The Feasibility of Dynamic Musculoskeletal Function Analysis of the Vastus Lateralis in Endurance Runners Using Continuous, Hands-Free Ultrasound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
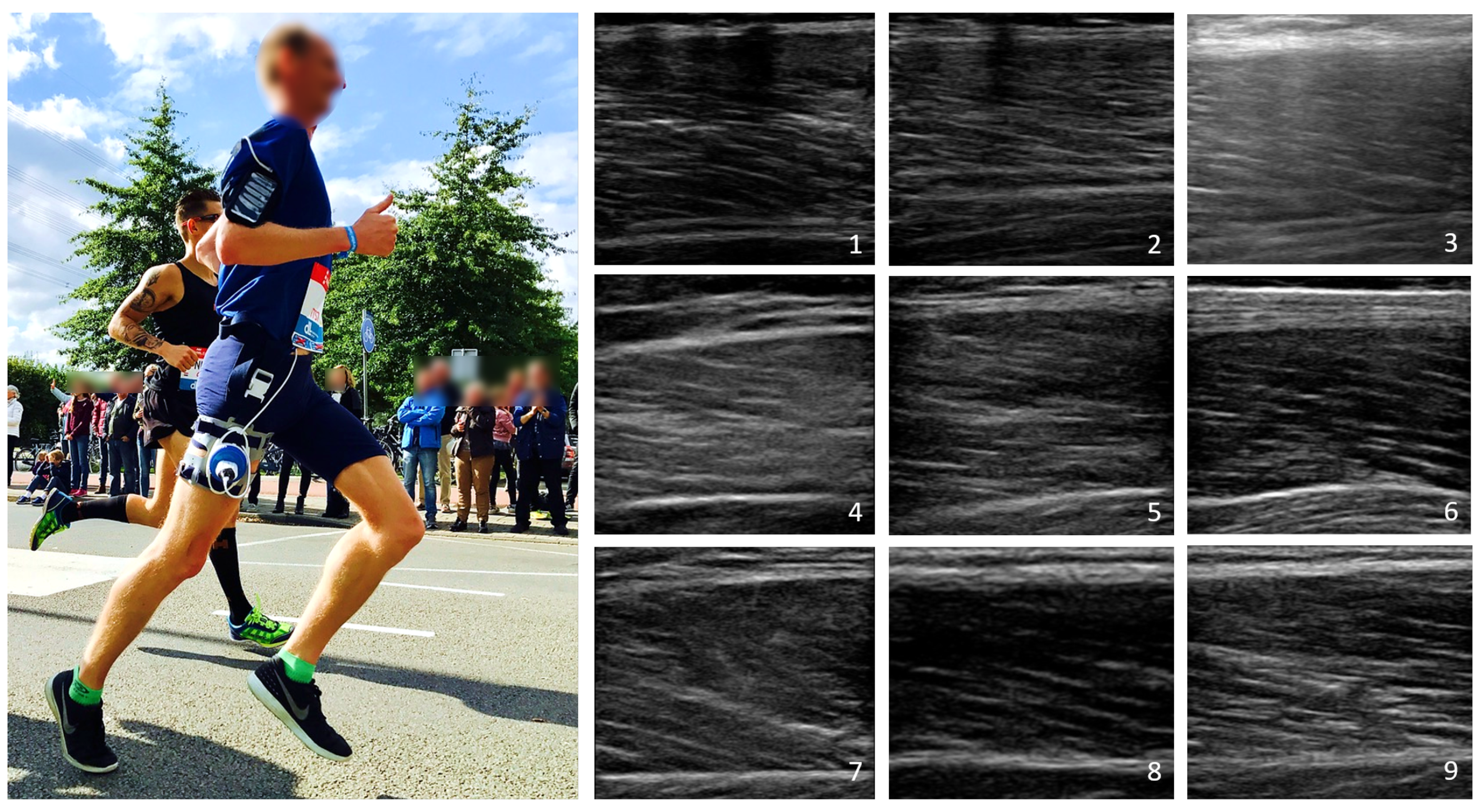
2.2. Acquisition Protocols and Equipment
2.3. Data Analysis
2.3.1. Image Decomposition
2.3.2. Image Quality
Probe-Skin Contact
Field-of-View Stability
2.3.3. Functional Evaluation
3. Results
3.1. Image Quality
3.2. Functional Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CW-SSIM | complex-wavelet structural similarity index method |
References
- For Health Statistics (US). National Health Interview Survey: Research for the 1995–2004 Redesign; Number 121–126; US Government Printing Office: Hyattsville, MA, USA, 1999.
- Reginster, J.Y. The prevalence and burden of arthritis. Rheumatology 2002, 41, 3–6. [Google Scholar] [CrossRef]
- Swentik, A. Pathophysiology of Skeletal Muscle Injury. In Muscular Injuries in the Posterior Leg; Springer: Boston, MA, USA, 2016; pp. 35–47. [Google Scholar]
- Eranki, A.; Cortes, N.; Ferenček, Z.G.; Sikdar, S. A novel application of musculoskeletal ultrasound imaging. J. Vis. Exp. 2013, 79, e50595. [Google Scholar] [CrossRef] [Green Version]
- Whiting, W.C.; Zernicke, R.F. Biomechanics of Musculoskeletal Injury; Human Kinetics: Stanningley, UK, 2008. [Google Scholar]
- Page, P. Pathophysiology of acute exercise-induced muscular injury: Clinical implications. J. Athl. Train. 1995, 30, 29. [Google Scholar] [PubMed]
- Järvinen, T.A.; Järvinen, T.L.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.; Sánchez, M.B.; May, G.; Loram, I. Estimating full regional skeletal muscle fibre orientation from B-mode ultrasound images using convolutional, residual, and deconvolutional neural networks. J. Imaging 2018, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; de Carvalho, I.A.; Bautmans, I.; Bernabei, R.; et al. Assessment of muscle function and physical performance in daily clinical practice. Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Spink, M.J.; Fotoohabadi, M.R.; Wee, E.; Hill, K.D.; Lord, S.R.; Menz, H.B. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch. Phys. Med. Rehabil. 2011, 92, 68–75. [Google Scholar] [CrossRef]
- McKean, M.R.; Burkett, B. The relationship between joint range of motion, muscular strength, and race time for sub-elite flat water kayakers. J. Sci. Med. Sport 2010, 13, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.; Bueno, D.R.; Gallego, J.Á.; Jaramillo, P.; Kilicarslan, A. Surface EMG in Neurorehabilitation and Ergonomics: State of the Art and Future Perspectives. In Emerging Therapies in Neurorehabilitation; Springer: Berlin, Germany, 2014; pp. 267–284. [Google Scholar]
- Martinez-Valdes, E.; Farina, D.; Negro, F.; Del Vecchio, A.; Falla, D. Early motor unit conduction velocity changes to high-intensity interval training versus continuous training. Med. Sci. Sports Exerc. 2018, 50, 2339–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.G.; Dingwell, J.B. Dynamics and stability of muscle activations during walking in healthy young and older adults. J. Biomech. 2009, 42, 2231–2237. [Google Scholar] [CrossRef] [Green Version]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.H. The variability in running gait caused by force plate targeting. J. Appl. Biomech. 2001, 17, 77–83. [Google Scholar] [CrossRef]
- Cappozzo, A.; Della Croce, U.; Leardini, A.; Chiari, L. Human movement analysis using stereophotogrammetry: Part 1: Theoretical background. Gait Posture 2005, 21, 186–196. [Google Scholar] [PubMed]
- Heres, H.M.; Sjoerdsma, M.; Schoots, T.; Rutten, M.C.; van de Vosse, F.N.; Lopata, R.G. Image acquisition stability of fixated musculoskeletal sonography in an exercise setting: A quantitative analysis and comparison with freehand acquisition. J. Med. Ultrason. 2020, 47, 47–56. [Google Scholar] [CrossRef]
- Nadzalan, A.M.; Mohamad, N.I.; Lee, J.L.F.; Chinnasee, C. Relationship between muscle architecture and badminton-specific physical abilities. Hum. Mov. 2018, 19, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Simpson, C.; Kim, B.; Bourcet, M.; Jones, G.; Jakobi, J. Stretch training induces unequal adaptation in muscle fascicles and thickness in medial and lateral gastrocnemii. Scand. J. Med. Sci. Sports 2017, 27, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Lento, P.H.; Primack, S. Advances and utility of diagnostic ultrasound in musculoskeletal medicine. Curr. Rev. Musculoskelet. Med. 2008, 1, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Pillen, S.; van Alfen, N. Skeletal muscle ultrasound. Neurol. Res. 2011, 33, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K. Effects of static stretching on mechanical properties and collagen fiber orientation of the Achilles tendon in vivo. Clin. Biomech. 2018, 60, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Czyrny, Z. Standards for musculoskeletal ultrasound. J. Ultrason. 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, E.; Aggeloussis, N.; Arampatzis, A. Reproducibility of gastrocnemius medialis muscle architecture during treadmill running. J. Electromyogr. Kinesiol. 2011, 21, 1081–1086. [Google Scholar] [CrossRef]
- Chalchat, E.; Gennisson, J.L.; Peñailillo, L.; Oger, M.; Malgoyre, A.; Charlot, K.; Bourrilhon, C.; Siracusa, J.; Garcia-Vicencio, S. Changes in the Viscoelastic Properties of the Vastus Lateralis Muscle With Fatigue. Front. Physiol. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Sjoerdsma, M.; Fixsen, L.S.; Schoots, T.; van de Vosse, F.N.; Lopata, R.G. A demonstration of high field-of-view stability in hands-free echocardiography. Cardiovasc. Ultrasound 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, S.; de Kleijn, M.; van Wijngaarden, J.; Houthuizen, P. The use of a probe stabilizer to reduce musculoskeletal overload of ultrasound operators in routine diagnostic echocardiographic imaging. J. Ultrason. 2019, 19, 193. [Google Scholar] [CrossRef]
- Farup, J.; Kjølhede, T.; Sørensen, H.; Dalgas, U.; Møller, A.B.; Vestergaard, P.F.; Ringgaard, S.; Bojsen-Møller, J.; Vissing, K. Muscle morphological and strength adaptations to endurance vs. resistance training. J. Strength Cond. Res. 2012, 26, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murach, K.; Greever, C.; Luden, N.D. Skeletal muscle architectural adaptations to marathon run training. Appl. Physiol. Nutr. Metab. 2015, 40, 99–102. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [Green Version]
- Portilla, J.; Simoncelli, E.P. A parametric texture model based on joint statistics of complex wavelet coefficients. Int. J. Comput. Vis. 2000, 40, 49–70. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Lim, J.S. The importance of phase in signals. Proc. IEEE 1981, 69, 529–541. [Google Scholar] [CrossRef]
- Sampat, M.P.; Wang, Z.; Gupta, S.; Bovik, A.C.; Markey, M.K. Complex wavelet structural similarity: A new image similarity index. IEEE Trans. Image Process. 2009, 18, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, X.; Cai, H.; Gao, Z. Full-reference image quality assessment-based b-mode ultrasound image similarity measure. arXiv 2017, arXiv:1701.02797. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, R.J.; Harding, P.J.; Loram, I.D. The application of deep convolutional neural networks to ultrasound for modelling of dynamic states within human skeletal muscle. arXiv 2017, arXiv:1706.09450. [Google Scholar]
- Kumar, A.; Anel, R.; Bunnell, E.; Habet, K.; Zanotti, S.; Marshall, S.; Neumann, A.; Ali, A.; Cheang, M.; Kavinsky, C.; et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit. Care Med. 2004, 32, 691–699. [Google Scholar] [CrossRef]
- Simonsen, J.G.; Axmon, A.; Nordander, C.; Arvidsson, I. Neck and upper extremity pain in sonographers–associations with occupational factors. Appl. Ergon. 2017, 58, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.; Roll, S.; Baker, J. Work-related musculoskeletal disorders (WRMSD) among registered diagnostic medical sonographers and vascular technologists: A representative sample. J. Diagn. Med. Sonogr. 2009, 25, 287–299. [Google Scholar] [CrossRef]
- Hodges, P.; Pengel, L.; Herbert, R.; Gandevia, S. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E.; Galanis, N.; Natsis, K.; Kapetanos, G. Validity of architectural properties of the hamstring muscles: Correlation of ultrasound findings with cadaveric dissection. J. Biomech. 2009, 42, 2549–2554. [Google Scholar] [CrossRef]




| Decomposition Level | Bandpass Filter Size Range (Pixels) |
|---|---|
| 1 | 1.5–2.6 |
| 2 | 3.0–5.3 |
| 3 | 5.8–10.4 |
| 4 | 11.4–20.2 |
| 5 | 21.6–38.9 |
| 6 | 41.7–73.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjoerdsma, M.; Caresio, C.; Tchang, B.; Meeder, A.; van de Vosse, F.; Lopata, R. The Feasibility of Dynamic Musculoskeletal Function Analysis of the Vastus Lateralis in Endurance Runners Using Continuous, Hands-Free Ultrasound. Appl. Sci. 2021, 11, 1534. https://doi.org/10.3390/app11041534
Sjoerdsma M, Caresio C, Tchang B, Meeder A, van de Vosse F, Lopata R. The Feasibility of Dynamic Musculoskeletal Function Analysis of the Vastus Lateralis in Endurance Runners Using Continuous, Hands-Free Ultrasound. Applied Sciences. 2021; 11(4):1534. https://doi.org/10.3390/app11041534
Chicago/Turabian StyleSjoerdsma, Marloes, Cristina Caresio, Benjamin Tchang, Amber Meeder, Frans van de Vosse, and Richard Lopata. 2021. "The Feasibility of Dynamic Musculoskeletal Function Analysis of the Vastus Lateralis in Endurance Runners Using Continuous, Hands-Free Ultrasound" Applied Sciences 11, no. 4: 1534. https://doi.org/10.3390/app11041534
APA StyleSjoerdsma, M., Caresio, C., Tchang, B., Meeder, A., van de Vosse, F., & Lopata, R. (2021). The Feasibility of Dynamic Musculoskeletal Function Analysis of the Vastus Lateralis in Endurance Runners Using Continuous, Hands-Free Ultrasound. Applied Sciences, 11(4), 1534. https://doi.org/10.3390/app11041534





