Machine-Learning-Based Elderly Stroke Monitoring System Using Electroencephalography Vital Signals
Abstract
1. Introduction
2. Related Works
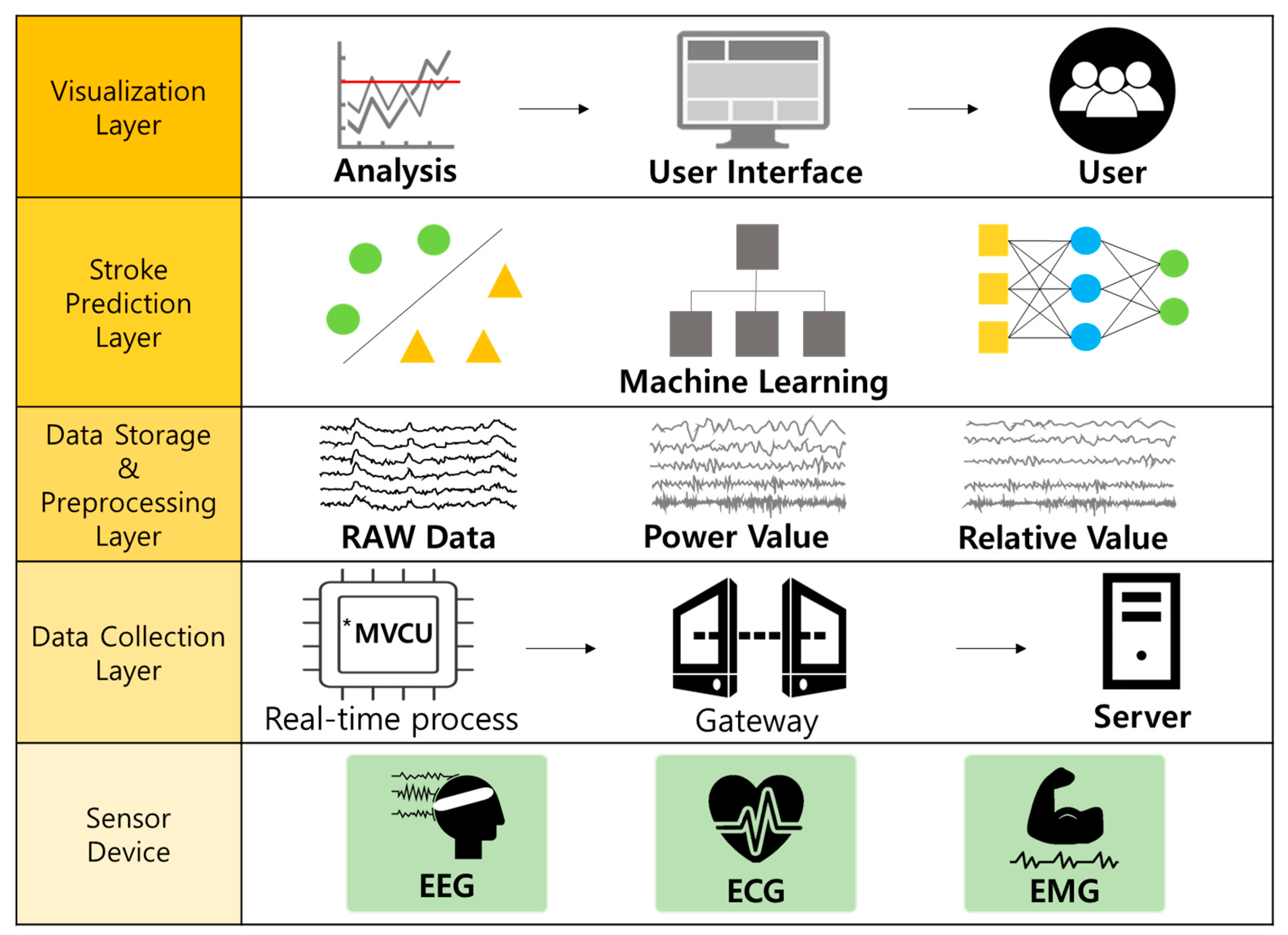
3. Elderly Stroke Monitoring System Based on Machine Learning and EEG
3.1. Real-Time EEG Data Collection
3.2. EEG Data Preprocessing
3.3. Attribute Definition and Extraction in EEG
3.4. Stroke Prediction Module for the Elderly Based on Machine Learning
4. Experiment and Analysis
4.1. Dataset and Experimental Environment
4.2. Performance Evaluation Measurement
4.3. Experiment Based on Machine-Learning Methodology
4.3.1. Predicting and Analyzing Stroke Diseases Based on Power Values
4.3.2. Predicting and Analyzing Stroke Diseases Based on Relative Values
4.4. An In-Depth Analysis Based on the Power and Relative Features of EEG
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Seo, K.-D.; Kang, M.J.; Kim, G.S.; Lee, J.H.; Suh, S.H.; Lee, K.-Y. National Trends in Clinical Outcomes of Endovascular Therapy for Ischemic Stroke in South Korea between 2008 and 2016. J. Stroke 2020, 22, 412–415. [Google Scholar] [CrossRef]
- Mackay, J.; Mensah, G.A. The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2004; pp. 22–43. [Google Scholar]
- Kim, J.Y.; Bae, H.-J. Spontaneous Intracerebral Hemorrhage: Management. J. Stroke 2017, 19, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B. Hypertension Mechanisms Causing Stroke. Clin. Exp. Pharmacol. Physiol. 1999, 26, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Hillis, A.E. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010, 9, 895–905. [Google Scholar] [CrossRef]
- Korpelainen, J.T.; Kauhanen, M.-L.; Kemola, H.; Malinen, U.; Myllylä, V.V. Sexual dysfunction in stroke patients. Acta Neurol. Scand. 1998, 98, 400–405. [Google Scholar] [CrossRef]
- Pikija, S.; Trkulja, V.; Ramesmayer, C.; Mutzenbach, J.S.; Killer-Oberpfalzer, M.; Hecker, C.; Bubel, N.; Füssel, M.U.; Sellner, J. Higher Blood Pressure during Endovascular Thrombectomy in Anterior Circulation Stroke Is Associated with Better Outcomes. J. Stroke 2018, 20, 373–384. [Google Scholar] [CrossRef]
- Boden-Albala, B.; Litwak, E.; Elkind, M.; Rundek, T.; Sacco, R.L. Social isolation and outcomes post stroke. Neurology 2005, 64, 1888–1892. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Bushnell, C.D.; Johnston, D.C.; Goldstein, L.B. Retrospective assessment of initial stroke severity: Comparison of the NIH stroke scale and the Canadian neurological scale. Stroke 2001, 32, 656–660. [Google Scholar] [CrossRef]
- Lee, M.; Ryu, J.; Kim, D. Automated epileptic seizure waveform detection method based on the feature of the mean slope of wavelet coefficient counts using a hidden Markov model and EEG signals. ETRI J. 2020, 42, 217–229. [Google Scholar] [CrossRef]
- Lyden, P.; Brott, T.; Tilley, B.; Welch, K.M.; Mascha, E.J.; Levine, S.; Haley, E.C.; Grotta, J.; Marler, J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994, 25, 2220–2226. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.M.; Park, T.H.; Lee, K.B.; Lee, S.J.; Cho, Y.J.; Lee, J.Y. Development of a stroke prediction model for Korean. J. Korean Neurol. Assoc. 2010, 28, 13–21. [Google Scholar]
- D’Agostino, R.B.; A Wolf, P.; Belanger, A.J.; Kannel, W.B. Stroke risk profile: Adjustment for antihypertensive medication. The Framingham Study. Stroke 1994, 25, 40–43. [Google Scholar] [CrossRef]
- Musuka, T.D.; Wilton, S.B.; Traboulsi, M.; Hill, M.D. Diagnosis and management of acute ischemic stroke: Speed is critical. Can. Med Assoc. J. 2015, 187, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.; McGee, D.; Castelli, W. Latest perspectives on cigarette smoking and cardiovascular disease: The Framingham Study. J. Card. Rehabil. 1984, 4, 267–277. [Google Scholar]
- Carroll, K.J. On the use and utility of the Weibull model in the analysis of survival data. Control. Clin. Trials 2003, 24, 682–701. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, P.; Liu, B.; Yao, Q.; Yan, K.; Zheng, Q.; Li, Y.; Zhang, L.; Li, M.; Wang, J.; et al. Time to recurrence after first-ever ischaemic stroke within 3 years and its risk factors in Chinese population: A prospective cohort study. BMJ Open 2019, 9, e032087. [Google Scholar] [CrossRef] [PubMed]
- Cicioğlu, M.; Çalhan, A. SDN-based wireless body area network routing algorithm for healthcare architecture. ETRI J. 2019, 41, 452–464. [Google Scholar] [CrossRef]
- Subasi, A.; Alkan, A.; Koklukaya, E.; Kiymik, M.K. Wavelet neural network classification of EEG signals by using AR model with MLE preprocessing. Neural Netw. 2005, 18, 985–997. [Google Scholar] [CrossRef]
- Guler, I.; Ubeyli, E.D. Multiclass Support Vector Machines for EEG-Signals Classification. IEEE Trans. Inf. Technol. Biomed. 2007, 11, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rim, B.; Sung, N.-J.; Min, S.; Hong, M. Deep Learning in Physiological Signal Data: A Survey. Sensors 2020, 20, 969. [Google Scholar] [CrossRef]
- Williams, G.W.; Lüders, H.O.; Brickner, A.; Goormastic, M.; Klass, D.W. Interobserver variability in EEG interpretation. Neurology 1985, 35, 1714. [Google Scholar] [CrossRef] [PubMed]
- Benbadis, S.R.; Lafrance, W.C.; Papandonatos, G.D.; Korabathina, K.; Lin, K.; Kraemer, H.C.; Workshop, F.T.N.T. Interrater reliability of EEG-video monitoring. Neurology 2009, 73, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Toraman, S.; Tuncer, S.A.; Balgetir, F. Is it possible to detect cerebral dominance via EEG signals by using deep learning? Med Hypotheses 2019, 131, 109315. [Google Scholar] [CrossRef]
- Sakhavi, S.; Guan, C.; Yan, S. Learning Temporal Information for Brain-Computer Interface Using Convolutional Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2018, 29, 5619–5629. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-H.; Shin, S.-B.; Kim, S.-D. Electroencephalography Based Fusion Two-Dimensional (2D)-Convolution Neural Networks (CNN) Model for Emotion Recognition System. Sensors 2018, 18, 1383. [Google Scholar] [CrossRef]
- Bălan, O.; Moise, G.; Moldoveanu, A.; Leordeanu, M.; Moldoveanu, F. Fear Level Classification Based on Emotional Dimensions and Machine Learning Techniques. Sensors 2019, 19, 1738. [Google Scholar] [CrossRef]
- Chambon, S.; Thorey, V.; Arnal, P.; Mignot, E.; Gramfort, A. DOSED: A deep learning approach to detect multiple sleep micro-events in EEG signal. J. Neurosci. Methods 2019, 321, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Faust, O.; Kannathal, N.; Chua, T.L.; Laxminarayan, S. Non-linear analysis of EEG signals at various sleep stages. Comput. Methods Programs Biomed. 2005, 80, 37–45. [Google Scholar] [CrossRef]
- Tian, X.; Deng, Z.; Ying, W.; Choi, K.-S.; Wu, D.; Qin, B.; Wang, J.; Shen, H.; Wang, S. Deep Multi-View Feature Learning for EEG-Based Epileptic Seizure Detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1962–1972. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H.; Subha, D.P. Automated EEG-based screening of depression using deep convolutional neural network. Comput. Methods Programs Biomed. 2018, 161, 103–113. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K. Detection of Early Stage Alzheimer’s Disease using EEG Relative Power with Deep Neural Network. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 352–355. [Google Scholar]
- Finnigan, S.; Wong, A.; Read, S.J. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clin. Neurophysiol. 2016, 127, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.; Jordan, K.G. Regional Attenuation without Delta (RAWOD): A distinctive EEG pattern that can aid in the diagnosis and management of severe acute ischemic stroke. Am. J. Electroneurodiagnostic Technol. 2005, 45, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Varelas, P.N.; Hacein-Bey, L. Ischemic Stroke, Hyperperfusion Syndrome, Cerebral Sinus Thrombosis, and Critical Care Seizures. Seizures Crit. Care 2017, 14, 155–186. [Google Scholar] [CrossRef]
- Ip, Z.; Rabiller, G.; He, J.W.; Yao, Z.; Akamatsu, Y.; Nishijima, Y.; Liu, J.; Yazdan-Shahmorad, A. Cortical stroke affects activity and stability of theta/delta states in remote hippocampal regions. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5225–5228. [Google Scholar]
- Shanthi, D.; Sahoo, G.; Saravanan, N. Designing an artificial neural network model for the prediction of thrombo-embolic stroke. Int. J. Biom. Bioinform. 2009, 3, 10–18. [Google Scholar]
- Nwosu, C.S.; Dev, S.; Bhardwaj, P.; Veeravalli, B.; John, D. Predicting Stroke from Electronic Health Records. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5704–5707. [Google Scholar]
- Bentley, P.; Ganesalingam, J.; Jones, A.L.C.; Mahady, K.; Epton, S.; Rinne, P.; Sharma, P.; Halse, O.; Mehta, A.; Rueckert, D. Prediction of stroke thrombolysis outcome using CT brain machine learning. NeuroImage Clin. 2014, 4, 635–640. [Google Scholar] [CrossRef]
- Hanifa, S.M.; Raja, S.K. Stroke risk prediction through non-linear support vector classification models. Int. J. Adv. Res. Comput. Sci. 2010, 1, 47–53. [Google Scholar]
- Yu, J.; Park, S.; Kwon, S.-H.; Ho, C.M.B.; Pyo, C.-S.; Lee, H. AI-based Stroke Disease Prediction System Using Real-Time Electromyography Signals. Appl. Sci. 2020, 10, 6791. [Google Scholar] [CrossRef]
- Yu, J.; Kim, D.; Park, H.; Chon, S.-C.; Cho, K.H.; Kim, S.-J.; Yu, S.; Park, S.; Hong, S. Semantic Analysis of NIH Stroke Scale using Machine Learning Techniques. In Proceedings of the International Conference on Platform Technology and Service (PlatCon), Jeju, Korea, 28–30 January 2019; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Yu, J.; Park, S.; Lee, H.; Pyo, C.-S.; Lee, Y.S. An Elderly Health Monitoring System Using Machine Learning and In-Depth Analysis Techniques on the NIH Stroke Scale. Mathematics 2020, 8, 1115. [Google Scholar] [CrossRef]
- Amini, L.; Azarpazhouh, R.; Farzadfar, M.T.; Mousavi, S.A.; Jazaieri, F.; Khorvash, F.; Norouzi, R.; Toghianfar, N. Prediction and Control of Stroke by Data Mining. Int. J. Prev. Med. 2013, 4, S245–S249. [Google Scholar] [PubMed]
- Oh, I.-S.; Lee, J.-S.; Moon, B.-R. Hybrid genetic algorithms for feature selection. IEEE Trans. Pattern Anal. Mach. Intell. 2004, 26, 1424–1437. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann: 225 Wyman Street, Waltham, MA 02451, USA, 2011. [Google Scholar]
- Hall, M. Correlation-based Feature Selection for Machine Learning. Ph.D. Thesis, Deptartment of Computer Science, Waikato University, Hamilton, NZ, USA, 1998. [Google Scholar]
- Grandini, M.; Bagli, E.; Visani, G. Metrics for multi-class classification: An overview. arXiv 2020, arXiv:2008.05756. [Google Scholar]








| Contents | Features | Meaning and Explanation | ||
|---|---|---|---|---|
| No. | ||||
| 1 ~ 66 | 6 Channel (Fz, Oz, T7, T8, C1, C2) | Delta () | Delta power (1~4 Hz) | |
| Theta () | Theta power (4~8 Hz) | |||
| Alpha () | Alpha power (8~13 Hz) | |||
| Beta () | Beta power (14~30 Hz) | |||
| Gamma () | Gamma power (30 Hz or more) | |||
| Low_Beta | Low beta power (12~25 Hz) | |||
| High_Beta | High beta power (25~30 Hz) | |||
| Theta_to_Beta | Value of the beta ratio in theta (extracting abnormal theta waves) | |||
| Delta divided by Alpha (DAR) IDAR | Ratio of mean power (Delta/Alpha) Inverse ratio of DAR (Alpha/Delta) | |||
| PRI | PRI power ratio index (delta+theta to alpha+beta), Low frequency to high frequency | |||
| 67 | Class Labeling | Normal or Stroke Elderly | ||
| No. | Features | Meaning and Explanation |
|---|---|---|
| 1 | Fz(Theta) | Theta power of Fz channel |
| 2 | Fz(Theta_to_Beta) | Beta ratio value in theta of Fz channel |
| 3 | Fz(IDAR) | Inverse ratio of DAR of Fz channel |
| 4 | Fz(RRI) | Power ratio index of Fz channel |
| 5 | T7(RRI) | Power ratio index of T7 channel |
| 6 | C1(DAR) | Ratio of mean power of C1 channel |
| 7 | Oz(IDAR) | Inverse ratio of DAR of Oz channel |
| 8 | T8(RRI) | Power ratio index of T8 channel |
| No. | Features | Meaning and Explanation |
|---|---|---|
| 1 | Fz(Alpha) | Alpha power of Fz channel (8~13 Hz) |
| 2 | Fz(Beta) | Beta power of Fz channel (14~30 Hz) |
| 3 | Fz(Gamma) | Gamma power of Fz channel (30 Hz or more) |
| 4 | Fz(IDAR) | Inverse ratio of DAR of Fz channel |
| 5 | T7(Theta) | Theta power of T7 channel (4~8 Hz) |
| 6 | T7(Alpha) | Alpha power of T7 channel (8~13 Hz) |
| 7 | T7(Gamma) | Gamma power of T7 channel (30 Hz or more) |
| 8 | C1(Theta) | Theta power of C1 channel (4~8 Hz) |
| 9 | Oz(Beta) | Beta power of Oz channel (14~30 Hz) |
| 10 | Oz(Gamma) | Gamma power of Oz channel (30 Hz or more) |
| 11 | C2(Beta) | Beta power of C2 channel (14~30 Hz) |
| 12 | C2(Gamma) | Gamma power of C2 channel (30 Hz or more) |
| 13 | C2(Low_Beta) | Low beta power of C2 channel (12~25 Hz) |
| 14 | T8(Theta) | Theta power of T8 channel (4~8 Hz) |
| 15 | T8(Gamma) | Gamma power of T8 channel (30 Hz or more) |
| True Condition | |||
| Stroke Elderly | Normal Elderly | ||
| Predicted Condition | Stroke Elderly | True Positive | False Positive |
| Normal Elderly | False Negative | True Negative | |
| Data Sets | Train (67)/ Test (33) | Train (80)/ Test (20) | 5-Fold CV 1 | 10-Fold CV | 20-Fold CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Acc. 2 | F1 3 | Acc. | F1 | Acc. | F1 | Acc. | F1 | Acc. | F1 | |
| RandomForest | 92.37 | 92.4 | 91.92 | 91.9 | 90.66 | 90.6 | 90.95 | 90.9 | 91.07 | 91.1 | |
| C4.5 DT 4 | 88.35 | 88.3 | 87.15 | 87.2 | 87.81 | 87.8 | 88.18 | 88.2 | 87.98 | 88.0 | |
| C5.0 DT | 84.89 | 84.8 | 83.14 | 83.2 | 83.92 | 83.9 | 84.67 | 84.5 | 84.43 | 84.4 | |
| Naive Bayes | 75.67 | 75.1 | 75.43 | 75.0 | 74.44 | 73.8 | 74.46 | 73.8 | 74.47 | 73.8 | |
| LR 5 | 84.90 | 84.9 | 85.29 | 85.3 | 83.95 | 83.9 | 83.96 | 84.0 | 84.06 | 84.1 | |
| MLP(ANN) 6 | 89.17 | 89.2 | 90.47 | 90.6 | 89.64 | 88.9 | 90.68 | 90.7 | 89.89 | 89.8 | |
| SVM | 81.56 | 81.5 | 82.08 | 82.1 | 82.44 | 82.4 | 82.72 | 82.7 | 82.68 | 82.7 | |
| ADTree | 85.63 | 85.6 | 86.82 | 86.8 | 88.86 | 88.9 | 89.91 | 89.9 | 89.66 | 89.7 | |
| C&RT | 83.09 | 83.1 | 83.22 | 83.2 | 83.27 | 83.3 | 84.28 | 84.2 | 84.32 | 84.3 | |
| QUEST | 78.57 | 78.6 | 77.92 | 77.9 | 78.36 | 78.4 | 79.72 | 79.7 | 79.59 | 79.6 | |
| Data Sets | Train (67)/ Test (33) | Train (80)/ Test (20) | 5-Fold CV | 10-Fold CV | 20-Fold CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Recall | Prec. 1 | Recall | Prec. | Recall | Prec. | Recall | Prec. | Recall | Prec. | |
| RandomForest | 92.4 | 92.6 | 91.9 | 92.1 | 90.7 | 90.9 | 91.0 | 91.2 | 91.1 | 91.3 | |
| C4.5 DT | 88.4 | 88.4 | 87.2 | 87.2 | 87.8 | 87.8 | 88.2 | 88.2 | 88.0 | 88.0 | |
| C5.0 DT | 84.9 | 84.9 | 83.2 | 83.1 | 83.9 | 83.9 | 84.7 | 84.6 | 84.4 | 84.4 | |
| Naive Bayes | 75.7 | 78.7 | 75.4 | 78.5 | 74.4 | 77.1 | 74.5 | 77.2 | 74.5 | 77.2 | |
| LR | 84.9 | 84.9 | 85.3 | 85.3 | 83.9 | 84.0 | 84.0 | 84.0 | 84.1 | 84.1 | |
| MLP(ANN) | 89.2 | 89.2 | 90.5 | 90.7 | 88.9 | 89.4 | 90.7 | 90.7 | 89.8 | 89.9 | |
| SVM | 81.6 | 81.5 | 82.1 | 82.1 | 82.4 | 82.3 | 82.7 | 82.6 | 82.6 | 82.7 | |
| ADTree | 85.6 | 85.7 | 86.9 | 86.8 | 88.8 | 88.9 | 89.9 | 89.9 | 89.7 | 89.7 | |
| C&RT | 83.1 | 83.1 | 83.2 | 83.2 | 83.3 | 83.3 | 84.3 | 84.2 | 84.3 | 84.3 | |
| QUEST | 78.5 | 78.6 | 77.9 | 77.9 | 78.3 | 78.4 | 79.7 | 79.7 | 79.5 | 79.6 | |
| Data Sets | Train (67)/ Test (33) | Train (80)/ Test (20) | 5-Fold CV | 10-Fold CV | 20-Fold CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Acc. | F1 | Acc. | F1 | Acc. | F1 | Acc. | F1 | Acc. | F1 | |
| RandomForest | 89.19 | 89.2 | 89.77 | 89.8 | 89.76 | 89.8 | 90.50 | 90.5 | 90.16 | 90.2 | |
| C4.5 DT | 81.38 | 81.4 | 83.56 | 83.6 | 82.49 | 82.5 | 82.86 | 82.8 | 82.49 | 82.5 | |
| C5.0 DT | 77.05 | 77.1 | 78.85 | 78.9 | 77.69 | 77.7 | 78.73 | 78.7 | 78.81 | 78.8 | |
| Naive Bayes | 72.99 | 72.9 | 73.74 | 73.6 | 73.53 | 83.4 | 73.45 | 73.3 | 73.39 | 73.2 | |
| LR | 78.54 | 78.5 | 79.11 | 79.1 | 78.70 | 78.7 | 78.68 | 78.7 | 78.60 | 78.6 | |
| MLP(ANN) | 84.35 | 84.4 | 86.41 | 86.4 | 86.06 | 86.1 | 87.11 | 87.1 | 87.03 | 87.0 | |
| SVM | 74.21 | 74.2 | 74.82 | 74.8 | 73.91 | 73.9 | 74.65 | 74.7 | 74.42 | 74.4 | |
| ADTree | 79.91 | 79.9 | 79.36 | 79.4 | 79.33 | 79.3 | 79.48 | 79.5 | 79.31 | 79.3 | |
| C&RT | 80.12 | 80.1 | 80.34 | 80.3 | 80.24 | 80.2 | 80.47 | 80.5 | 80.42 | 80.4 | |
| QUEST | 73.88 | 73.9 | 73.91 | 73.9 | 73.55 | 73.6 | 73.94 | 73.7 | 73.75 | 73.8 | |
| Data Sets | Train (67)/ Test (33) | Train (80)/ Test (20) | 5-Fold CV | 10-Fold CV | 20-Fold CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Recall | Prec. | Recall | Prec. | Recall | Prec. | Recall | Prec. | Recall | Prec. | |
| RandomForest | 89.2 | 89.2 | 89.8 | 89.8 | 89.7 | 89.9 | 90.5 | 90.6 | 90.1 | 90.3 | |
| C4.5 DT | 81.4 | 81.4 | 83.6 | 83.6 | 82.5 | 82.6 | 82.9 | 83.0 | 82.4 | 82.6 | |
| C5.0 DT | 77.0 | 77.4 | 78.8 | 78.9 | 77.7 | 77.7 | 78.6 | 78.9 | 78.8 | 78.9 | |
| Naive Bayes | 73.0 | 73.5 | 73.7 | 74.3 | 73.5 | 74.2 | 73.4 | 74.1 | 73.4 | 74.0 | |
| LR | 78.5 | 78.5 | 79.1 | 79.1 | 78.7 | 78.7 | 78.7 | 78.7 | 78.6 | 78.6 | |
| MLP(ANN) | 84.4 | 84.4 | 86.4 | 86.5 | 86.1 | 86.1 | 87.1 | 87.1 | 86.9 | 87.1 | |
| SVM | 74.0 | 74.6 | 74.6 | 74.9 | 73.7 | 74.3 | 74.5 | 74.8 | 74.2 | 74.6 | |
| ADTree | 79.9 | 80.0 | 79.2 | 79.5 | 79.2 | 79.4 | 79.5 | 79.6 | 79.1 | 79.4 | |
| C&RT | 80.1 | 80.1 | 80.3 | 80.3 | 80.2 | 80.3 | 80.5 | 80.6 | 80.4 | 80.5 | |
| QUEST | 73.9 | 73.9 | 73.9 | 73.9 | 73.4 | 73.7 | 73.8 | 73.9 | 73.5 | 73.9 | |
| Rules | The Rule and In-Depth Analysis |
|---|---|
| 1 | IF Fz_PRI 102.4606 then Stroke. |
| 2 | IF Fz_PRI > 102.4606 and Fz_Theta 0.000039 and Fz_PRI 2,526,416.637 and C1_DAR 84.787 then Normal. |
| 3 | IF Fz_PRI > 102.4606 and Fz_Theta 0.000039 and Fz_PRI 2,526,416.637 and C1_DAR > 84.787 then Stroke. |
| 4 | IF Fz_PRI > 102.4606 and Fz_Theta 0.000039 and Fz_PRI > 2,526,416.637 and T7_PRI 4.483 then Normal. |
| 5 | IF Fz_PRI > 102.4606 and Fz_Theta 0.000039 and Fz_PRI > 2,526,416.637 and T7_PRI > 4.483 and Fz_PRI 2,836,110.757 then Normal. |
| 6 | IF Fz_PRI > 102.4606 and Fz_Theta 0.000039 and Fz_PRI > 2,526,416.637 and T7_PRI > 4.483 and Fz_PRI > 2,836,110.757 then Stroke. |
| 7 | IF Fz_PRI > 102.4606 and Fz_Theta > 0.000039 then Stroke. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-A.; Park, S.; Jun, J.-A.; Ho, C.M.B.; Pyo, C.-S.; Lee, H.; Yu, J. Machine-Learning-Based Elderly Stroke Monitoring System Using Electroencephalography Vital Signals. Appl. Sci. 2021, 11, 1761. https://doi.org/10.3390/app11041761
Choi Y-A, Park S, Jun J-A, Ho CMB, Pyo C-S, Lee H, Yu J. Machine-Learning-Based Elderly Stroke Monitoring System Using Electroencephalography Vital Signals. Applied Sciences. 2021; 11(4):1761. https://doi.org/10.3390/app11041761
Chicago/Turabian StyleChoi, Yoon-A, Sejin Park, Jong-Arm Jun, Chee Meng Benjamin Ho, Cheol-Sig Pyo, Hansung Lee, and Jaehak Yu. 2021. "Machine-Learning-Based Elderly Stroke Monitoring System Using Electroencephalography Vital Signals" Applied Sciences 11, no. 4: 1761. https://doi.org/10.3390/app11041761
APA StyleChoi, Y.-A., Park, S., Jun, J.-A., Ho, C. M. B., Pyo, C.-S., Lee, H., & Yu, J. (2021). Machine-Learning-Based Elderly Stroke Monitoring System Using Electroencephalography Vital Signals. Applied Sciences, 11(4), 1761. https://doi.org/10.3390/app11041761






