Finally, condensation polymerization can also be used for inorganic shells, such as SiO2, and it is usually part of other synthesis techniques, such as sol-gel [32].
4.1.3. Nanoencapsulation
Recent developments in nanotechnology, and specifically in synthesis methods of nanoparticles, allow the encapsulation of PCMs on nanoscale. Systems on nanoscale have essentially different physical and chemical properties to their counterparts on macroscale. The size effects of nanoscale provide outstanding properties to nanoencapsulated PCMs, which can make improvements to the efficiency of existing applications as well as meeting the requirements for new ones. For example, nanoencapsulation is proved to dramatically reduce the supercooling effect. Furthermore, the nanoencapsulated PCMs, due to their size, are able to form stable dispersions in fluids, known as nanofluids, without rapidly increasing their viscosity, which opens the path for new applications. In addition, nanoencapsulated PCMs have proven to be more durable to mechanical stress than larger capsules, which in some applications is very important [22,32].
The nanocapsule diameter is expected to be less than 1 μm, while in microencapsulated PCMs it is most typically 5 to 400 μm. As a result, not all the fabrication methods of microencapsulation that are presented in the previous section can be adopted for PCM capsules on nanoscale. The methods that are suitable for producing nanocapsules are mainly chemical. Specifically, the efficient synthesis techniques are emulsion polymerization, miniemulsion polymerization, interfacial polymerization, in situ polymerization, and sol-gel technique [22,32].
4.2. Form-stable PCMs
Solid-liquid PCMs, as known, present some disadvantages related to the liquid phase, such as leakage, volume change, supercooling, etc. To overcome these obstacles, composite PCMs are being investigated. A modification approach for pure PCMs, in order to enhance their performance and stability, is the fabrication of form-stable PCMs. Form-stable, or shape-stabilized, PCMs consist of a 3D matrix, or a porous structure, where the pure PCMs are entrapped. The matrix or porous structure can be in macro, micro, or nano scale [2,33]. The advantages of this technology are the limitation of liquid phase leakage, the enhancement of the thermal conductivity, the controlling of volume change during melting/freezing cycles, the reducing of PCM interaction with the environment, the avoidance of additional device requirement, and the ability of manufacturing the thermal energy system in the desired forms and dimensions for easy application. The materials used as substrates can be polymers or inorganic porous minerals [5,34].
4.2.1. Polymer Form-stable PCMs
In polymer-based form-stable PCM composites, the PCM is entrapped inside a cross-linked polymer matrix. The synthesis of the polymer matrix can be achieved with many different methods, providing structural diversity and, as a result, the ability of fabricating a product with special specifications, properly designed for each application. Additionally, polymeric cross-linked materials, as substrates, have shown great encapsulation rate results, which is a very important property [5].
There is a wide variety of the polymeric materials that can be used for this purpose. Materials that have been commonly used are polyethylene [35], polypropylene [36], polyurethane [37], poly methyl methacrylate [38], poly vinyl chloride [39], styrene-butadiene-styrene triblock copolymer [40], etc. Nevertheless, due to environmental concerns, there is great necessity for reducing the utilization of nonrenewable petroleum-based polymers. This has led to research for raw materials that can be received from natural sources, such as biomass, and they are renewable, biodegradable, environmentally friendly, of high abundance, and low cost. Such materials are celluloses, starches, and vegetable oils. Specifically, cellulose, agarose, chitosan [41], castor oil [42], etc. have been used for the fabrication of form-stable PCMs.
4.2.2. Inorganic Form-stable PCMs
Form-stable PCMs can also be fabricated with inorganic mineral porous materials such as kaolin, diatomite, vermiculite, sepiolite, bentonite, attapulgite, fly ash, opal, etc., as well as with expanded materials such as expanded graphite and expanded perlite. The porous structure of these materials makes them suitable for their utilization as carries for the encapsulation of the PCM. The fabrication of inorganic porous form-stable PCMs can be performed with impregnation method, melting intercalation technology, and melting adsorption method [43].
There is chemical compatibility between PCMs and the porous materials. The interactions developing between them are mainly surface tension, hydrogen bond, Van der Waals, etc. Due to these interactions, the form-stable composites show increased thermal stability and reliability. The absorbance rate, which is connected with latent heat capacity, is high in the case of expanded graphite, diatomite, and expanded perlite carriers. Thermal conductivity is vital for the energy storage and release efficiency. In mineral porous materials, thermal conductivity is relatively low but it can be easily enhanced with the addition of expanded graphite as an additive [43,44].
4.3. Thermal Conductivity Enhancement Techniques
Thermal conductivity in a thermal energy storage system is a very important factor for the efficiency of energy storing and releasing. PCMs’, especially organics’, main disadvantage is their low thermal conductivity. Low thermal conductivity decreases the heat transfer rate which means that the stored energy cannot be used efficiently, putting limitations on plenty of applications. As a result, the enhancement of thermal conductivity of PCMs is tremendously important for the amplification of system’s performance [45]. The research that has been held for the improvement of thermal conductivity is mainly based on the improvement that encapsulation offers [10,46,47], the addition of highly-thermal conductive materials, such as carbon-based nanomaterials [48,49], metallic [50], or inorganic nanoparticles [51], and in the fabrication of form-stable PCMs with highly-thermal conductive carrier materials such as metallic foams [52], expanded graphite [53], etc. Another approach is the fabrication of nanofluid PCMs, which are dispersions of thermal conductive nanoparticles, for example, TiO2, in the PCM liquid [54] (Table 5).

Table 5.
Recent applications of micro- and nanoencapsulated PCMs with improved thermal conductivity.
4.3.1. Enhancement of Thermal Conductivity by Encapsulation
According to the literature, organic PCMs have low thermal conductivity, which does not allow their efficient use in several applications. The preparation of core-shell or form-stable composite organic PCMs increases the thermal conductivity, as well as the heat transfer rate, due to increment of the surface area. A very important factor for the improvement of thermal conductivity is the size of the microcapsules. The extreme reduction of capsule size increases the thermal resistance because of the high contact rate of the capsules [47]. Another notable factor is the shell-to-core ratio. A higher shell-to-core ratio causes more increment on thermal conductivity but reduces the rate of energy storage because of the smaller amount of PCM inside the capsule [46]. The optimum shell-to-core ratio considering thermal conductivity, thermal storage, and mechanical strength is around 1:3 [64].
Inorganic shells, such as SiO2 and CaCO3, present better improvement on thermal conductivity than organic shells, such as PMMA, though they have decreased mechanical properties. For that reason, there has been investigation for fabricating core-shell PCMs with double shell: polymer shell for mechanical strength and inorganic for thermal conductivity enhancement [46]. Additionally, another point of view is the forming of a metallic second shell, which can improve thermal conductivity along with mechanical strength and agglomeration problems. Recent studies of micro/nanoencapsulation and form-stable composites are tabulated in Table 5 and Table 6, respectively.

Table 6.
Recent applications of form-stable composites PCMs.
4.3.2. Enhancement of Thermal Conductivity with Nanoparticle Additives
The enhancement of thermal conductivity of PCMs can be efficiently achieved with the fabrication of composite PCMs, which consist of the PCM material and a dispersion of high-thermal-conductivity additives. The PCMs mostly enhanced are organic PCMs such as paraffin, fatty acids, fatty acid ester, alcohols, and their eutectic mixtures. The additives can be carbon-based nanostructures [73,74], metallic nanoparticles, or nonmetallic nanoparticles. Besides the nature of the additives materials, the size, the shape, and the aspect ratio are the main factors that define the thermal conductivity improvement.
Carbon-based materials, such as carbon fibers (CF), carbon nanotubes (CNTs), graphene, graphite, and their derivatives, are widely being investigated for thermal conductivity enhancement, with very promising results. Carbon-based materials can be used as additives directly in the organic PCM, or they can be added inside a composite PCM, such as a form-stable PCM or a microcapsule, for further thermal conductivity improvement. CNTs’ disadvantage is agglomeration, and as a result, CNTs show limited dispersion ability inside the PCM. This problem can be overcome with treatment of the CNTs, such as oxidation or doping, which weakens the intramolecular forces. On the other hand, carbon fibers show better dispersion ability, and they can be added efficiently without further treatment. Addition of graphite particles can also achieve great enhancement, although graphene nanoparticles are the most effective due to low interfacial thermal resistance and large aspect ratio [45,74].
Metallic nanoparticles can also be used as additives for the enhancement of thermal conductivity. The metallic nanoparticles that have been used are silver, copper, nickel, and magnesium nanoparticles. All of these metallic additives enhance efficiency of the thermal conductivity, but their practical use is limited because of a number of drawbacks that they show, such as low dispersion due to high agglomeration, low thermal stability, and high density [45,50].
Nonmetallic nanoparticles that can be added for thermal conductivity improvement are alumina (Al2O3), magnetite (Fe3O4), titania (TiO2), mesoporous SiO2, aluminum nitride (AlN), boron nitride (BN), etc. Nanoalumina particles improve the thermal conductivity, and the heat transfer, and they reduce the supercooling effect, although nanoalumina, along with titania, suffers from low dispersion problems [51]. Samaneh Sami and Nasrin Etesami [75] controlled the aggregation of TiO2 nanoparticles inside paraffin PCM by adding the surfactant reagent stearoyl lactylate.
4.3.3. Enhancement of Thermal Conductivity with Metallic Foams and Expanded Graphite
Another perspective for thermal conductivity enhancement is the fabrication of a composite material consisting of the PCM and metallic foam as a carrier. Metallic foams that have been used for this purpose are copper foam [76], nickel foam [77], and porous graphite foam [78]. The composites can be prepared with vacuum infiltration or vacuum impregnation. These structures have plenty of advantages, such as high thermal conductivity, high thermal and chemical stability, high porosity, low density, and high aspect ratio. Their low density and high aspect ratio are the properties that make metallic foams prevail over metallic nanoparticles. Taking into account that thermal conductivity depends on the arrangement of the two components in the composite, it is mentioned that thermal conductivity is higher when the porosity is low because there are less interfacial interactions, and, as a result, the thermal resistance is lower. Although the energy storage capacity is improved when the porosity is higher, it is highlighted that both of those effects have to be considered for the optimization of the final material. Porous graphite and copper foam composite PCMs exhibit higher thermal conductivity in comparison with nickel foam composites [45].
Expanded graphite (EG) is a structure that comes from the oxidation and desiccation of graphite that has all the desired properties to be used for enhancement of thermal conductivity: chemical stability, high thermal conductivity, low density, and aspect ratio. The fabrication of the form-stable composite PCM can be achieved by impregnation of the molten PCM in the EG. The thermal conductivity of the composite depends on mass fraction, packing density, aspect ratio, surface area, and thickness of EG. High mass fraction and packing density leads to higher thermal conductivity [45,79,80].
5. Applications of PCMs in Buildings
In the past few decades, PCM technologies and the feasibility of their incorporation for thermal energy storage in the building sector have been investigated thoroughly. Thermal energy systems using PCMs are a sustainable and environmentally friendly solution that can definitely diminish the required energy consumption for the provision of comfort conditions of the building envelope. The PCM applications for thermal energy storage in this sector are divided in two categories: active and passive systems [12,81].
Active application systems based on PCMs require mechanical equipment or a source of additional energy for their operation, for example electricity to pumps or fans [82]. These systems are more suitable for cases where there is need for more heat transfer performance or better control of the application [64]. Active thermal storage systems with PCMs include systems located inside the building envelope for heating, ventilation, and air conditioning and systems that are integrated in the building structure and operating through air or water distribution, as well as systems located outside the building envelope, such as storage containers for domestic hot water, etc. [81,83]. On the other hand, passive application systems aim to improve the thermal performance of the construction systems such as the walls, the floor, the ceiling, etc. [84]. The most noteworthy passive applications of TES systems in building are presented in this section.
5.1. PCM Passive Application Systems in Building
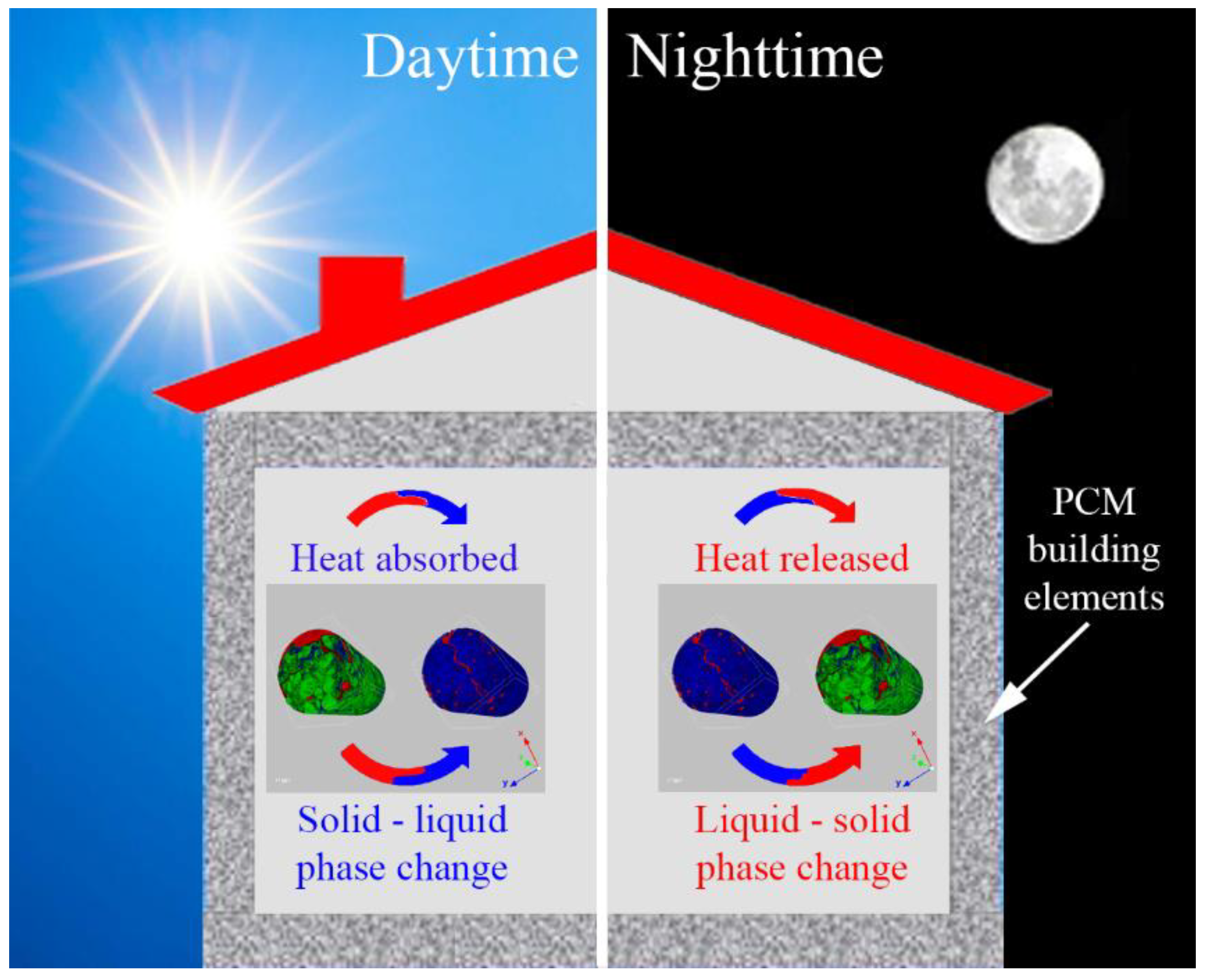
Passive application PCM systems exploit the naturally-available energy sources along with the architectural design of building components to minimize the energy requirements of building operations. The main characteristic of these systems is that there is no requirement for mechanical equipment or additional energy, due to the fact that the heat is charged or discharged only because of temperature fluctuations when the environment’s temperature rises or falls beyond the PCM’s phase change temperature (Figure 4). Passive application PCM systems in the building sector can be classified as systems that are integrated into the building materials, such as materials for the walls, the floor, the ceiling, etc., and as systems that are implemented as components to the building envelope, such as blinds, suspended ceilings, etc. [16].
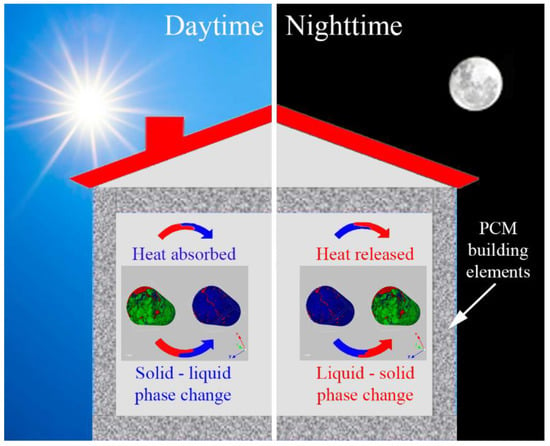
Figure 4.
Charging process (working of PCM during daytime) and discharging process (working of PCM during nighttime).
5.1.1. PCMs in Wall
The most general and effective solution for building energy saving is the incorporation of PCM into constructional materials that are applied either to the internal or the external surface of the walls. PCMs can be integrated into mortars, gypsum boards, concrete blocks, bricks, and panels.
Mortars and Plasters
Mortars and plasters have been thoroughly investigated for PCM applications due to their use as coating materials for most of the building surfaces, along with their ability to have different compositions and their low cost. There are different kind of mortars, depended on the binder, such as gypsum, lime, and cement mortars [14]. The research that has been carried out about integration of PCMs in mortars and plasters is mostly about encapsulated PCMs, which involve high costs. As Cunha et al. [85] reported, nonencapsulated PCMs incorporated into mortar do not cause any significant enhancement to the mortar’s properties. On the other hand, there are many studies showing that encapsulated PCMs into mortar and plaster have notable results for energy storage.
Sandra Cunha et al. [86] investigated the effects on mechanical, physical, and thermal properties of four different innovative mortars, by the direct integration of pure PCM. The physical and mechanical properties were affected, for example water absorption was reduced and mechanical strength was significantly changed above 10% of PCM. Thermal behavior amplifies in proportion with PCM amount. A total of 20% of PCM showed very efficient thermal performance and mechanical properties suitable for interior mortar application. Cynthia Guardia et al. [87] incorporated an encapsulated PCM to cement-lime mortar and studied both its thermal and mechanical properties. The addition of 10% and 20% of the PCMs remarkably increased the thermal properties for energy storage, while decreased the mechanical properties within the permissible limits. Hamed Abbasi Hattan et al. [88] studied the integration of polyethylene glycol (PEG) shape-stabilized in silica fume PCM in cement mortar for plastering building external brick walls. The experiment included thermal performance tests on outdoor temperature walls in two different cities. The thermal performance of the mortar was high, and the demands could be fulfilled without incorporating an amount of PCM that could cause decrease in mechanical strength, density, and water absorption.
Gypsum Boards
Another constructional element that can be used for TES are gypsum boards. The implementation of PCM technology into gypsum boards has been studied since 1990 because gypsum boards are low-cost and they are used widely in plenty of constructional applications. Srinivasaraonaik et al. [89] incorporated microencapsulated PCMs into gypsum mixture for gypsum boards. The addition of PCMs caused reduction in the compressive and flexural strength, while the porosity of the gypsum composites increased. As long as it concerns the thermal properties, thermal conductivity of the gypsum composites decreased in proportion with the amount of PCM incorporation. Similar results were obtained from the integration of graphene oxide microencapsulated PCM composites [73]. Additionally, in another study, Juan Pablo Bravo et al. [90] modified gypsum boards with a paraffinic microencapsulated PCM (20 wt%) and implemented a two-month enclosure test. The results were analyzed in a computer simulation program and they showed that microencapsulated PCMs with phase change temperature from 26.5 °C to 29.2 °C allow the charge and discharge of the material and, thus, the provision of indoor thermal comfort.
Concrete Blocks and Bricks
Another technology that has been investigated is the incorporation of PCMs into building elements such as concrete blocks and bricks. Vinh Duy Cao et al. [91] incorporated microencapsulated PCMs in Portland cement and in geopolymer concrete. The results obtained from thermal and mechanical analysis show that latent heat capacity increases, while thermal conductivity and compressive strength decreases, in proportion with the conciseness of PCMs. The addition of 3.2 wt.% and 2.7 wt.% to Portland cement and geopolymer concrete, respectively, can reduce the power consumption of the building in 23 °C by 11% and 15%, respectively. PCMs can also be successfully incorporated in bricks. Rajat Saxena et al. [92] integrated eicosane and OM35 PCMs into bricks. The results show an essential reduction of temperature fluctuation (4.5 °C to 7 °C) between incorporated and conventional bricks. A recent study from Mahmudul Hasan Mizan et al. [93] investigated the enhancement of interfacial bonding between concrete and PCM. The experiment included the preparation of 36 specimens with different surface preparation techniques each. The strength improvement was achieved by using silica fume.
PCM Panels and Wallboards
There are numerous applications of panels and wallboards of incorporated PCMs with conventional construction materials. In addition, there has also been research for the manufacturing of panels and wallboards of different materials, such as polymers [94,95] and metals [96]. Hang Yu et al. [97] investigated the fabrication of diatomite-based form-stable composite PCM wallboard for external building walls. Sayilacksha Gnanachelvam et al. [98] studied the utilization of tree wallboards (gypsum plasterboard, fiber cement board, and magnesium sulphate board) and a bio-PCM mat as fire resistant wallboards for their application to light-gauge steel-framed (LSF) wall systems made of cold-formed steel studs. Gypsum plasterboards provide better fire resistance that the other wallboards, and fiber cement boards provided the lowest. Behzad Maleki et al. [99] investigated the fabrication of plaster wallboards enhanced with nanocapsules with CuO nanoparticles. The PCM plaster wallboard reduced the thermal load of building by 13.83%.
5.1.2. Floor Applications
Building floors are another area where PCM technology can be applied for energy saving by thermal regulation. The applications that have been investigated for floor solutions are very varied. The addition of PCMs in the construction can be by adding a single layer of PCM [100,101] or by adding a multilayer of different types of PCMs [102,103]. Additionally, another approach is the development of floor panels with capillary network for PCM circulation [104], or the use of PCMs along with an electrical powered system for diminishing the demanded energy consumption [105]. Finally, PCMs can also be integrated in the raw materials used for construction, such as concrete [91]. Wanchun Sun et al. [84] investigated the thermal performance of a double-layer PCM radiant floor system containing two types of inorganic composite PCMs. The composites PCMs used were Na2HPO4•12H2O@EG and CaCl2•6H2O@EG. During winter, the thermal comfort lasted 2.2 times more than the reference room, which contained pebbles in the floor. During the summer, the thermal comfort lasted 1.7 times more. In both cases, the system can achieve shifting of the peak load. Shilei Lu et al. [86] studied the manufacturing of a PCM floor heating system consisting of a double pipe with three different modules. Regardless of the module, indoor thermal comfort was achieved. The average indoor temperature fluctuation was 1.8–3.0 °C.
5.1.3. Ceiling Applications
The development of ceiling solutions based on PCM technology has also been a field of research. There are many approaches for PCM ceiling applications, such as the incorporation of PCM ceiling panels into the roof construction [106,107], the development of ceiling panels with capillary network for PCM circulation [108], and the coupling of PCM technology with other technologies [109,110]. Mariana Velasco-Carrasco et al. [106] investigated the thermal performance of three different PCM blister panels for ceiling tiles application. The PCM used was INERTEK 23. The PCM was incorporated in three blister panels, two of which were enhanced with the addition of aluminum and steel wool particles, respectively. The panels were embedded between the roof slabs. The results obtained showed that the overall thermal performance of the aluminum- and steel-wool-particle-enhanced panels was improved, especially in the case of steel wool particles. Shilei Lu et al. [109] developed an innovative building cooling system of PCM ceiling coupled with earth-air heat exchanger (EAHE). The evaluation of experimental methods and data analysis indicated that indoor room temperature with this system could reduce peak temperature by 2.1 °C under 8-h timed cold storage experiment and 2.7 °C under 12-h timed cold storage experiment. Hansol Lim et al. [110] proposed a PCM integrated thermoelectric radiant cooling panel, which consists of thermoelectric modules, heat sinks, insulation, and PCM layer between two aluminum panels. The PCM layer provides passive cooling by freezing the PCM during the operation period for shifting the electrical load to the off-peak period demand periods.
5.1.4. Windows and Glazed Applications
Modern building designs contain large, glazed areas such as windows, glazed façade, and, sometimes, glazed roof, as they allow natural room lighting and vision, although glazing materials have very poor thermal performance which leads to increased energy consumption of the building. This indicates the necessity of expanding the PCM technologies for thermal energy storage to glazed materials also. Applications of PCM technology in glazed materials has been investigated. Changyu Liu et al. [82] studied the optical and thermal performance of a PCM-filled glazed unit in comparison with an air-filled glazed unit. The thermal performance of the PCM filled unit was highly improved, although the optical properties were degraded as the transparency was very low, especially when the PCM was at the liquid phase. An important factor to this matter is the thickness of the PCM in the glazed unit. Increased thickness improves the thermal performance but decreases the transparency. In this study it was found that the thickness should not be more than 16 mm in order to satisfy both thermal and optical demands. Corresponding study was held in reference [111,112,113].
6. Conclusions
A thermal energy storage system is a type of a sustainable energy storage system that is based on the utilization of materials that can store thermal energy when increasing their temperature and release it when the temperature is reduced. Latent heat storage systems using PCM are based on the absorption or release of heat that takes place during the material’s phase transition (latent heat of fusion). There is a wide variety of compounds that are used as phase PCMs. For building applications, solid-liquid PCMs are preferred because of their ability to absorb and release large amounts of energy within a narrow range of temperature. Solid-liquid PCMs utilized in buildings can be classified into three major groups: organic compounds, inorganic compounds, and eutectics of organic and/or inorganic compounds. Organic PCMs gather the most advantageous properties.
The fabrication of composite PCMs enhances the thermal conductivity, thermal stability, and heat transfer rate, as well as impedes the leakage of the liquid phase. The encapsulated PCMs are composed of the core material (PCM) and the shell material, which can be inorganic or organic. A core-shell composite can have single or several cores within the capsule and multiple shells. The PCMs can be characterized by their size. The most interesting encapsulated PCMs are microencapsulated and nanoencapsulated PCMs, as the higher surface area per volume unit facilitates the thermal transfer and leads to better thermal performance. Encapsulation can be achieved with physical, chemical, or physicochemical methods. The most widely-used methods for micro- and nanoencapsulation are chemical methods, such as emulsion, interfacial, and suspension polymerization, and by the physicochemical sol-gel technique.
Form-stable, or shape-stabilized, PCMs consist of a 3D matrix, or a porous structure, where the pure PCMs are entrapped. In polymer-based form-stable PCM composites, the PCM is entrapped inside a cross-linked polymer matrix. The synthesis of the polymer matrix can be achieved with many different methods, providing structural diversity and, as a result, the ability of fabricating a product with special specifications. Form-stable PCMs can also be fabricated with inorganic mineral porous materials, as well as with expanded graphite and perlite.
Thermal conductivity in a TES system is a very important factor for the efficiency of energy storing and releasing. As a result, the enhancement of thermal conductivity of PCMs is requisite. The improvement of thermal conductivity can be achieved with encapsulation, addition of highly-thermal-conductive materials such as carbon-based nanomaterials, metallic or inorganic nanoparticles, and the fabrication of form-stable PCM.
The application of TES systems in the building sector is a promising technology with many advantages. TES systems applied in buildings can be either active or passive. Active TES systems are characterized by forced convection heat transfer and, in some cases, also mass transfer. On the other hand, passive TES systems aim to create or maintain comfort conditions in the building, exploiting naturally-available energy sources, such as the solar energy or the wind, along with architectural design of building components. The main characteristic of these systems is that there is no requirement for mechanical equipment or additional energy due to the fact that the heat is charged or discharged only because of temperature fluctuations when the environment’s temperature rises or falls beyond the PCM’s phase change temperature.
Author Contributions
Conceptualization: C.V.P. and I.A.K.; methodology: C.V.P. and I.A.K.; software: C.V.P. and I.A.K.; validation: C.V.P. and I.A.K.; formal analysis: C.V.P. and I.A.K.; investigation: C.V.P. and I.A.K.; resources: C.V.P. and I.A.K.; data curation: I.A.K.; writing—original draft preparation: C.V.P. and I.A.K.; writing—review and editing: I.A.K. and C.A.C.; visualization: I.A.K. and C.A.C.; supervision: C.A.C.; project administration: C.A.C.; funding acquisition: C.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H.; Eddhahak, A. A review of microencapsulated and composite phase change materials: Alteration of strength and thermal properties of cement-based materials. Renew. Sustain. Energy Rev. 2019, 110, 467–484. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A review on phase change material application in building. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Zhu, N.; Li, S.; Hu, P.; Wei, S.; Deng, R.; Lei, F. A review on applications of shape-stabilized phase change materials embedded in building enclosure in recent ten years. Sustain. Cities Soc. 2018, 43, 251–264. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Keshteli, A.N.; Sheikholeslami, M. Nanoparticle enhanced PCM applications for intensification of thermal performance in building: A review. J. Mol. Liq. 2019, 274, 516–533. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.-C.; Mo, K.H. Thermal efficiency and durability performances of paraffinic phase change materials with enhanced thermal conductivity—A review. Thermochim. Acta 2019, 673, 198–210. [Google Scholar] [CrossRef]
- Leong, K.Y.; Rahman, M.R.A.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Aziz, N.A.; Amin, N.A.M.; Majid, M.S.A.; Zaman, I. Thermal energy storage (TES) technology for active and passive cooling in buildings: A Review. Matec Web Conf. 2018, 225, 03022. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Advanced energy storage materials for building applications and their thermal performance characterization: A review. Renew. Sustain. Energy Rev. 2016, 57, 916–928. [Google Scholar] [CrossRef]
- da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Edible Oils as Practical Phase Change Materials for Thermal Energy Storage. Appl. Sci. 2019, 9, 1627. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.-H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- Ghadim, H.B.; Shahbaz, K.; Al-Shannaq, R.; Farid, M.M. Binary mixtures of fatty alcohols and fatty acid esters as novel solid-liquid phase change materials. Int. J. Energy Res. 2019. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Rodríguez-Cumplido, F.; Pabón-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutiérrez, A.; Grágeda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Morphology-controlled synthesis of microencapsulated phase change materials with TiO2 shell for thermal energy harvesting and temperature regulation. Energy 2019, 172, 599–617. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Alkan, C.; Özcan, A.N. Thermal energy storage characteristics of myristic acid-palmitic eutectic mixtures encapsulated in PMMA shell. Sol. Energy Mater. Sol. Cells 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Sánchez, L.; Sánchez, P.; de Lucas, A.; Carmona, M.; Rodríguez, J.F. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym. Sci. 2007, 285, 1377–1385. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Damlıoğlu, Y.; Alkan, C. Poly(styrene-co-divinylbenzene-co-acrylamide)/n-octadecane microencapsulated phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 198, 5–10. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernandez, A.I.; Farid, M.M. Preparation and Characterization of Microencapsulated Phase Change Materials for Use in Building Applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294. [Google Scholar] [CrossRef]
- Onder, E.; Sarier, N.; Cimen, E. Encapsulation of phase change materials by complex coacervation to improve thermal performances of woven fabrics. Thermochim. Acta 2008, 467, 63–72. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Sobolciak, P.; Karkri, M.; Al-Maadeed, M.A.; Krupa, I. Thermal characterization of phase change materials based on linear low-density polyethylene, paraffin wax and expanded graphite. Renew. Energy 2016, 88, 372–382. [Google Scholar] [CrossRef]
- Alkan, C.; Kaya, K.; Sari, A. Preparation, Thermal Properties and Thermal Reliability of Form-Stable Paraffin/Polypropylene Composite for Thermal Energy Storage. J. Polym. Environ. 2009, 17, 254–258. [Google Scholar] [CrossRef]
- Tang, B.; Wang, L.; Xu, Y.; Xiu, J.; Zhang, S. Hexadecanol/phase change polyurethane composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 144, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Jin, X.; Li, J.; Xue, P.; Jia, M. Preparation and characterization of PVC-based form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2014, 130, 435–441. [Google Scholar] [CrossRef]
- Chen, P.; Gao, X.; Wang, Y.; Xu, T.; Fang, Y.; Zhang, Z. Metal foam embedded in SEBS/paraffin/HDPE form-stable PCMs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2016, 149, 60–65. [Google Scholar] [CrossRef]
- Şentürk, S.B.; Kahraman, D.; Alkan, C.; Gökçe, İ. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, Y.; Liu, Q.; Zhou, C.; Zhang, X.; Lei, J. Form-stable phase change materials based on castor oil and palmitic acid for renewable thermal energy storage. J. Therm. Anal. Calorim. 2019, 137, 1225–1232. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Harish, S.; Ishikawa, K.; Chiashi, S.; Shiomi, J.; Maruyama, S. Anomalous Thermal Conduction Characteristics of Phase Change Composites with Single-Walled Carbon Nanotube Inclusions. J. Phys. Chem. C 2013, 117, 15409–15413. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Fang, G. Synthesis and properties of microencapsulated stearic acid/silica composites with graphene oxide for improving thermal conductivity as novel solar thermal storage materials. Sol. Energy Mater. Sol. Cells 2019, 189, 197–205. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y.M. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Huang, X.; Lin, Y.; Alva, G.; Fang, G. Thermal properties and thermal conductivity enhancement of composite phase change materials using myristyl alcohol/metal foam for solar thermal storage. Sol. Energy Mater. Sol. Cells 2017, 170, 68–76. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Liu, Y.-D.; Zhou, Y.-G.; Tong, M.-W.; Zhou, X.-S. Experimental study of thermal conductivity and phase change performance of nanofluids PCMs. Microfluid. Nanofluidics 2009, 7, 579–584. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Ji, R.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Huang, P.; Li, B.; Sun, L. Design and synthesis of novel microencapsulated phase change materials with enhancement of thermal conductivity and thermal stability: Self-assembled boron nitride into shell materials. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124225. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Wu, H.; Guo, S. Construction of hybrid graphene oxide/graphene nanoplates shell in paraffin microencapsulated phase change materials to improve thermal conductivity for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124780. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Y.; Fang, G. Preparation and thermal properties of microencapsulated stearyl alcohol with silicon dioxide shell as thermal energy storage materials. Appl. Therm. Eng. 2020, 169, 114943. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Hu, D.; Wu, L. Synthesis and characterization of microencapsulated methyl laurate with polyurethane shell materials via interfacial polymerization in Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124958. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Liang, S.; Chen, K.; Tian, C.; Wang, J.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Kong, X. Preparation and characterization of a novel composite phase change material with double phase change points based on nanocapsules. Renew. Energy 2020, 147, 374–383. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, Y.; Wei, C.; Liang, S.; Luo, X.; Wang, J.; Zhang, L. Nanoencapsulated phase change materials with polymer-SiO2 hybrid shell materials: Compositions, morphologies, and properties. Energy Convers. Manag. 2018, 164, 83–92. [Google Scholar] [CrossRef]
- Liao, H.; Chen, W.; Liu, Y.; Wang, Q. A phase change material encapsulated in a mechanically strong graphene aerogel with high thermal conductivity and excellent shape stability. Compos. Sci. Technol. 2020, 189, 108010. [Google Scholar] [CrossRef]
- Su, J.; Ren, L.; Wang, L. Preparation and mechanical properties of thermal energy storage microcapsules. Colloid Polym. Sci. 2005, 284, 224–228. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zhang, X.; Yin, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X.; Huang, Z. Lauric-stearic acid eutectic mixture/carbonized biomass waste corn cob composite phase change materials: Preparation and thermal characterization. Thermochim. Acta 2019, 674, 21–27. [Google Scholar] [CrossRef]
- Song, S.; Qiu, F.; Zhu, W.; Guo, Y.; Zhang, Y.; Ju, Y.; Feng, R.; Liu, Y.; Chen, Z.; Zhou, J.; et al. Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 193, 237–245. [Google Scholar] [CrossRef]
- Tan, N.; Xie, T.; Feng, Y.; Hu, P.; Li, Q.; Jiang, L.-M.; Zeng, W.-B.; Zeng, J.-L. Preparation and characterization of erythritol/sepiolite/exfoliated graphite nanoplatelets form-stable phase change material with high thermal conductivity and suppressed supercooling. Sol. Energy Mater. Sol. Cells 2020, 217, 110726. [Google Scholar] [CrossRef]
- Wu, B.; Lao, D.; Fu, R.; Su, X.; Liu, H.; Jin, X. Novel PEG/EP form-stable phase change materials with high thermal conductivity enhanced by 3D ceramics network. Ceram. Int. 2020, 46, 25285–25292. [Google Scholar] [CrossRef]
- Tan, N.; Xie, T.; Hu, P.; Feng, Y.; Li, Q.; Zhao, S.; Zhou, H.-N.; Zeng, W.-B.; Zeng, J.-L. Preparation and characterization of capric-palmitic acids eutectics/silica xerogel/exfoliated graphite nanoplatelets form-stable phase change materials. J. Energy Storage 2021, 34, 102016. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lahiri, B.B.; Philip, J. Carbon black nano particle loaded lauric acid-based form-stable phase change material with enhanced thermal conductivity and photo-thermal conversion for thermal energy storage. Energy 2020, 191, 116572. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Tao, W.; Li, D. Preparation of microencapsulated phase change materials used graphene oxide to improve thermal stability and its incorporation in gypsum materials. Constr. Build. Mater. 2019, 224, 48–56. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Li, W.-W.; Nian, Y.-L.; Xia, W.-d. Study of thermal conductive enhancement mechanism and selection criteria of carbon-additive for composite phase change materials. Int. J. Heat Mass Transf. 2018, 116, 507–511. [Google Scholar] [CrossRef]
- Sami, S.; Etesami, N. Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl. Therm. Eng. 2017, 124, 346–352. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Hussain, A.; Tso, C.Y.; Chao, C.Y.H. Experimental investigation of a passive thermal management system for high-powered lithium ion batteries using nickel foam-paraffin composite. Energy 2016, 115, 209–218. [Google Scholar] [CrossRef]
- Sedeh, M.M.; Khodadadi, J.M. Thermal conductivity improvement of phase change materials/graphite foam composites. Carbon 2013, 60, 117–128. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Jia, Y.; Fang, G. Improved thermal properties of stearyl alcohol/high density polyethylene/expanded graphite composite phase change materials for building thermal energy storage. Energy Build. 2017, 153, 41–49. [Google Scholar] [CrossRef]
- Gholamibozanjani, G.; Farid, M. A comparison between passive and active PCM systems applied to buildings. Renew. Energy 2020, 162, 112–123. [Google Scholar] [CrossRef]
- Meng, E.; Cai, R.; Sun, Z.; Yang, J.; Wang, J. Experimental study of the passive and active performance of real-scale composite PCM room in winter. Appl. Therm. Eng. 2021, 185, 116418. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on building energy performance improvement using phase change materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Cunha, S.; Lima, M.; Aguiar, J.B. Influence of adding phase change materials on the physical and mechanical properties of cement mortars. Constr. Build. Mater. 2016, 127, 1–10. [Google Scholar] [CrossRef]
- Cunha, S.; Leite, P.; Aguiar, J. Characterization of innovative mortars with direct incorporation of phase change materials. J. Energy Storage 2020, 30, 101439. [Google Scholar] [CrossRef]
- Guardia, C.; Barluenga, G.; Palomar, I.; Diarce, G. Thermal enhanced cement-lime mortars with phase change materials (PCM), lightweight aggregate and cellulose fibers. Constr. Build. Mater. 2019, 221, 586–594. [Google Scholar] [CrossRef]
- Hattan, H.A.; Madhkhan, M.; Marani, A. Thermal and mechanical properties of building external walls plastered with cement mortar incorporating shape-stabilized phase change materials (SSPCMs). Constr. Build. Mater. 2021, 270. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.P.; Sinha, S.; Tyagi, I.; Rawat, A. Studies on the mechanical properties and thermal behavior of microencapsulated eutectic mixture in gypsum composite board for thermal regulation in the buildings. J. Build. Eng. 2020, 31, 101400. [Google Scholar] [CrossRef]
- Bravo, J.P.; Venegas, T.; Correa, E.; Álamos, A.; Sepúlveda, F.; Vasco, D.A.; Barreneche, C. Experimental and Computational Study of the Implementation of mPCM-Modified Gypsum Boards in a Test Enclosure. Buildings 2020, 10, 15. [Google Scholar] [CrossRef]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.-L. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Saxena, R.; Rakshit, D.; Kaushik, S.C. Phase change material (PCM) incorporated bricks for energy conservation in composite climate: A sustainable building solution. Sol. Energy 2019, 183, 276–284. [Google Scholar] [CrossRef]
- Mizan, M.H.; Ueda, T.; Matsumoto, K. Enhancement of the concrete-PCM interfacial bonding strength using silica fume. Constr. Build. Mater. 2020, 259, 119774. [Google Scholar] [CrossRef]
- El Omari, K.; Le Guer, Y.; Bruel, P. Analysis of micro-dispersed PCM-composite boards behavior in a building’s wall for different seasons. J. Build. Eng. 2016, 7, 361–371. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, F.; Liu, P.; Hu, P.; Wu, M. Energy saving potential of a novel phase change material wallboard in typical climate regions of China. Energy Build. 2016, 128, 360–369. [Google Scholar] [CrossRef]
- Meng, E.; Yu, H.; Zhou, B. Study of the thermal behavior of the composite phase change material (PCM) room in summer and winter. Appl. Therm. Eng. 2017, 126, 212–225. [Google Scholar] [CrossRef]
- Yu, H.; Li, C.; Zhang, K.; Tang, Y.; Song, Y.; Wang, M. Preparation and thermophysical performance of diatomite-based composite PCM wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101753. [Google Scholar] [CrossRef]
- Gnanachelvam, S.; Ariyanayagam, A.; Mahendran, M. Fire resistance of LSF wall systems lined with different wallboards including bio-PCM mat. J. Build. Eng. 2020, 32, 101628. [Google Scholar] [CrossRef]
- Maleki, B.; Khadang, A.; Maddah, H.; Alizadeh, M.; Kazemian, A.; Ali, H.M. Development and thermal performance of nanoencapsulated PCM/ plaster wallboard for thermal energy storage in buildings. J. Build. Eng. 2020, 32, 101727. [Google Scholar] [CrossRef]
- Entrop, A.G.; Brouwers, H.J.H.; Reinders, A.H.M.E. Experimental research on the use of micro-encapsulated Phase Change Materials to store solar energy in concrete floors and to save energy in Dutch houses. Sol. Energy 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Royon, L.; Karim, L.; Bontemps, A. Optimization of PCM embedded in a floor panel developed for thermal management of the lightweight envelope of buildings. Energy Build. 2014, 82, 385–390. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Ling, Z.; Fang, X.; Zhang, Z. Experimental investigation on the thermal performance of double-layer PCM radiant floor system containing two types of inorganic composite PCMs. Energy Build. 2020, 211, 109806. [Google Scholar] [CrossRef]
- Ansuini, R.; Larghetti, R.; Giretti, A.; Lemma, M. Radiant floors integrated with PCM for indoor temperature control. Energy Build. 2011, 43, 3019–3026. [Google Scholar] [CrossRef]
- Lu, S.; Xu, B.; Tang, X. Experimental study on double pipe PCM floor heating system under different operation strategies. Renew. Energy 2020, 145, 1280–1291. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, Y.; Xu, X.; Di, H.; Yang, R.; Qin, P. Experimental study of under-floor electric heating system with shape-stabilized PCM plates. Energy Build. 2005, 37, 215–220. [Google Scholar] [CrossRef]
- Velasco-Carrasco, M.; Chen, Z.; Aguilar-Santana, J.L.; Riffat, S. Experimental Evaluation of Phase Change Material Blister Panels for Building Application. Future Cities Environ. 2020, 6. [Google Scholar] [CrossRef]
- Pasupathy, A.; Athanasius, L.; Velraj, R.; Seeniraj, R.V. Experimental investigation and numerical simulation analysis on the thermal performance of a building roof incorporating phase change material (PCM) for thermal management. Appl. Therm. Eng. 2008, 28, 556–565. [Google Scholar] [CrossRef]
- Griffiths, P.W.; Eames, P.C. Performance of chilled ceiling panels using phase change material slurries as the heat transport medium. Appl. Therm. Eng. 2007, 27, 1756–1760. [Google Scholar] [CrossRef]
- Lu, S.; Liang, B.; Li, X.; Kong, X.; Jia, W.; Wang, L. Performance Analysis of PCM Ceiling Coupling with Earth-Air Heat Exchanger for Building Cooling. Materials (Basel) 2020, 13, 2890. [Google Scholar] [CrossRef]
- Lim, H.; Kang, Y.-K.; Jeong, J.-W. Application of a phase change material to a thermoelectric ceiling radiant cooling panel as a heat storage layer. J. Build. Eng. 2020, 32, 101787. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Zhu, Y.; Li, D.; Ma, L. Experimental investigation of optical and thermal performance of a PCM-glazed unit for building applications. Energy Build. 2018, 158, 794–800. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Henríquez, J.R. Thermally effective windows with moving phase change material curtains. Appl. Therm. Eng. 2001, 21, 1909–1923. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Amaral, C.; Figueiredo, A. Thermal performance of a window shutter containing PCM: Numerical validation and experimental analysis. Appl. Energy 2016, 179, 64–84. [Google Scholar] [CrossRef]
- Fokaides, P.A.; Kylili, A.; Kalogirou, S.A. Phase change materials (PCMs) integrated into transparent building elements: A review. Mater. Renew. Sustain. Energy 2015, 4, 1–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).