Different Approaches to the Regeneration of Dental Tissues in Regenerative Endodontics
Abstract
1. Introduction
- Dental pulp stem cells (DPSCs): Clonogenic cells with high proliferation potential and long-term self-renewal [11], isolated from permanent third molars in 2000 by Gronthos et al. They reside within niches in pulp chambers [8] in a stable microenvironment, which depends on the interplay between growth factors, extracellular matrix proteins, receptor molecules, and stem cells [5]. Research has indicated that dental pulp stem cells have the ability to become odontoblast-like cells and generate ectopic dentin in the subcutaneous tissues of immunocompromised mice [12,13]. Furthermore, it was shown that DPSCs can differentiate into other non-dental cells, such as osteoblasts, odontoblast, chondrocytes (thus, they can produce bone and cartilage tissues), neuron cells, adipocyte, cardiomyocytes, and insulin-secreting Beta cells [5,14].
- Stem cells from exfoliated deciduous teeth (SHED): Isolated by Miura et al., exhibiting multipotential differentiation properties and increased cell-population doublings in comparison to DPSCs [15]. It is hypothesized that SHED cells have an extensive proliferation ability higher than DPSCs and MSCs derived from bone marrow, due to being a more immature population [16].
- Stem cells from apical papillae (SCAP): MSC-like cells located in the tooth root apex, discovered for the first time by Sonoyama et al. in the apical papilla of human immature permanent teeth [12]. Studies performed in immunocompromised rodents showed the odontogenic potential of SCAP cells when multipotent stem cells were transplanted with hydroxyapatite/tricalcium phosphate particles. The regeneration of pulp-like tissue and dentin structure were observed [17]. According to the conducted scientific studies, it is believed that SCAP cells are involved in the formation of root dentin, as a source of primary odontoblast [18], opposed to DPSCs, which take part in reparative dentin formation, providing replacement odontoblast [19]. It is also hypothesized that a positive result of endodontic treatment of infected immature permanent tooth may be achieved due to the reservoir of SCAP in the apical papilla, and their ability to produce primary odontoblasts involved in apexogenesis [12,20,21].
- Periodontal ligament stem cells (PDLSCs): These multipotent cells have the potential to develop into cementoblast-like cells, adipocytes, and chondrogenic cells. In vivo experiments have exhibited PDLSC’s capacity to form cementum/PDL-like structures [22,23]. Therefore, using these cells in periodontal regeneration protocols is being considered [24].
- Dental follicle precursor cells (DFPCs): Localized in a dental sac, also known as a dental follicle, a loose connective tissue that surrounds developing teeth, and also impacted teeth. The latter are usually extracted and disposed of, therefore there are no controversial ethical issues linked to the sourcing of DFPCs [25]. Some studies have shown that DFPCs can transform into fibroblasts, osteoblasts, periodontal ligament, and cementoblasts [26], thus these cells may be useful in regeneration therapies of periodontal tissues [5].
2. Materials and Methods
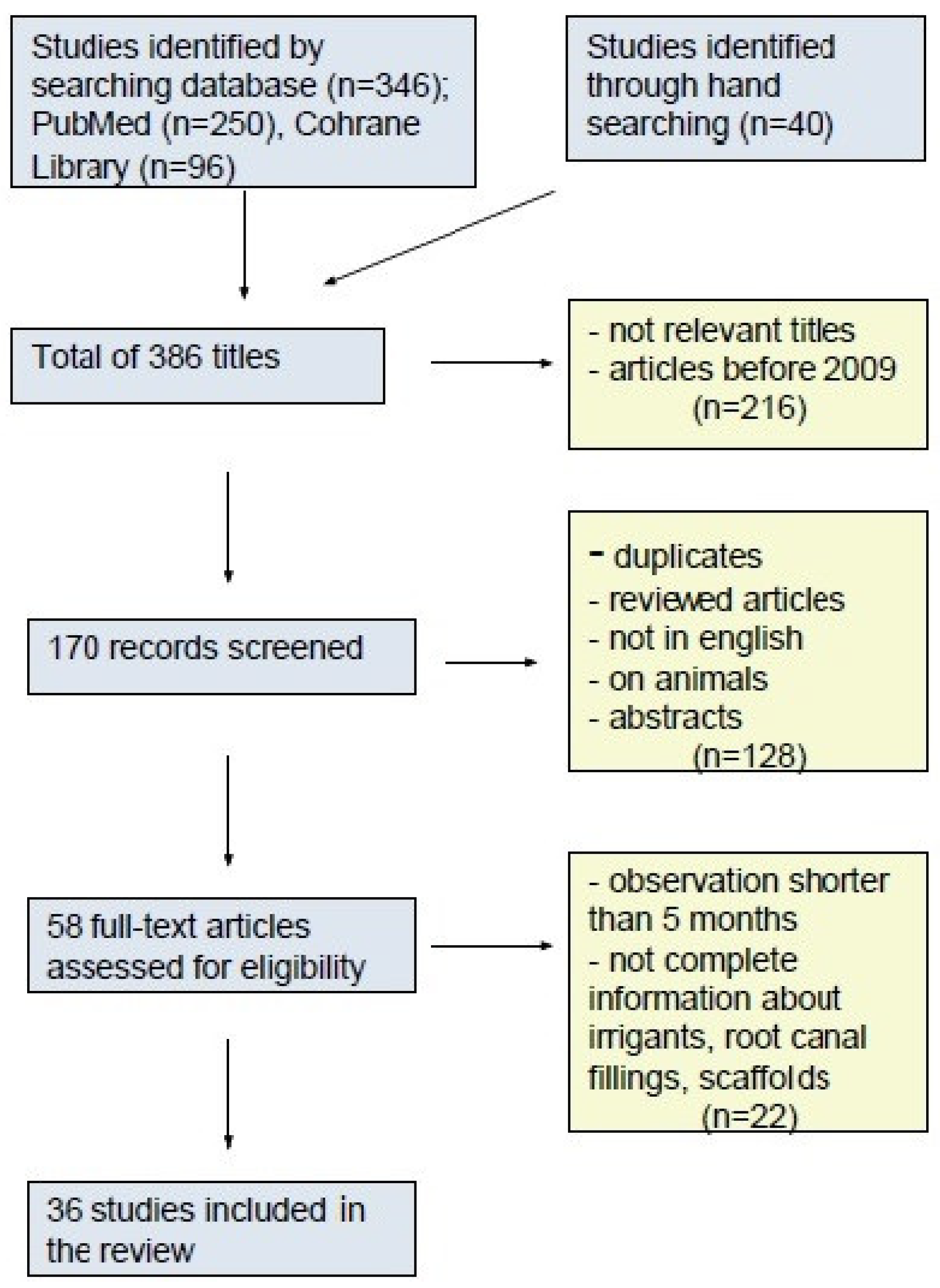
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Parveen, S.; Krishnakumar, K.; Sahoo, S.K. New era in health care: Tissue engineering. J. Stem Cells Regen. Med. 2006, 1, 8–24. [Google Scholar] [PubMed]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S. Stem Sense: A Proposal for the Classification of Stem Cells. Stem Cells Dev. 2004, 13, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Sangappa, S.K.; Javanaiahar, N.; Kumar, A.P.; Shruti, S. Regenerative endodontic: Current progress. IOSR J. Dent. Med. Sci. 2014, 13, 88–95. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
- Chandki, R.; Kala, M.; Banthia, P.; Banthia, R. From Stem to Roots: Tissue engineering in Endodontics. J. Clin. Exp. Dent. 2012, 4, e66–e71. [Google Scholar] [CrossRef]
- Gong, T.; Heng, B.C.; Lo, E.C.M.; Zhang, C. Current Advance and Future Prospects of Tissue Engineering Approach to Dentin/Pulp Regenerative Therapy. Stem Cells Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Kim, S.; Shin, S.-J.; Song, Y.; Kim, E. In Vivo Experiments with Dental Pulp Stem Cells for Pulp-Dentin Complex Regeneration. Mediat. Inflamm. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem Cell Proliferation Pathways Comparison between Human Exfoliated Deciduous Teeth and Dental Pulp Stem Cells by Gene Expression Profile from Promising Dental Pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Kaushik, S.N.; Kim, B.; Walma, A.M.C.; Choi, S.C.; Wu, H.; Mao, J.J.; Jun, H.-W.; Cheon, K. Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 2016, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Chueh, L.-H.; Huang, G.T.-J. Immature Teeth with Periradicular Periodontitis or Abscess Undergoing Apexogenesis: A Paradigm Shift. J. Endod. 2006, 32, 1205–1213. [Google Scholar] [CrossRef]
- Banchs, F.; Trope, M. Revascularization of Immature Permanent Teeth with Apical Periodontitis: New Treatment Protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Komaki, M.; Sekiya, I.; Sakaguchi, Y.; Noguchi, K.; Oda, S.; Muneta, T.; Ishikawa, I. Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 2006, 41, 303–310. [Google Scholar] [CrossRef]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human Periodontal Ligament Cell Sheets Can Regenerate Periodontal Ligament Tissue in an Athymic Rat Model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef]
- Bojic, S.; Volarevic, V.; Ljujic, B.; Stojkovic, M. Dental stem cells—Characteristics and potential. Histol. Histopathol. 2014, 29, 699–706. [Google Scholar] [CrossRef]
- Kémoun, P.; Laurencin-Dalicieux, S.; Rue, J.; Farges, J.-C.; Gennero, I.; Conte-Auriol, F.; Briand-Mesange, F.; Gadelorge, M.; Arzate, H.; Narayanan, A.S.; et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007, 329, 283–294. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; Abubakr, N.; Dörfer, C.E.; El-Sayed, K.F. Tissue Engineering Approaches for Enamel, Dentin, and Pulp Regeneration: An Update. Stem Cells Int. 2020, 2020, 5734539. [Google Scholar] [CrossRef]
- Kim, S.G.; Zhou, J.; Solomon, C.; Zheng, Y.; Suzuki, T.; Chen, M.; Song, S.; Jiang, N.; Cho, S.; Mao, J.J. Effects of Growth Factors on Dental Stem/Progenitor Cells. Dent. Clin. N. Am. 2012, 56, 563–575. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Musson, D.S.; McLachlan, J.L.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Adrenomedullin is expressed during rodent dental tissue development and promotes cell growth and mineralization. Biol. Cell 2010, 102, 145–157. [Google Scholar] [CrossRef]
- Zeng, Q.; Nguyen, S.; Zhang, H.; Chebrolu, H.P.; Alzebdeh, D.; Badi, M.A.; Kim, J.R.; Ling, J.; Yang, M. Release of Growth Factors into Root Canal by Irrigations in Regenerative Endodontics. J. Endod. 2016, 42, 1760–1766. [Google Scholar] [CrossRef]
- Malik, Z.; Alexiou, M.; Hallgrímsson, B.; Economides, A.; Luder, H.; Graf, D. Bone Morphogenetic Protein 2 Coordinates Early Tooth Mineralization. J. Dent. Res. 2018, 97, 835–843. [Google Scholar] [CrossRef]
- Aksel, H.; Öztürk, Ş.; Serper, A.; Ulubayram, K. VEGF/BMP-2 loaded three-dimensional model for enhanced angiogenic and odontogenic potential of dental pulp stem cells. Int. Endod. J. 2017, 51, 420–430. [Google Scholar] [CrossRef]
- Yadlapati, M.; Biguetti, C.; Cavalla, F.; Nieves, F.; Bessey, C.; Bohluli, P.; Garlet, G.P.; Letra, A.; Fakhouri, W.D.; Silva, R.M. Characterization of a Vascular Endothelial Growth Factor–loaded Bioresorbable Delivery System for Pulp Regeneration. J. Endod. 2017, 43, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Duncan, H.F.; Diogenes, A.; Simon, S.; Cooper, P.R. Exploiting the Bioactive Properties of the Dentin-Pulp Complex in Regenerative Endodontics. J. Endod. 2016, 42, 47–56. [Google Scholar] [CrossRef]
- Rao, S.M.; Ugale, G.M.; Warad, S.B. Bone Morphogenetic Proteins: Periodontal Regeneration. N. Am. J. Med. Sci. 2013, 5, 161–168. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zheng, Y.; Yuan, G.; Yang, G.; He, F.; Chen, Y.-P. BMP Activity Is Required for Tooth Development from the Lamina to Bud Stage. J. Dent. Res. 2012, 91, 690–695. [Google Scholar] [CrossRef]
- Saber, S.E.-D. Tissue engineering in endodontics. J. Oral Sci. 2009, 51, 495–507. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Chang, Q.; Lu, J.; Wang, C. Growth factors and platelet-rich plasma: Promising biological strategies for early intervertebral disc degeneration. Int. Orthop. 2015, 39, 927–934. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma, V. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; De Angelis, M.G.C.; Lupi, S.M.; Baena, R.R.Y. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Raghavendra, S.S.; Gathani, K.M. Scaffolds in regenerative endodontics: A review. Dent. Res. J. 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Shah, N.; Logani, A.; Jadhav, G.R. Comparative outcome of revascularization in bilateral, non-vital, immature maxillary anterior teeth supplemented with or without platelet rich plasma: A case series. J. Conserv. Dent. 2013, 16, 568–572. [Google Scholar] [CrossRef]
- Vyas, T. Stem Cell in Modern Dentistry: A Review Article. Int. J. Res. Health Allied Sci. 2017, 3, 51–59. [Google Scholar]
- Albuquerque, M.T.P.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based Strategies for Regenerative Endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent. Clin. N. Am. 2017, 61, 689–711. [Google Scholar] [CrossRef]
- Huang, G.T.-J. A paradigm shift in endodontic management of immature teeth: Conservation of stem cells for regeneration. J. Dent. 2008, 36, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Saoud, T.M.A.; Ricucci, D.; Lin, L.M.; Gaengler, P. Regeneration and Repair in Endodontics—A Special Issue of the Regenerative Endodontics—A New Era in Clinical Endodontics. Dent. J. 2016, 4, 3. [Google Scholar] [CrossRef]
- Galler, K. Clinical procedures for revitalization: Current knowledge and considerations. Int. Endod. J. 2016, 49, 926–936. [Google Scholar] [CrossRef]
- Valsan, D.; Pulyodan, M.K.; Mohan, S.P.; Divakar, N.; Moyin, S.; Thayyil, S. Regenerative endodontics: A paradigm shift in clinical endodontics. J. Pharm. Bioallied Sci. 2020, 12, S20–S26. [Google Scholar] [CrossRef]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- American Association of Endodontists. Clinical Considerations for a Regenerative Procedure; Revised 6-8-16.; American Association of Endodontists: Chicago, IL, USA, 2016. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Ajram, J.; Khalil, I.; Gergi, R.; Zogheib, C. Management of an Immature Necrotic Permanent Molar with Apical Periodontitis Treated by Regenerative Endodontic Protocol Using Calcium Hydroxide and MM-MTA: A Case Report with Two Years Follow Up. Dent. J. 2019, 7, 1. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z. Regenerative Endodontic Treatment of a Maxillary Mature Premolar. Case Rep. Dent. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Nagas, E.; Uyanik, M.O.; Cehreli, Z.C. Revitalization of necrotic mature permanent incisors with apical periodontitis: A case report. Restor. Dent. Endod. 2018, 43, e31. [Google Scholar] [CrossRef] [PubMed]
- Žižka, R.; Šedý, J.; Voborná, I. Retreatment of failed revascularization/revitalization of immature permanent tooth—A case report. J. Clin. Exp. Dent. 2018, 10, e185–e188. [Google Scholar] [CrossRef]
- Suresh, N.; Arul, B.; Kowsky, D.; Natanasabapathy, V. Successful Regenerative Endodontic Procedure of a Nonvital Immature Permanent Central Incisor Using Amniotic Membrane as a Novel Scaffold. Dent. J. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Samra, R.A.A.; El Backly, R.M.; Aly, H.M.; Nouh, S.R.; Moussa, S.M. Revascularization in Mature Permanent Teeth with Necrotic Pulp and Apical Periodontitis: Case Series. Alex. Dent. J. 2018, 43, 7–12. [Google Scholar] [CrossRef]
- Moodley, D.S.; Peck, C.; Moodley, T.; Patel, N. Management of necrotic pulp of immature permanent incisor tooth: A regenerative endodontic treatment protocol: Case report. S. Afr. Dent. J. 2017, 72, 122–125. [Google Scholar]
- Gürhan, C.; Köseler, İ.; Güneri, P.; Çalışkan, K. Diagnosis and Regenerative Endodontic Treatment of Mandibular Premolar with Type II Dens Invaginatus: A Rare Case Report. J. Dent. Oral Biol. 2017, 2, 1081. [Google Scholar]
- Carmen, L.; Asunción, M.; Beatriz, S.; Rosa, Y.V. Revascularization in Immature Permanent Teeth with Necrotic Pulp and Apical Pathology: Case Series. Case Rep. Dent. 2017, 2017, 3540159. [Google Scholar] [CrossRef]
- Alagl, A.; Bedi, S.; Hassan, K.; Alhumaid, J. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: Clinical and cone-beam computed tomography evaluation. J. Int. Med Res. 2017, 45, 583–593. [Google Scholar] [CrossRef]
- Llaquet, M.; Mercadé, M.; Plotino, G. Regenerative endodontic procedures: A review of the literature and a case report of an immature central incisor. G. Ital. Endod. 2017, 31, 65–72. [Google Scholar] [CrossRef]
- Kashikar, R.; Chandak, M.; Mukherjee, P.; Patel, A. Regenerative Endodontic Treatment: A Case Report. IOSR J. Dent. Med. Sci. 2017, 16, 59–61. [Google Scholar] [CrossRef]
- Erkmen Almaz, M.; Akyıldız, M.B.; Şaroglu Sönmez, I. Healing with Incomplete Root Development After Forty Months Fol-lowing: A Case Report. Meandros Med. Dent. J. 2017, 18, 153–157. [Google Scholar] [CrossRef]
- Amitava, B.; Deepashree, P.; Sayani, D.; Ritam, K.; Shabnam, Z.; Gautam Kumar, K. Regenerative pulp therapy for immature non-vital tooth: A case report. Int. J. Appl. Dent. Sci. 2016, 2, 83–86. [Google Scholar]
- Saoud, T.M.; Martin, G.; Chen, Y.-H.M.; Chen, K.-L.; Chen, C.-A.; Songtrakul, K.; Malek, M.; Sigurdsson, A.; Lin, L.M. Treatment of Mature Permanent Teeth with Necrotic Pulps and Apical Periodontitis Using Regenerative Endodontic Procedures: A Case Series. J. Endod. 2016, 42, 57–65. [Google Scholar] [CrossRef] [PubMed]
- She, C.M.L.; Cheung, G.S.P.; Zhang, C. Long-Term Follow-Up of a Revascularized Immature Necrotic Tooth Evaluated by CBCT. Case Rep. Dent. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Al-Tammami, M.F.; Al-Nazhan, S.A. Retreatment of failed regenerative endodontic of orthodontically treated immature per-manent maxillary central incisor: A case report. Restor. Dent. Endod. 2017, 42, 65–71. [Google Scholar] [CrossRef]
- Subash, D.; Shoba, K.; Aman, S.; Bharkavi, S.K.I. Revitalization of an Immature Permanent Mandibular Molar with a Necrotic Pulp Using Platelet-Rich Fibrin: A Case Report. J. Clin. Diagn. Res. 2016, 10, ZD21–ZD23. [Google Scholar] [CrossRef] [PubMed]
- Aldakak, M.M.N.; Capar, I.D.; Rekab, M.S.; Abboud, S. Single-Visit Pulp Revascularization of a Nonvital Immature Permanent Tooth Using Biodentine. Iran. Endod. J. 2016, 11, 246–249. [Google Scholar]
- Silva, M.H.; Campos, C.N.; Coelho, M.S. Revascularization of an Immature Tooth with Apical Periodontitis Using Calcium Hy-droxide: A 3-year Follow-up. Open Dent. J. 2015, 9, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Guniganti, S.S.; Faizuddin, U.; Solomon, R.V.; Mattapathi, J. Revitalization of traumatized immature tooth with platelet-rich fibrin. Contemp. Clin. Dent. 2015, 6, 574–576. [Google Scholar] [CrossRef]
- Al-Nazhan, S.; Al-Ghamdi, N.S. Pulp revascularization of immature maxillary first premolar. J. Conserv. Dent. 2015, 18, 496–499. [Google Scholar] [CrossRef]
- Miltiadous, M.-E.A.; Floratos, S.G. Regenerative Endodontic Treatment as a Retreatment Option for a Tooth with Open Apex—A Case Report. Braz. Dent. J. 2015, 26, 552–556. [Google Scholar] [CrossRef]
- Chandran, V.; Chacko, V.; Sivadas, G. Management of a Nonvital Young Permanent Tooth by Pulp Revascularization. Int. J. Clin. Pediatr. Dent. 2014, 7, 213–216. [Google Scholar] [CrossRef]
- Aggarwal, G.; Bogra, P.; Singh, S.V.; Gupta, S.; Manchanda, S.; Saini, N. Regeneration of Human Dental Pulp: A Myth or Reality? A Case Report. Endodontology 2014, 26, 317–322. [Google Scholar]
- Kaya-Büyükbayram, I.; Ozalp, S.; Aytugar, E.; Aydemir, S. Regenerative Endodontic Treatment of an Infected Immature Dens Invaginatus with the Aid of Cone-Beam Computed Tomography. Case Rep. Dent. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Johns, D.A.; Shivashankar, V.Y.; Krishnamma, S.; Johns, M. Use of photoactivated disinfection and platelet-rich fibrin in regenerative Endodontics. J. Conserv. Dent. 2014, 17, 487–490. [Google Scholar] [CrossRef]
- Güven Polat, G.; Yıldırım, C.; Akgün, O.M.; Altun, C.; Dinçer, D.; Ozkan, C.K. The use of platelet rich plasma in the treatment of immature tooth with periapical lesion: A case report. Restor. Dent. Endod. 2014, 39, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.M.K.; Yadav, S.S.; Kumar, S.R.M. Revascularization of Immature Mandibular Premolar with Pulpal Necrosis—A Case Report. J. Clin. Diagn. Res. 2014, 8, ZD29–ZD31. [Google Scholar]
- Forghani, M.; Parisay, I.; Maghsoudlou, A. Apexogenesis and revascularization treatment procedures for two traumatized immature permanent maxillary incisors: A case report. Restor. Dent. Endod. 2013, 38, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Narang, I.; Mittal, N.; Mishra, N. Platelet-rich fibrin-mediated revitalization of immature necrotic tooth. Contemp. Clin. Dent. 2013, 4, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Park, H.-J.; Yeom, J.-H.; Seo, J.-S.; Ryu, G.-J.; Park, K.-H.; Shin, S.-I.; Kim, S.-Y. Long-term follow-ups of revascularized immature necrotic teeth: Three case reports. Int. J. Oral Sci. 2012, 4, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Miglani, S.; Singla, M. Conventional apexification and revascularization induced maturogenesis of two non-vital, immature teeth in same patient: 24 months follow up of a case. J. Conserv. Dent. 2012, 15, 68–72. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Isbitiren, B.; Sara, S.; Erbas, G. Regenerative Endodontic Treatment (Revascularization) of Immature Necrotic Molars Medicated with Calcium Hydroxide: A Case Series. J. Endod. 2011, 37, 1327–1330. [Google Scholar] [CrossRef]
- Thomson, A.; Kahler, B. Regenerative endodontics—Biologically-based treatment for immature permanent teeth: A case report and review of the literature. Aust. Dent. J. 2010, 55, 446–452. [Google Scholar] [CrossRef]
- Petrino, J.A.; Boda, K.K.; Shambarger, S.; Bowles, W.R.; McClanahan, S.B. Challenges in Regenerative Endodontics: A Case Series. J. Endod. 2010, 36, 536–541. [Google Scholar] [CrossRef]
- Levin, L.G. Pulp and Periradicular Testing. J. Endod. 2013, 39 (Suppl. S3), S13–S19. [Google Scholar] [CrossRef]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Mao, T.; Neelakantan, P. Three-dimensional imaging modalities in endodontics. Imaging Sci. Dent. 2014, 44, 177–183. [Google Scholar] [CrossRef]
- Sberna, M.T.; Rizzo, G.; Zacchi, E.; Capparè, P.; Rubinacci, A. A preliminary study of the use of peripheral quantitative computed tomography for investigating root canal anatomy. Int. Endod. J. 2009, 42, 66–75. [Google Scholar] [CrossRef]
- Kim, S.G. Infection and Pulp Regeneration. Dent. J. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Hargreaves, K.M. Microbial Modulation of Stem Cells and Future Directions in Regenerative Endodontics. J. Endod. 2017, 43, S95–S101. [Google Scholar] [CrossRef]
- Ayoub, S.; Cheayto, A.; Bassam, S.; Najar, M.; Berbéri, A.; Fayyad-Kazan, M. The Effects of Intracanal Irrigants and Medicaments on Dental-Derived Stem Cells Fate in Regenerative Endodontics: An update. Stem Cell Rev. Rep. 2020, 16, 650–660. [Google Scholar] [CrossRef]
- Bezgin, T.; Sönmez, H. Review of current concepts of revascularization/revitalization. Dent. Traumatol. 2015, 31, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.P.; Walsh, L.J. Optimizing Antimicrobial Agents in Endodontics. In Antibacterial Agents; Kumavath, R., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Elnaggar, S.E.; El Backly, R.M.; Zaazou, A.M.; Elshabrawy, S.M.; Abdallah, A.A. Effect of different irrigation protocols for applications in regenerative endodontics on mechanical properties of root dentin. Aust. Endod. J. 2020. [Google Scholar] [CrossRef]
- Martin, D.E.; De Almeida, J.F.A.; Henry, M.A.; Khaing, Z.Z.; Schmidt, C.E.; Teixeira, F.B.; Diogenes, A. Concentration-dependent Effect of Sodium Hypochlorite on Stem Cells of Apical Papilla Survival and Differentiation. J. Endod. 2014, 40, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Chugal, N.; Lin, L.M. Alkaline Materials and Regenerative Endodontics: A Review. Materials 2017, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Lin, L.M. A Review of Regenerative Endodontics: Current Protocols and Future Directions. J. Istanb. Univ. Fac. Dent. 2017, 51 (Suppl. S1), S41–S51. [Google Scholar] [CrossRef]
- Aksel, H.; Albanyan, H.; Bosaid, F.; Azim, A.A. Dentin Conditioning Protocol for Regenerative Endodontic Procedures. J. Endod. 2020, 46, 1099–1104. [Google Scholar] [CrossRef]
- Meschi, N.; Hilkens, P.; Van Gorp, G.; Strijbos, O.; Mavridou, A.; Perula, M.C.D.L.; Lambrichts, I.; Verbeken, E.; Lambrechts, P. Regenerative Endodontic Procedures Posttrauma: Immunohistologic Analysis of a Retrospective Series of Failed Cases. J. Endod. 2019, 45, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kontakiotis, E.G.; Filippatos, C.G.; Tzanetakis, G.N.; Agrafioti, A. Regenerative Endodontic Therapy: A Data Analysis of Clinical Protocols. J. Endod. 2015, 41, 146–154. [Google Scholar] [CrossRef]
- Hameed, M.H.; Gul, M.; Ghafoor, R.; Badar, S.B. Management of Immature Necrotic Permanent Teeth with Regenerative Endodontic Procedures—A Review of Literature. J. Pak. Med. Assoc. 2019, 69, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhujiang, A.; Kim, S.G. Regenerative Endodontic Treatment of an Immature Necrotic Molar with Arrested Root Development by Using Recombinant Human Platelet-derived Growth Factor: A Case Report. J. Endod. 2016, 42, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Laureys, W.G.; Cuvelier, C.A.; Dermaut, L.R.; De Pauw, G.A. The Critical Apical Diameter to Obtain Regeneration of the Pulp Tissue after Tooth Transplantation, Replantation, or Regenerative Endodontic Treatment. J. Endod. 2013, 39, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Saoud, T.M.A.; Sigurdsson, A.; Rosenberg, P.A.; Lin, L.M.; Ricucci, D. Treatment of a Large Cystlike Inflammatory Periapical Lesion Associated with Mature Necrotic Teeth Using Regenerative Endodontic Therapy. J. Endod. 2014, 40, 2081–2086. [Google Scholar] [CrossRef]
- Paryani, K.; Kim, S.G. Regenerative Endodontic Treatment of Permanent Teeth after Completion of Root Development: A Report of 2 Cases. J. Endod. 2013, 39, 929–934. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, X.; Zhu, J.; Su, C.; Yang, Y.; Meng, L. Influence of Apical Diameter on the Outcome of Regenerative Endodontic Treatment in Teeth with Pulp Necrosis: A Review. J. Endod. 2018, 44, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Yaripour, S.; Sharifi, F.; Kinoshita, J.-I. A Review on Triple Antibiotic Paste as a Suitable Material Used in Regenerative Endodontics. Iran. Endod. J. 2018, 13, 1–6. [Google Scholar]
- Kirchhoff, A.L.; Raldi, D.P.; Salles, A.C.; Cunha, R.S.; Mello, I. Tooth discolouration and internal bleaching after the use of triple antibiotic paste. Int. Endod. J. 2015, 48, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, B.; Trope, M. Pulp revascularization of a necrotic infected immature permanent tooth: Case report and review of the literature. Pediatr. Dent. 2007, 29, 47–50. [Google Scholar]
- Iwaya, S.-I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.-H.; Chen, K.-L.; Chen, C.-A.; Tayebaty, F.; Rosenberg, P.A.; Lin, L.M. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int. Endod. J. 2011, 45, 294–305. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of Dentin Conditioning with Intracanal Medicaments on Survival of Stem Cells of Apical Papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Tandan, M.; Hegde, M.N.; Hegde, P. Effect of four different intracanal medicaments on the apical seal of the root canal system: A dye extraction study. Indian J. Dent. Res. 2014, 25, 607. [Google Scholar] [CrossRef]
- Kharchi, A.S.; Tagiyeva-Milne, N.; Kanagasingam, S. Regenerative Endodontic Procedures, Disinfectants and Outcomes: A Sys-tematic Review. Prim. Dent. J. 2020, 9, 65–84. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Farik, B.; Munksgaard, E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002, 18, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Kahler, S.L.; Shetty, S.; Andreasen, F.M.; Kahler, B. The Effect of Long-term Dressing with Calcium Hydroxide on the Fracture Susceptibility of Teeth. J. Endod. 2018, 44, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Latham, J.; Fong, H.; Jewett, A.; Johnson, J.D.; Paranjpe, A. Disinfection Efficacy of Current Regenerative Endodontic Protocols in Simulated Necrotic Immature Permanent Teeth. J. Endod. 2016, 42, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chen, L.-P. Radiographic outcome of necrotic immature teeth treated with two endodontic techniques: A retrospective analysis. Biomed. J. 2016, 39, 366–371. [Google Scholar] [CrossRef]
- Chueh, L.H.; Ho, Y.C.; Kuo, T.C.; Lai, W.H.; Chen, Y.H.; Chiang, C.P. Regenerative endodontic treatment for necrotic immature permanent teeth. J. Endod. 2009, 35, 160–164. [Google Scholar] [CrossRef]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Estrada, M.M.; Álvarez López, B. Biomateriales tissue engineering and treatment of tooth with apex unripe: Revascularization. J. Dent. Health Oral Disord. Ther. 2018, 9, 1. [Google Scholar] [CrossRef]
- Lourenço Neto, N.; Marques, N.C.; Fernandes, A.P.; Rodini, C.O.; Sakai, V.T.; Abdo, R.C.; Machado, M.A.; Santos, C.F.; Oliveira, T.M. Immunolocalization of dentin matrix protein-1 in human primary teeth treated with different pulp capping materials. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 165–169. [Google Scholar] [CrossRef]
- Staniowski, T.; Brzęcka, D.M. Novel Bioceramic Root Repair Materials: Review of the Literature. Dent. Med. Probl. 2016, 53, 551–558. [Google Scholar] [CrossRef]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.K.; Case, P.; Thomson, A.; Holcombe, T. Revascularization Outcomes: A Prospective Analysis of 16 Consecutive Cases. J. Endod. 2014, 40, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Harlamb, S.C. Management of incompletely developed teeth requiring root canal treatment. Aust. Dent. J. 2016, 61, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Shokouhinejad, N.; Nekoofar, M.H.; Pirmoazen, S.; Shamshiri, A.R.; Dummer, P.M. Evaluation and Comparison of Occurrence of Tooth Discoloration after the Application of Various Calcium Silicate–based Cements: An Ex Vivo Study. J. Endod. 2016, 42, 140–144. [Google Scholar] [CrossRef]
- Yoldaş, S.E.; Bani, M.; Atabek, D.; Bodur, H. Comparison of the Potential Discoloration Effect of Bioaggregate, Biodentine, and White Mineral Trioxide Aggregate on Bovine Teeth: In Vitro Research. J. Endod. 2016, 42, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Marconyak, L.J., Jr.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef]
- Altunsoy, M.; Tanrıver, M.; Ok, E.; Kucukyilmaz, E. Shear Bond Strength of a Self-adhering Flowable Composite and a Flowable Base Composite to Mineral Trioxide Aggregate, Calcium-enriched Mixture Cement, and Biodentine. J. Endod. 2015, 41, 1691–1695. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Antunes, M.; Falacho, R.I.; Sequeira, D.B.; Roseiro, L.; Santos, J.M.; Ramos, J.C. Effect of restorative timing on shear bond strength of composite resin/calcium silicate–based cements adhesive interfaces. Clin. Oral Investig. 2020, 1–9. [Google Scholar] [CrossRef]
- Meraji, N.; Camilleri, J. Bonding over Dentin Replacement Materials. J. Endod. 2017, 43, 1343–1349. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Does Delayed Restoration Improve Shear Bond Strength of Different Restorative Protocols to Calcium Silicate-Based Cements? Materials 2018, 11, 2216. [Google Scholar] [CrossRef]
- Shetty, H.; Shetty, S.; Kakade, A.; Mali, S.; Shetty, A.; Neelakantan, P. Three-dimensional qualitative and quantitative analyses of the effect of periradicular lesions on the outcome of regenerative endodontic procedures: A prospective clinical study. Clin. Oral Investig. 2021, 25, 691–700. [Google Scholar] [CrossRef]
- Bose, R.; Nummikoski, P.; Hargreaves, K. A Retrospective Evaluation of Radiographic Outcomes in Immature Teeth with Necrotic Root Canal Systems Treated With Regenerative Endodontic Procedures. J. Endod. 2009, 35, 1343–1349. [Google Scholar] [CrossRef]
| Case No. | Patient Age | Tooth/Tooth Number | Root Canal Irrigants | Inter-visit Root Canal Filling | Scaffold; Capping Material | Recall | Results | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | Permanent immature with apical periodontitis/#36 | 2.5% NaOCl, 20% EDTA activated with EndoActivator in the coronal third | Calcium hydroxide | Blood clot; MM-MTA (Micro-Mega, Besançon CEDEX, France) | 2 y | The patient was reviewed after 3, 9, 12 and 24 months: -asymptomatic -physiological mobility -normal reaction to percussion and palpation -9 m: complete periapical healing, apical closure -1 y: increase in root length, dentin thickness -2 y: CBCT(cone-beam computed tomography): complete periapical healing, apical foramen closure of the both mesial canals and distolingual canal, resolution of the periapical lesion of the distobuccal canal, not completed apical closure | Ajram J et al. [55] |
| 2 | 15 | Permanent mature/#13 | 5.25% NaOCl, 17% EDTA | Double Antibiotics Paste (DAP) | Blood clot; Mineral trioxide aggregate (MTA) | 30 m | -3 m: asymptomatic, no response to vitality testing -6 m: nearly normal reaction to the electric pulp tester -12 m: positive response to pulp vitality testing, root wall thickening -30 m: normal reaction of pulp vitality | Qingan Xu et al. [56] |
| 3 | 21 | Permanent mature with apical periodontitis / #21, #22 | 5.25% NaOCl, saline, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; ProRoot MTA (Dentsply Tulsa Dental, Tulsa, OK, USA) | 5 y | -1 m: asymptomatic, decreased apical radiolucency, negative response to the cold test and the electric pulp test -5 y: asymptomatic, complete resolution of the apical lesion | Nagas E et al. [57] |
| 4 | 7 | Permanent immature with apical periodontitis—retreatment of failed revitalization / #11 | 5% NaOCl ultrasonically activated for 5 min, saline, 17% EDTA | Calcium hydroxide | Blood clot; White MTA (ProRoot MTA; Dentsply Tulsa Dental, Johanson City, TN) | 15 m | The recall visits were performed after 3, 5, 9, 12, and 15 months. -asymptomatic -negative response to cold or heat vitality tests -normal reaction to percussion and palpation tests -decrease in periapical lesion and root development -new mineralized tissue in contact with MTA | Žižka R et al. [58] |
| 5 | 18 | Permanent immature with apical periodontitis / #11 | 1% NaOCl, 17% EDTA | Calcium hydroxide (Prime Dental products, Mumbai, India) | Human Amniotic Membrane (ACTREC, Tata memorial hospital tissue bank, Mumbai, India); Biodentine (Septodont, France) | 3 y | The patient was reviewed after 15 days, 3 months, 19 months, and 36 months. -asymptomatic -pulp responded to vitality tests -healing of apical lesion and thickening of dentinal walls | Suresh N et al. [59] |
| 6-I | 24 | Permanent mature with apical periodontitis/#21 | 1.5% NaOCl, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; MTA (MTA Angelus) | 9 m | -a decrease in size of radiolucency -asymptomatic -no sensitivity to percussion and palpation -sinus tract healed -normal probing depths -physiological mobility -negative response to pulp tests | Rasha A. Abou Samra et al. [60] |
| 6-II | 25 | Permanent mature with apical periodontitis / #11 | 1.5% NaOCl, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; MTA (MTA Angelus) | 9 m | The patient was recalled at 3, 6 and 9 months after treatment. -asymptomatic -negative reaction to pulp vitality tests -healing of apical lesion -normal reaction to percussion | Rasha A. Abou Samra et al. [60] |
| 6-III | 20 | Permanent mature with apical periodontitis / #31 | 1.5% NaOCl, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; MTA (MTA Angelus) | 9 m | The follow-up visits were performed at 3, 6 and 9 months after treatment. -reduction of apical radiolucency size -no reaction to pulp vitality tests -normal probing depths | Rasha A. Abou Samra et al. [60] |
| 7 | 10 | Permanent immature with apical periodontitis / #11 | 1.5% NaOCl, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; Mineral trioxide aggregate (MTA; Dentsply Tulsa Dental, Tulsa, USA) | 5 m | -2 m: resolution of the apical lesion, apex closure, increased width of root walls, positive response to pulp vitality testing. -5 m: apex closure, positive response for vitality testing | Moodley, Desi Patel et al. [61] |
| 8 | 23 | Permanent immature with apical periodontitis / #45 | 2.5% NaOCl, 17% EDTA, sterile saline | Triple Antibiotics Paste (TAP) | Blood clot and PRF(platelet rich fibrin); MTA(ProRoot MTA, Dentsply) | 1 y | -8 m: asymptomatic, healing of periapical radiolucency -12 m: apical radiolucency not completely eradicated | Gürhan C et al. [62] |
| 9 | 8 | Permanent immature with apical periodontitis / #46 | 5% NaOCl | Triple Antibiotics Paste (TAP) | Blood clot; Mineral trioxide aggregate (MTA) | 1 y | -complete healing apical lesion -root canal walls thickening and -foramen apex progressed in closing | López Carmen et al. [63] |
| 10 | 8–11 | Permanent immature teeth with apical periodontitis / 24 incisors, 6 premolars | 2.5% NaOCl, sterile saline, 0.12% CHX, 17% EDTA | Triple Antibiotics Paste (TAP) | PRF; White MTA Dentsply Tulsa Dental, Tulsa, OK) | 1 y | -1 m: asymptomatic -12 m: resolution or decreasing in the apical lesion, negative response to percussion and palpation tests | Alagl A et al. [64] |
| 11 | 8 | Permanent immature with apical periodontitis / #21 | 5.25% NaOCl, sterile saline, 17% EDTA | Calcium hydroxide | Blood clot; Biodentine | 2 y | Time points for recall were at 1, 3, 6 and 12 months. -apical closure -no response to cold or electric test | Marc Llaquet et al. [65] |
| 12 | 16 | Permanent immature with apical periodontitis / #21 | 5.25% NaOCl, 0.2% CHX | Triple Antibiotics Paste (TAP) | Blood clot; MTA(ProRoot MTA, Dentsply) | 6 m | -3 m: asymptomatic -6 m: root maturation, healing of the radiolucent lesion | Rasika Kashikar et al. [66] |
| 13 | 13 | Permanent immature with apical periodontitis / #35 | 5.25% NaOCl | Triple Antibiotics Paste (TAP) | Blood clot; MTA(ProRoot MTA, Dentsply) | 1 y | -asymptomatic -positive response to electrical test -apical radiolucency resolved completely -no increase in root length and root wall thickness | Merve Erkmen Almaz et al. [67] |
| 14 | 8 | Permanent immature with apical periodontitis / #11 | 1.5% NaOCl, normal saline | Triple Antibiotics Paste (TAP) | Blood clot; White MTA (Proroot MTA, Densply) | 1 y | Recall visits were after 1, 3, 6, 9, and 12 months. -negative response to percussion and palpation tests -negative response to heat or an electric pulp tester (EPT) -root lengthening -thickening of the dentinal walls - apical closure -healing of the periapical lesion | Amitava Bora et al. [68] |
| 15 | 8–21 | Permanent mature with apical periodontitis / 4 anterior and 3 molar teeth | 2.5% NaOCl, 17% EDTA | Metapaste (Meta Biomed Co, Ltd., Chungbuk, Korea) | Blood clot; MTA (ProRoot MTA, Dentsply) | 8–26 m | -apical lesion healed in two teeth and healing in 5 teeth -no response to cold or electric pulp tester tests -asymptomatic | Tarek Mohamed Saoud et al. [69] |
| 16 | 12 | Permanent immature with apical periodontitis / #15 | 3% NaOCl, sterile saline | Calcium hydroxide (Calasept, Nordiska Dental, Ängelholm, Sweden) | Blood clot; Grey MTA (ProRoot, Dentsply Tulsa Dental, Johnson City, TN, USA) | 5.5 y | The patient was recalled after 4, 7, 18, 36, and 66 months -asymptomatic -4 m: decreasing of the periapical lesion -7 m: positive response to cold and electric pulp tests, root growth - 18 m: apical closure -3 y and 5.5 y: severe calcification of the canal, greyish discoloration of the cervical region of the crown | She CM et al. [70] |
| 17 | 12 | Permanent immature with apical periodontitis, endodontically treated / #11 | 5.25% NaOCl, sterile saline, 0.12% CHX | Double Antibiotics Paste (DAP) | Blood clot; CollaPlug (Zimmer Dental, Carlsbad, CA, USA), MTA (MTA-Angelus, Angelus, Londrina, PR, Brazil) | 3 y | -3 m and 6 m: asymptomatic -1 y: radiolucency healing -3 y: asymptomatic, root apex closed | Al-Tammami MF et al. [71] |
| 18 | 13 | Permanent immature with apical periodontitis / #37 | 5.25% NaOCl, saline | Triple Antibiotics Paste (TAP) | PRF; Biodentine (Septodont, France) | 1 y | Follow-up visits conducted after 3, 6, 9, and 12 months. -asymptomatic -9 m: positive response to sensibility tests -root lengthening and canal walls thickening - apical closure -decreasing of periapical lesion | Subash D et al. [72] |
| 19 | 11 | Permanent immature / #45 | 5.25% NaOCl, 17% EDTA, saline, 2% CHX | (one visit) | Blood clot; Biodentine (BD, Septodont, Saint Maur des Fosses, France) | 2 y | -complete root maturation -no pain -healed sinus tract | Aldakak MM et al. [73] |
| 20 | 6 | Permanent immature with apical periodontitis / #21 | 3% NaOCl | Calcium hydroxide (Fórmula & Ação, São Paulo, Brazil) | Blood clot; Collagen matrix (Hemospon; Technew Ind. & Comercio, Rio de Janeiro, Brazil), MTA (Angelus; Londrina, Paraná, Brazil) | 3 y | -6 m: root canal lengthening, absence of radiolucency -3 y: asymptomatic, crown discoloration, closed root apex | Silva MH et al. [74] |
| 21 | 14 | Permanent immature with apical periodontitis / #11 | 5.25% NaOCl, saline, 0.2% CHX | Triple Antibiotics Paste (TAP) | PRF; Gray MTA (ProRoot MTA, Dentsply) | 14 m | The patient was recalled after 3, 6, 9, 12, and 14 months. -asymptomatic -negative reaction to palpation and percussion tests -regression of periapical lesion -root apex closure | Faizuddin U et al. [75] |
| 22 | 8 | Permanent immature / #14 | 5.25% NaOCl | Calcium hydroxide (Vitapex; Neo Dental Chemical Products, Tokyo, Japan) | Blood clot; ProRoot MTA (Dentsply Maillefer, Ballaigues, Switzerland) | 3 y | -3 m: asymptomatic -6 m: root length increasing -12 m: asymptomatic, complete root development -36 m: apical closure | Al-Ghamdi NS et al. [76] |
| 23 | 14 | Permanent immature with apical periodontitis, endodontically treated / #11 | 2.5% NaOCl ultrasonically activated for 60 s, 17% EDTA | Triple Antibiotics Paste (TAP) | Blood clot; a sterile collagen membrane barrier (Collacote, Zimmer Dental, Carlsbad, CA, USA), MTA Dentsply Tulsa Dental, Tulsa, OK) | 3 y | -2 y: radiolucent lesion healed -3 y: asymptomatic, apical closure -no signs of thickening of canal walls and root lengthening -negative response to the cold test | Maria-Elpida A. Miltiadous et al. [77] |
| 24 | 10 | Permanent immature / # 11 | 5.25% NaOCl | Triple Antibiotics Paste (TAP) | Blood clot; White MTA (Angelus) | 1 y | -asymptomatic-normal response to percussion -normal probing depths -normal tooth mobility -negative response to cold test or EPT -increased root length -root wall thickening | Chandran V et al. [78] |
| 25 | 11 | Permanent immature with apical periodontitis / #11, #21 | 3% NaOCl, saline | Triple Antibiotics Paste (TAP) | Blood clot; MTA | 6 m | -no swelling, no pain -reduced tooth mobility -negative response to electrical or thermal tests -decrease of radiolucency -progressive root maturation | Aggarwal, Gaurav et al. [79] |
| 26 | 9 | Permanent immature dens invaginatus with apical periodontitis / #12 | 2.5% NaOCl, saline | Calcium hydroxide (Sultan Chemists Inc., Englewood, NJ, USA), after 3 weeks—Triple Antibiotics Paste (TAP) | Blood clot; MTA (MTA-A; Angelus, Londrina, Brazil) | 20 m | -12 m: complete healing of the periapical lesion, the apical foramen remain open, negative response to vitality testing -20 m: apical closure. | Kaya-Büyükbayram I et al. [80] |
| 27 | 9 | Permanent immature with apical periodontitis / #11 #21 | 5.25% NaOCl, saline | (one visit) | PRF; MTA(ProRoot MTA, Dentsply) | 10 m | -6 m: negative response to percussion and palpation tests, asymptomatic, root lengthening, thickening of the dentinal walls, decreasing of the apical lesion. -10 m: complete apical closure, negative response to electric pulp test | Johns DA et al. [81] |
| 28 | 10 | Permanent immature with apical periodontitis / #35 | 2.5% NaOCl | Triple Antibiotics Paste (TAP) | PRP (platelet rich plasma); MTA(ProRoot MTA, Dentsply) | 2 y | -6 m: asymptomatic, root development, healed apical radiolucency -2 y: root fully developed | Güven Polat G et al. [82] |
| 29 | 12 | Permanent immature with apical periodontitis / # 35 | 2.5% NaOCl, sterile saline, 2% CHX | Triple Antibiotics Paste (TAP) | Blood clot; MTA | 1 y | -3 m: asymptomatic, no sinus tract, healing of the radiolucency -6 m: continuation of apex development -1 y: closure of the apex, and dentinal walls thickening, below the MTA a mineralized bridge developed | Raju SM et al. [83] |
| 30 | 9 | Permanent immature with apical periodontitis / #11, #21 | 5.25% NaOCl, saline | Triple Antibiotics Paste (TAP) | Blood clot; White MTA | 18 m | The patient was follow-up after 6, 12, and 18. -asymptomatic -apical closure -increased root length -complete healing of periapical lesion | Forghani M et al. [84] |
| 31 | 11 | Permanent immature with apical periodontitis / #21 | 2.5% NaOCl | Triple Antibiotics Paste (TAP) | PRF; MTA | 1 y | The tooth was reviewed after 6 and 12 months. -asymptomatic -negative response to palpation and percussion tests -positive reaction to cold and electric sensitivity tests -root development -apical closure -resolution of apical lesion | Mishra N et al. [85] |
| 32 | 12 | Permanent immature with apical periodontitis / #35 | 3% NaOCl, sterile saline | Triple Antibiotics Paste (TAP) | Blood clot; MTA (Dentsply Tulsa Dental, Johnson City, TN, USA) | 2 y | -6 w: reduced the periapical lesion -2 y: complete root apex closure, increase in root wall thickness and in root length | Kim DS et al. [86] |
| 33 | 24 | Permanent immature with apical periodontitis / #21 | 5.25% NaOCl, distilled water, with 2% CHX | Triple Antibiotics Paste (TAP) | Blood clot; White MTA (ProRoot, Dentsply/Tulsa Dental, Tulsa, OK, USA) | 2 y | -dentinal walls thickening-root end closure and root elongation -apical lesion healed | Aggarwal V et al. [87] |
| 34 | 8–11 | Permanent immature with apical periodontitis / #26, #46, #47 | 2.5% NaOCl, sterile saline | Ca(OH)2 powder (Merck, Darmstadt, Germany) | Blood clot; MTA (Dentsply Tulsa Dental, Tulsa, OK) | 10 m | -thickening of root canal walls -continued apical development -asymptomatic -periapical lesion healing | Cehreli ZC et al. [88] |
| 35 | 12 | Permanent immature with apical periodontitis / #35 | 1% NaOCl | Triple Antibiotics Paste (TAP) | Blood clot; ProRoot white MTA (Dentsply Tulsa Dental, TN, USA) | 18 m | -asymptomatic -positive response to electric pulp vitality testing -normal reaction to percussion and palpation -root maturation -healing of the apical lesion | Thomson A et al. [89] |
| 36-I | 13 | Permanent immature with apical periodontitis / #11, #21 | 5.25% NaOCl, saline, 0,12% CHX | Triple Antibiotics Paste (TAP) | Blood clot; White MTA (Dentsply Tulsa Dental, Tulsa, OK) | 1 y | -asymptomatic -normal reaction to percussion and palpation -probing depths in normal limits -healing of the radiolucent lesions -#21: increased thickness of the apical area -#11: lack of increase in the thickness of the root walls or in the length of the root; no response to vitality testing | Petrino J et al. [90] |
| 36-II | 11 | Permanent immature with apical periodontitis / #45, #35 | 5.25% NaOCl, saline, 0,12% CHX | Triple Antibiotics Paste (TAP) | Blood clot; CollaPlug (Zimmer Dental, Carlsbad, CA, USA), MTA (Dentsply Tulsa Dental, Tulsa, OK) | 1 y | -asymptomatic -normal reaction to percussion or palpation -periapical lesions decreasing -root walls thickening -root length increasing -positive reaction to vitality testing | Petrino J et al. [90] |
| 36-III | 6 | Permanent immature with apical periodontitis / #11, #21 | 5.25% NaOCl, saline, 0.12% CHX | Triple Antibiotics Paste (TAP) | Blood clot; CollaPlug (Zimmer Dental, Carlsbad, CA, USA), MTA (Dentsply Tulsa Dental, Tulsa, OK) | 6 m | -asymptomatic -no reaction to palpation and percussion -healing of apical lesion -root walls thickening -increase in root length -negative response to vitality testing | Petrino J et al. [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupińska, A.M.; Skośkiewicz-Malinowska, K.; Staniowski, T. Different Approaches to the Regeneration of Dental Tissues in Regenerative Endodontics. Appl. Sci. 2021, 11, 1699. https://doi.org/10.3390/app11041699
Krupińska AM, Skośkiewicz-Malinowska K, Staniowski T. Different Approaches to the Regeneration of Dental Tissues in Regenerative Endodontics. Applied Sciences. 2021; 11(4):1699. https://doi.org/10.3390/app11041699
Chicago/Turabian StyleKrupińska, Anna M., Katarzyna Skośkiewicz-Malinowska, and Tomasz Staniowski. 2021. "Different Approaches to the Regeneration of Dental Tissues in Regenerative Endodontics" Applied Sciences 11, no. 4: 1699. https://doi.org/10.3390/app11041699
APA StyleKrupińska, A. M., Skośkiewicz-Malinowska, K., & Staniowski, T. (2021). Different Approaches to the Regeneration of Dental Tissues in Regenerative Endodontics. Applied Sciences, 11(4), 1699. https://doi.org/10.3390/app11041699






