Relationship between the Difference in Oxygenated Hemoglobin Concentration Changes in the Left and Right Prefrontal Cortex and Cognitive Function during Moderate-Intensity Aerobic Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Cognitive Function Test
2.4. fNIRS
2.5. Mean Arterial Pressure (MAP), Heart Rate (HR), and Skin Blood Flow (SBF)
2.6. Statistical Analysis
3. Results
3.1. Related Parameters of Exercise Intensity
3.2. 2-Back Test
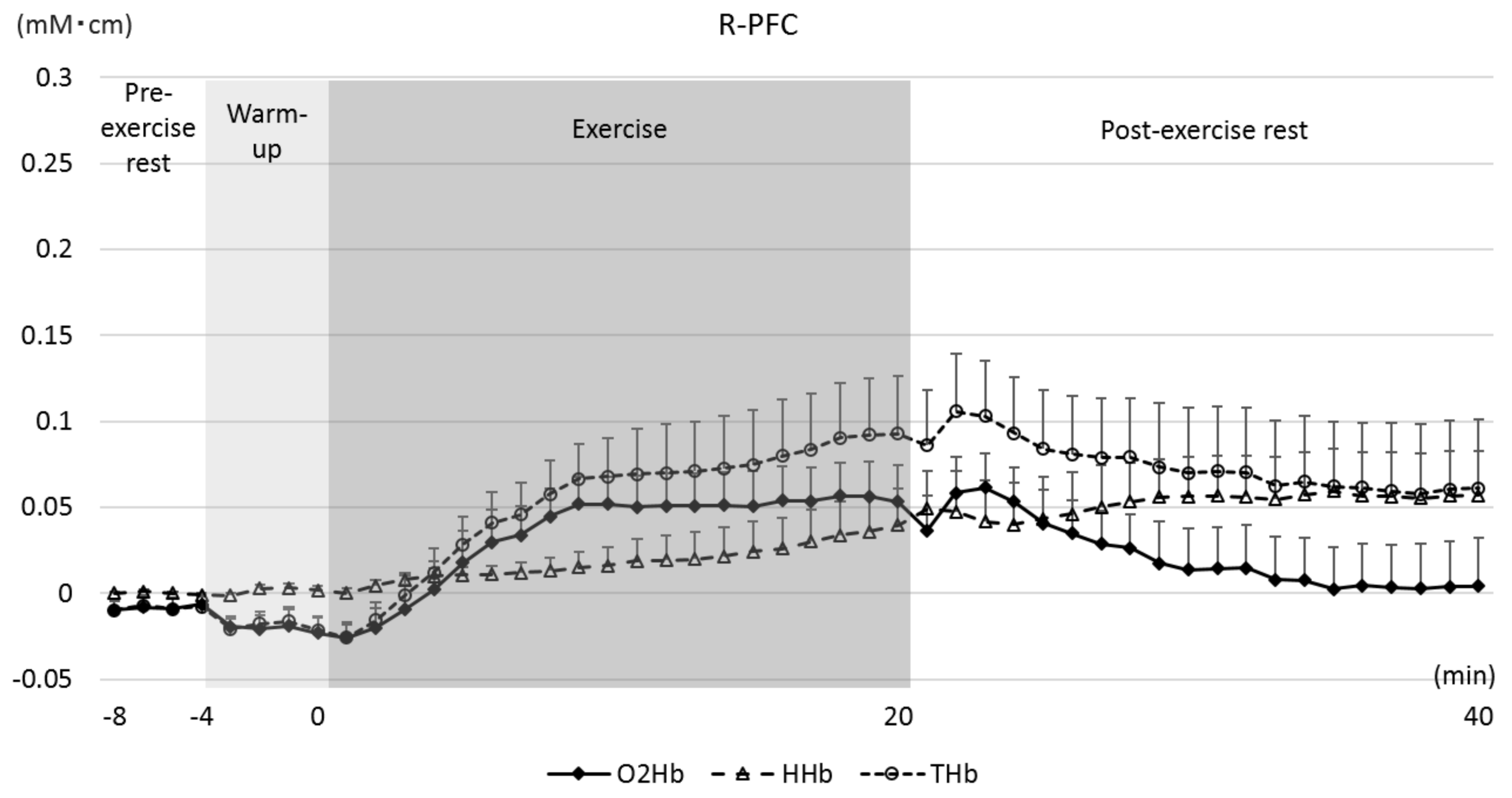
3.3. O2Hb in the L-PFC and R-PFC
3.4. MAP, HR, and SBF
3.5. Correlation Coefficients
4. Discussion
4.1. Related Parameters of Exercise Intensity
4.2. Reaction Time
4.3. PFC oxygen Dynamics
4.4. Peak O2Hb Levels, AUC O2Hb, and Increase in the Slope of O2Hb
4.5. MAP, HR, and SBF
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebowitz, A.; Nemoto, K.; Ma, N.Y.; Usuniwa, H.; Tamura, M.; Ishikawa, K.; Arai, T. Exercise habit could modulate cognitive benefits from physical and cognitive intervention. Psychiatry Clin. Neurosci. 2018, 72, 189–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herting, M.M.; Chu, X. Exercise, cognition, and the adolescent brain. Birth Defects Res. 2017, 109, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Torbeyns, T.; De Geus, B.; Bailey, S.; Decroix, L.; Van Cutsem, J.; De Pauw, K.; Meeusen, R. Bike desks in the classroom: Energy expenditure, physical health, cognitive performance, brain functioning, and academic performance. J. Phys. Act. Health 2017, 14, 429–439. [Google Scholar] [CrossRef]
- Sacker, A.; Cable, N. Do adolescent leisure-time physical activities foster health and well-being in adulthood? Evidence from two British birth cohorts. Eur. J. Public Health 2005, 16, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wortmann, M. Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimer’s Res. Ther. 2012, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Murayama, Y.; Sato, Y.; Hu, L.; Brugnera, A.; Compare, A.; Sakatani, K. Relation between cognitive function and baseline concentrations of Hemoglobin in prefrontal cortex of elderly people measured by time-resolved near-infrared spectroscopy. In Oxygen Transport to Tissue XXXIX; Springer: Cham, Switzerland, 2017; pp. 269–276. [Google Scholar]
- Takeda, T.; Kawakami, Y.; Konno, M.; Matsuda, Y.; Nishino, M.; Suzuki, Y.; Kawano, Y.; Nakajima, K.; Ozawa, T.; Kondo, Y.; et al. PFC blood oxygenation changes in four different cognitive tasks. In Oxygen Transport to Tissue XXXIX; Springer: Cham, Switzerland, 2017; pp. 199–204. [Google Scholar]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Herrmann, M.J.; Walter, A.; Ehlis, A.C.; Fallgatter, A.J. Cerebral oxygenation changes in the prefrontal cortex: Effects of age and gender. Neurobiol. Aging 2006, 27, 888–894. [Google Scholar] [CrossRef]
- Tomasi, D.; Ernst, T.; Caparelli, E.C.; Chang, L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum. Brain Mapp. 2006, 27, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Banaji, M.; Mallet, A.; Elwell, C.E.; Nicholls, P.; Cooper, C.E. A model of brain circulation and metabolism: NIRS signal changes during physiological challenges. PLoS Comput. Biol. 2008, 4, e1000212. [Google Scholar] [CrossRef] [Green Version]
- Ainslie, P.N.; Barach, A.; Murrell, C.; Hamlin, M.; Hellemans, J.; Ogoh, S. Alterations in cerebral autoregulation and cerebral blood flow velocity during acute hypoxia: Rest and exercise. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H976–H983. [Google Scholar] [CrossRef]
- Raichle, M.E.; Grubb, R.L.; Gado, M.H.; Eichling, J.O.; Ter-Pogossian, M.M. Correlation between regional cerebral blood flow and oxidative metabolism: In vivo studies in man. Arch. Neurol. 1976, 33, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S. Relationship between cognitive function and regulation of cerebral blood flow. J. Physiol. Sci. 2017, 67, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, M.; Fukuda, M.; Yamagishi, Y.; Sato, T.; Uehara, T.; Ito, M.; Suto, T.; Mikuni, M. Frontal lobe function in bipolar disorder: A multichannel near-infrared spectroscopy study. NeuroImage 2006, 29, 172–184. [Google Scholar] [CrossRef]
- Phillips, A.; Chan, F.H.N.; Zheng, M.Z.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.H.; Seo, J.P.; Lee, S.H.; Jin, S.H.; Yeo, S.S. The cortical activation pattern during bilateral arm raising movements. Neural Regen. Res. 2017, 12, 317–320. [Google Scholar] [PubMed]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Iwamoto, K.; Kawano, N.; Noda, Y.; Ozaki, N.; Noda, A. Differential effects of physical activity and sleep duration on cognitive function in young adults. J. Sport Health Sci. 2018, 7, 227–236. [Google Scholar] [CrossRef]
- Perrey, S. NIRS for Measuring Cerebral Hemodynamic Responses during Exercise; Springer: Berlin/Heidelberg, Germany, 2012; pp. 335–349. [Google Scholar]
- Stute, K.; Hudl, N.; Stojan, R.; Voelcker-Rehage, C. Shedding Light on the Effects of Moderate Acute Exercise on Working Memory Performance in Healthy Older Adults: An fNIRS Study. Brain Sci. 2020, 10, 813. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- Chang, H.; Kim, K.; Jung, Y.-J.; Kato, M. Effects of acute high-intensity resistance exercise on cognitive function and oxygenation in prefrontal cortex. J. Exerc. Nutr. Biochem. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Mekari, S.; Fraser, S.; Bosquet, L.; Bonnéry, C.; Labelle, V.; Pouliot, P.; Lesage, F.; Bherer, L. The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur. J. Appl. Physiol. 2015, 115, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Akila, V.; Christaline, J.A. A review of cognitive brain activation using near-infrared spectroscopy (NIRS). AIP Conf. Proc. 2020. [Google Scholar]
- Lefferts, W.K.; Babcock, M.C.; Tiss, M.J.; Ives, S.J.; White, C.N.; Brutsaert, T.D.; Heffernan, K.S. Effect of hypoxia on cerebrovascular and cognitive function during moderate intensity exercise. Physiol. Behav. 2016, 165, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, A.; Morishita, S.; Tokunaga, Y.; Sato, D.; Qin, W.; Kojima, S.; Onishi, H. Laterality of cortical oxygenation in the prefrontal cortex during 20 min of moderate-intensity cycling exercise: A near-infrared spectroscopy study. Ann. Phys. Rehabil. Med. 2018, 61, e460. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.E.; D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Aron, A.R.; Fletcher, P.C.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003, 6, 115–116. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Morinaga, K.; Akiyoshi, J.; Matsushita, H.; Ichioka, S.; Tanaka, Y.; Tsuru, J.; Hanada, H. Anticipatory anxiety-induced changes in human lateral prefrontal cortex activity. Biol. Psychol. 2007, 74, 34–38. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M.; Torricelli, A.; Spinelli, L.; Pifferi, A.; Cubeddu, R. Bilateral prefrontal cortex oxygenation responses to a verbal fluency task: A multichannel time-resolved near-infrared topography study. J. Biomed. Opt. 2005, 10, 011012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlaoui, K.; Di Sante, G.; Barbeau, J.; Maheux, M.; Lesage, F.; Ska, B.; Joanette, Y. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang. 2012, 121, 164–173. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Sherwood, R.J.; Henriques, J.B.; Davidson, R.J. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychol. Sci. 2005, 16, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Hallett, M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin. Neurophysiol. 2001, 112, 107–113. [Google Scholar] [CrossRef]
- Tomporowski, P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003, 112, 297–324. [Google Scholar] [CrossRef]
- Kamijo, K.; Hayashi, Y.; Sakai, T.; Yahiro, T.; Tanaka, K.; Nishihira, Y. Acute effects of aerobic exercise on cognitive function in older adults. J. Gerontol. Ser. B 2009, 64, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chu, C.-H.; Chu, I.-H. Executive function during acute exercise: The role of exercise intensity. J. Sport Exerc. Psychol. 2013, 358–367. [Google Scholar] [CrossRef]
- Brisswalter, J.; Collardeau, M.; René, A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 2002, 32, 555–566. [Google Scholar] [CrossRef]
- Chen, F.T.; Etnier, J.L.; Wu, C.H.; Cho, Y.M.; Hung, T.M.; Chang, Y.K. Dose-response relationship between exercise duration and executive function in older adults. J. Clin. Med. 2018, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Morishita, S.; Tsubaki, A.; Nashimoto, S.; Fu, J.B.; Onishi, H. Face scale rating of perceived exertion during cardiopulmonary exercise test. BMJ Open Sport Exerc. Med. 2018, 4, e00047. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Stephane, P. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur. J. Appl. Physiol. 2008, 102, 153–163. [Google Scholar] [CrossRef]
- Fellman, D.; Jylkkä, J.; Warris, O.; Soveri, A.; Ritakallio, L.; Haga, S.; Salmi, J.; Nyman, T.J.; Laine, M. The role of strategy use in working memory training outcomes. Journal Mem. Lang. 2020, 104064. [Google Scholar] [CrossRef]
- Hazamy, A.; Altmann, L.J.P.; Stegemöller, E.; Bowers, D.; Lee, H.K.; Wilson, J.; Okun, M.S.; Hass, C.J. Improved cognition while cycling in Parkinson’s disease patients and healthy adults. Brain Cogn. 2017, 113, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.; Forsyth, B.; Lee, A.; Machado, L. Repeated computerized cognitive testing: Performance shifts and test–retest reliability in healthy young adults. Psychol. Assess. 2018, 30, 539. [Google Scholar] [CrossRef]
- Yaple, Z.; Arsalidou, M. N-back working memory task: Meta-analysis of normative fMRI studies with children. Child Dev. 2018, 89, 2010–2022. [Google Scholar] [CrossRef]
- Miró-Padilla, A.; Bueichekú, E.; Ventura-Campos, N.; Flores-Compañ, M.-J.; Parcet, M.A.; Ávila, C. Long-term brain effects of N-back training: An fMRI study. Brain Imaging Behav. 2019, 13, 1115–1127. [Google Scholar] [CrossRef]
- Lefferts, W.K.; DeBlois, J.P.; Receno, C.N.; Barreira, T.V.; Brutsaert, T.D.; Carhart, R.L.; Heffernan, K.S. Effects of acute aerobic exercise on arterial stiffness and cerebrovascular pulsatility in adults with and without hypertension. J. Hypertens. 2018, 36, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Deschuyteneer, M.; Vandierendonck, A.; Muyllaert, I. Does solution of mental arithmetic problems such as 2 + 6 and 3 × 8 rely on the process of “memory updating”? Exp. Psychol. 2006, 53, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Schmiedek, F.; Lövdén, M.; Lindenberger, U. On the relation of mean reaction time and intraindividual reaction time variability. Psychol. Aging 2009, 24, 841. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.M.; Price, C.; Okun, M.; Montijo, H.; Bowers, D. Is the n-back task a valid neuropsychological measure for assessing working memory? Arch. Clin. Neuropsychol. 2009, 24, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Schoofs, D.; Preuß, D.; Wolf, O.T. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 2008, 33, 643–653. [Google Scholar] [CrossRef]
- Byun, K.; Hyodo, K.; Suwabe, K.; Ochi, G.; Sakairi, Y.; Kato, M.; Dan, I.; Soya, H. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: An fNIRS study. Neuroimage 2014, 98, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef]
- Kono, T.; Matsuo, K.; Tsunashima, K.; Kasai, K.; Takizawa, R.; Rogers, M.A.; Yamasue, H.; Yano, T.; Taketani, Y.; Kato, N. Multiple-time replicability of near-infrared spectroscopy recording during prefrontal activation task in healthy men. Neurosci. Res. 2007, 57, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sasai, S.; Homae, F.; Watanabe, H.; Taga, G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. Neuroimage 2011, 56, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Frederick, B.D. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. Neuroimage 2010, 53, 553–564. [Google Scholar] [CrossRef] [Green Version]
- Hatakenaka, M.; Miyai, I.; Mihara, M.; Sakoda, S.; Kubota, K. Frontal regions involved in learning of motor skill—A functional NIRS study. Neuroimage 2007, 34, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hada, Y.; Abo, M.; Kaminaga, T.; Mikami, M. Detection of cerebral blood flow changes during repetitive transcranial magnetic stimulation by recording hemoglobin in the brain cortex, just beneath the stimulation coil, with near-infrared spectroscopy. Neuroimage 2006, 32, 1226–1230. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.; Chang, P.-H. There is No test–retest reliability of brain activation induced by robotic passive hand movement: A functional NIRS study. Brain Behav. 2020, 10, e01788. [Google Scholar] [CrossRef]
- Maki, A.; Yamashita, Y.; Ito, Y.; Watanabe, E.; Mayanagi, Y.; Koizumi, H. Spatial and temporal analysis of human motor activity using noninvasive NIR topography. Med. Phys. 1995, 22, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Okibayashi, M.; Danjo, N.; Ishikawa, A.; Inoue, Y.; et al. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS ONE 2012, 7, e32234. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.; Secher, N.H.; Siebenmann, C.; Nielsen, H.B.; Kohl-Bareis, M.; Lundby, C.; Rasmussen, P. Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiol. J. Am. Soc. Anesthesiol. 2012, 117, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, T.; Lian, C.-S.; Afroundeh, R.; Shirakawa, K.; Yunoki, T. Comparison of oscillations of skin blood flow and deoxygenation in vastus lateralis in light exercise. Biol. Sport 2014, 31, 15. [Google Scholar] [CrossRef] [Green Version]
- Willie, C.K.; Tzeng, Y.-C.; Fisher, J.A.; Ainslie, P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-B.; Zhu, P.-F.; Yin, Z.-X.; Kraus, V.B.; Threapleton, D.; Chei, C.-L.; Brasher, M.S.; Zhang, J.; Qian, H.-Z.; Mao, C.; et al. A U-shaped association between blood pressure and cognitive impairment in Chinese elderly. J. Am. Med. Dir. Assoc. 2017, 18, 193.e7–193.e13. [Google Scholar] [CrossRef] [Green Version]
- Sesso, H.D.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Gaziano, J.M.; Manson, J.E.; Glynn, R.J. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension 2000, 36, 801–807. [Google Scholar] [CrossRef]
- Martini, M.; Röhrig, A.; Wenghoefer, M.; Schindler, E.; Messing-Jünger, M. Cerebral oxygenation and hemodynamic measurements during craniosynostosis surgery with near-infrared spectroscopy. Child’s Nerv. Syst. 2014, 30, 1367–1374. [Google Scholar] [CrossRef]
- Miyata, S.; Noda, A.; Ozaki, N.; Hara, Y.; Minoshima, M.; Iwamoto, K.; Takahashi, M.; Iidaka, T.; Koike, Y. Insufficient sleep impairs driving performance and cognitive function. Neurosci. Lett. 2010, 469, 229–233. [Google Scholar] [CrossRef]
- Agbangla, N.F.; Audiffren, M.; Albinet, C.T. Assessing muscular oxygenation during incremental exercise using near-infrared spectroscopy: Comparison of three different methods. Physiol. Res. 2017, 66, 979–985. [Google Scholar] [CrossRef]
- Bailey, C.S.; Wooster, L.T.; Buswell, M.; Patel, S.; Pappagianopoulos, P.P.; Bakken, K.; White, C.; Tanguay, M.; Blodgett, J.B.; Baggish, A.L.; et al. Post-exercise oxygen uptake recovery delay: A novel index of impaired cardiac reserve capacity in heart failure. Heart Fail. 2018, 6, 329–339. [Google Scholar]
- Cunningham, D.A.; St Croix, C.M.; Paterson, D.H.; Özyener, F.; Whipp, B.J. The Off-Transient Pulmonary Oxygen Uptake (VO2) Kinetics Following Attainment of a Particular VO2 During Heavy-Intensity Exercise in Humans. Exp. Physiol. 2000, 85, 339–347. [Google Scholar] [CrossRef] [PubMed]
- De Feo, P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Metab. Cardiovasc. Dis. 2013, 23, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Devin, J.L.; Sax, A.T.; Hughes, G.I.; Jenkins, D.G.; Aitken, J.F.; Chambers, S.K.; Dunn, J.C.; Bolam, K.A.; Skinner, T.L. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: A randomised controlled trial. J. Cancer Surviv. 2016, 10, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Jung, M.E.; Wright, A.E.; Wright, W.; Manders, R.J. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014, 39, 835–841. [Google Scholar] [CrossRef]
- Barbero-Alvarez, J.C.; Soto, V.M.; Barbero-Alvarez, V.; Granda-Vera, J. Match analysis and heart rate of futsal players during competition. J. Sports Sci. 2008, 26, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bediz, C.S.; Oniz, A.; Guducu, C.; Demirci1, E.U.; Ogut, H.; Gunay, E.; Cetinkaya, C.; Ozgoren, M. Acute supramaximal exercise increases the brain oxygenation in relation to cognitive workload. Front. Hum. Neurosci. 2016, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefferts, W.K.; DeBlois, J.P.; White, C.N.; Heffernan, K.S. Effects of acute aerobic exercise on cognition and constructs of decision-making in adults with and without hypertension. Front. Aging Neurosci. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Hsieh, S.; Chang, Y.K.; Hung, T.M.; Fang, C.L. The effects of acute resistance exercise on young and older males’ working memory. Psychol. Sport Exerc. 2016, 22, 286–293. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, T.; Mei, L.; Li, A.; Zhang, C. Influence of acute combined physical and cognitive exercise on cognitive function: An NIRS study. PeerJ 2019, 7, e7418. [Google Scholar] [CrossRef]
- Chang, Y.K.; Tsai, C.L.; Hung, T.M.; So, E.C.; Chen, F.T.; Etnier, J.L. Effects of acute exercise on executive function: A study with a Tower of London Task. J. Sport Exerc. Psychol. 2011, 33, 847–865. [Google Scholar] [CrossRef] [Green Version]
- Torrens-Burton, A.; Basoudan, N.; Bayer, A.J.; Tales, A. Perception and reality of cognitive function: Information processing speed, perceived memory function, and perceived task difficulty in older adults. J. Alzheimer’s Dis. 2017, 60, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Giles, G.E.; Brunyé, T.; Eddy, M.D.; Mahoney, C.R.; Gagnon, S.A.; Taylor, H.A.; Kanarek, R.B. Acute exercise increases oxygenated and deoxygenated hemoglobin in the prefrontal cortex. Neuroreport 2014, 25, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.; Nagel, B.J. Differences in brain activity during a verbal associative memory encoding task in high-and low-fit adolescents. J. Cogn. Neurosci. 2013, 25, 595–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Tian, J.; Hao, R.; Tian, L.; Liu, Q. Transcranial direct current stimulation over the right DLPFC selectively modulates subprocesses in working memory. PeerJ 2018, 6, e4906. [Google Scholar] [CrossRef] [Green Version]
- Bosch, B.M.; Bringard, A.; Ferretti, G.; Schwartz, S.; Iglói, K. Effect of cerebral vasomotion during physical exercise on associative memory, a near-infrared spectroscopy study. Neurophotonics 2017, 4, 041404. [Google Scholar] [CrossRef]
- Helton, W.S.; Warm, J.S.; Tripp, L.D.; Matthews, G.; Parasuraman, R.; Hancock, P.A. Cerebral lateralization of vigilance: A function of task difficulty. Neuropsychologia 2010, 1683–1688. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85. [Google Scholar] [CrossRef]
- Dalsgaard, M.K. Fuelling cerebral activity in exercising man. J. Cereb. Blood Flow Metab. 2006, 26, 731–750. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.P.; Meijer, R.I.; Newman, J.; Stehouwer, C.D.; Eringa, E.C.; Smulders, Y.M.; Serné, E.H. Insulin-induced changes in microvascular vasomotion and capillary recruitment are associated in humans. Microcirculation 2014, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Memory function and brain glucose metabolism. Pharmacopsychiatry 2003, 36 (Suppl. S1), 62–67. [Google Scholar]
- Kime, R.; Fujioka, M.; Osawa, T.; Takagi, S.; Niwayama, M.; Kaneko, Y.; Murase, N.; Katsumura, T.; Osada, T. Which is the best indicator of muscle oxygen extraction during exercise using NIRS? Evidence that HHb is not the candidate. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; pp. 163–169. [Google Scholar]
- Glowacki, S.P.; Martin, S.E.; Maurer, A.; Baek, W.; Green, J.S.; Crouse, S.F. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med. Sci. Sports Exerc. 2004, 36, 2119–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, G.S.; Poyares, D.; Santana, M.G.; Garbuio, S.A.; Tufik, S.; Mello, M.T. Effect of acute physical exercise on patients with chronic primary insomnia. J. Clin. Sleep Med. 2010, 270–275. [Google Scholar] [CrossRef] [Green Version]






| Peak Value during CPET | Averaged Value during 20 min Exercise at Moderate Intensity | |
|---|---|---|
| VO2 (mL/kg/min) | 36.6 ± 6.5 | 21.5 ± 2.9 |
| HR (bpm) | 171.0 ± 26.6 | 133.3 ± 13.6 |
| Load (Watt) | 191.8 ± 32.7 | 92.8 ± 17.3 |
| Timepoint | Reaction Time (Average Time Spent on Each Question) |
|---|---|
| pre | 2.3 ± 0.3 s |
| post1 | 1.9 ± 0.3 * s |
| post2 | 1.8 ± 0.3 * s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, W.; Kojima, S.; Yamazaki, Y.; Morishita, S.; Hotta, K.; Inoue, T.; Tsubaki, A. Relationship between the Difference in Oxygenated Hemoglobin Concentration Changes in the Left and Right Prefrontal Cortex and Cognitive Function during Moderate-Intensity Aerobic Exercise. Appl. Sci. 2021, 11, 1643. https://doi.org/10.3390/app11041643
Qin W, Kojima S, Yamazaki Y, Morishita S, Hotta K, Inoue T, Tsubaki A. Relationship between the Difference in Oxygenated Hemoglobin Concentration Changes in the Left and Right Prefrontal Cortex and Cognitive Function during Moderate-Intensity Aerobic Exercise. Applied Sciences. 2021; 11(4):1643. https://doi.org/10.3390/app11041643
Chicago/Turabian StyleQin, Weixiang, Sho Kojima, Yudai Yamazaki, Shinichiro Morishita, Kazuki Hotta, Tatsuro Inoue, and Atsuhiro Tsubaki. 2021. "Relationship between the Difference in Oxygenated Hemoglobin Concentration Changes in the Left and Right Prefrontal Cortex and Cognitive Function during Moderate-Intensity Aerobic Exercise" Applied Sciences 11, no. 4: 1643. https://doi.org/10.3390/app11041643







