Abstract
Recent success of systemic therapeutic agents, including combination immunotherapy, could promote a change in the treatment strategy in patients with advanced hepatocellular carcinoma (HCC). Although hepatic arterial infusion chemotherapy (HAIC) is a treatment option for advanced HCC in Japan, it is not recommended by other guidelines. We discuss the clinical benefits of HAIC compared to sorafenib. The clinical benefits of HAIC are as follows: (1) even a patient with Child–Pugh B HCC (7 or 8 points) is a candidate for HAIC (2) Child–Pugh scores barely decline with the use of HAIC compared with sorafenib (3) HAIC is highly effective in patients with vascular invasion compared with sorafenib; and (4) survival in patients receiving HAIC may not be associated with skeletal muscle volume. In contrast, the disadvantages are problems related with the reservoir system. HAIC has clinical benefits in a subpopulation of patients without extrahepatic metastasis with Child–Pugh A HCC and vascular invasion (especially primary branch invasion or main portal vein invasion) or with Child–Pugh B HCC.
1. Introduction
The introduction of sorafenib, a molecular-targeted agent (MTA), in 2007, has been a landmark in the history of systemic therapy for advanced hepatocellular carcinoma (HCC). After the success of the SHARP and Asia-Pacific trials [1,2], several clinical trials of new MTAs (e.g., sunitinib, brivanib, and linifanib, among others) conducted from 2007 until 2016 have failed [3,4]. However, the recent success of treatments in clinical trials, such as regorafenib, lenvatinib, cabozantinib, and ramucirumab, has changed the treatment strategy for advanced HCC [5,6,7,8]. Furthermore, the combination of atezolizumab with bevacizumab improved overall and progression-free survival outcomes compared with sorafenib in patients with advanced HCC [9]. This combination therapy was approved for unresectable HCC in clinical practice in the United States (US) and Japan in May 2020 and September 2020, respectively. Therefore, combination therapy is likely considered the first-line therapy for advanced HCC, and current first-line MTAs (sorafenib and lenvatinib) and second-line MTAs (regorafenib, ramucirumab, and cabozantinib) are likely to be shifted to second- and third-line therapies, respectively [10]. However, as these above-mentioned drugs have been recommended to HCC patients with preserved liver, those with deteriorated liver function are generally not candidates for such drugs.
In contrast, hepatic arterial infusion chemotherapy (HAIC), which has been performed since the 1990s in Japan, may be a candidate for addressing an unmet medical need. Although several studies showed the efficacy of HAIC in a subpopulation of patients with advanced HCC [11,12,13,14,15,16,17], various guidelines from Asia, Europe, and the US do not recommend HAIC as a treatment option for advanced HCC due to low evidence levels, except for the Japanese guideline [18,19,20,21]. In addition, technical difficulties and medical care are needed to institute and maintain the reservoir system. Therefore, although HAIC has been used in East Asia, especially Japan, it has low feasibility as a treatment. Sorafenib has been widely used as a standard systemic therapeutic agent for more than 10 years, whereas adoption of HAIC has been limited. In this review, we discuss the current status and clinical benefits of HAIC for advanced HCC compared with sorafenib, based on articles published between 2008 and 2020.
2. Overview of HAIC
2.1. Concept
HAIC involves two procedures as follows: as scheduled, chemotherapeutic regimens are administered through a reservoir port connected to a catheter, which is implanted under the skin, and a catheter is inserted each time without implantation of the reservoir port. As HAIC is expected to accumulate drug concentrations in the local liver and reduce systemic toxicity of anti-cancer drugs, it is considered to have a more favorable antitumor effect and less influence on other organs than systemic chemotherapy. However, currently, no randomized controlled trials (RCTs) have compared HAIC with systemic chemotherapy in a large number of HCC patients.
2.2. Regimens
As the anti-cancer drugs that can be used in HAIC differ across countries, it may be difficult to adopt these HAIC regimens. In Japan, three regimens have been used for HAIC treatment: 5-fluorouracil (5-FU) combined with low-dose cisplatin (CDDP) (low-dose FP) [22,23,24], 5-FU combined with interferon (FAIT) [25,26,27,28], and CDDP alone [29,30,31] (Table 1). The response rates (complete response [CR] + partial response [PR]/all patients) of the regimens comprising low-dose FP or FAIT and the CDDP regimen were approximately 30–40% and 20–30%, respectively. Recently, HAIC regimens comprising low-dose FP or CDDP alone have been generally used in Japan [32].

Table 1.
Regimens of hepatic arterial infusion chemotherapy.
2.3. Indications
HAIC is commonly used to treat advanced HCC, whether naive or recurrent tumors. According to the Clinical Practice Guidelines for Hepatocellular Carcinoma (2017 version) established by the Japan Society of Hepatology (JSH) [21], HAIC or MTA is recommended as a second-line treatment in HCC patients with ≥4 nodules, without vascular invasion and extrahepatic metastasis (EHM); whereas transcatheter arterial chemoembolization (TACE), hepatectomy, HAIC, and MTA are recommended as first-line treatments in HCC patients with vascular invasion, without EHM. Furthermore, patients with Child–Pugh A or B HCC are candidates for HAIC [21]. In this regard, the guidelines from Korea and Taiwan demonstrated that HAIC may be considered an optional treatment in a subpopulation of patients [33,34].
2.4. Clinical Outcomes
As shown in Table 1, the median survival time (MST) was different based on the degree of vascular invasion. Radiological responders (CR or PR) show significantly longer survival than radiological non-responders (stable or progressive disease). A Japanese nationwide survey reported that the MST was significantly longer in patients who received HAIC (n = 341, 14 months) than in those who did not receive active treatment (n = 341, 5.2 months) in a propensity score-matched analysis [23]. In Child–Pugh A or B HCC patients with portal vein tumor thrombus, the MST was similarly significantly longer in patients receiving HAIC (7.9 months) than in those without therapy (3.1 months) [23]. A recent report demonstrated that none of the HAIC regimens (low-dose FP, FAIT, and CDDP alone) had no effect on survival in patients with advanced HCC [11].
3. HAIC Versus SORAFENIB
3.1. Clinical Response and Outcomes
As mentioned earlier, no RCTs have compared HAIC with sorafenib in a large number of patients with advanced HCC. A few retrospective studies have compared HAIC with sorafenib as well as one RCT with a small population [11,12,13,14,15,16,17] (Table 2). The previous prospective and retrospective studies showed that the overall survival (OS) and response rate of HAIC were significantly higher than those of sorafenib in HCC patients with vascular invasion [12,14,15,16], and other studies indicated that the progression-free survival of HAIC was better than that of sorafenib [13,17]. However, these studies had small sample sizes. A recent retrospective cohort study with a large population (2006 patients: 541 patients with HAIC; 1465 patients with sorafenib) demonstrated that the MST of patients with vascular invasion without EHM was significantly longer in the HAIC group than in the sorafenib group (10.1 versus 9.1 months) after propensity score matching, although no significant difference in OS was observed between both groups in patients without both vascular invasion and EHM after propensity score matching (12.2 and 15.4 months for the HAIC and sorafenib groups, respectively) [11]. Similarly, a meta-analysis indicated that HAIC is superior to sorafenib in HCC patients with vascular invasion [35]. Furthermore, Hatooka et al. reported that HAIC showed worse OS than sorafenib in the treatment of patients with HCC refractory to TACE [36]. Therefore, HAIC may be a potential first-line treatment in advanced HCC refractory to TACE with vascular invasion and without EHM.

Table 2.
Summary of hepatic arterial infusion chemotherapy versus sorafenib.
Although sorafenib was introduced more than 10 years ago, the long-term survival at 10 years has not been established in a large number of patients with advanced HCC who received sorafenib. Rimola et al. reported that the CR rate and MST for CR patients receiving sorafenib were 1% (12 of 1119 patients) and 85.8 months, respectively [37]. In contrast, a Japanese nationwide follow-up survey indicated that the survival rate at 10 years was 5.0% in Child–Pugh A HCC patients treated with HAIC using a reservoir port [32]. Similarly, we showed that three of six CR patients who received HAIC using a low-dose FP-based regimen survived for over 10 years [38]. Further studies with a large sample size are necessary to compare long-term survival between the two treatments.
3.2. RCTs Comparing Sorafenib Plus HAIC with Sorafenib
As sorafenib has shown a clinical benefit [1,2], the combination of sorafenib with HAIC would be expected to have a synergistic effect on clinical outcomes. Currently, three RCTs comparing sorafenib plus HAIC with sorafenib have been conducted [39,40,41] (Table 3). The SILIUS study, which compared sorafenib plus HAIC using CDDP + 5-FU with sorafenib, demonstrated a significant difference in the response rate; however, no significant difference in OS was observed between the two groups [40]. Nevertheless, subgroup analyses of this study showed that sorafenib plus HAIC showed a survival benefit in advanced HCC patients with main portal vein tumor thrombus (so-called main portal vein invasion [Vp4]) (MST, 1.4 vs. 6.5 months; hazard ratio [HR], 0.493; p = 0.050), no significant differences in OS was observed among patients with Vp0 or Vp1-3 between the sorafenib plus HAIC group and the sorafenib group (Vp0: 11.3 and 11.9 months, HR, 1.001, p = 0.996; Vp1-3: 12.6 and 14.4 months, HR, 1.367, p = 0.423, respectively). Other studies indicated that survival was significantly longer in the sorafenib plus HAIC group than in the sorafenib group [39,41]. Especially, subgroup analyses stratified by the grade of portal vein invasion showed similar results (Vp1-2: 18.17 vs. 10.87 months, HR, 0.33, p = 0.002; primary branch portal vein invasion [Vp3]: 13.47 vs. 6.27 months, HR, 0.29, p < 0.001; Vp4: 9.47 vs. 5.5 months, HR, 0.40, p < 0.001) [39]. Based on these reports, the combination of sorafenib and HAIC may be expected to have a survival benefit compared with sorafenib alone in advanced HCC with vascular invasion.

Table 3.
Randomized controlled trials comparing sorafenib plus hepatic arterial infusion chemotherapy with sorafenib.
3.3. HAIC versus Sorafenib Based on Liver Function
For patients with HCC, preserving liver function during and after several treatments is extremely important to achieve positive long-term prognoses. MTAs, including sorafenib, are generally used in patients with Child–Pugh A HCC, whereas HAIC is administered in patients with Child–Pugh A or B HCC. Terashima et al. reported that the Child–Pugh scores at 4 and 12 weeks after HAIC did not significantly decline compared with those after sorafenib treatment among patients with Child–Pugh A HCC [42]. The Child–Pugh score of responders to HAIC with Child–Pugh B HCC was significantly improved, unlike that of non-responders [41]. In addition, patients with a Child–Pugh B of 7 or 8 points were candidates for HAIC, and the clinical benefit of HAIC was extremely limited for patients with a Child–Pugh B score of 9 points [43]. Similarly, our previous report demonstrated that among HAIC responders, the Child–Pugh class of most patients showed no decline after HAIC, and the Child–Pugh class significantly improved after HAIC among responders with Child–Pugh B HCC before HAIC [44].
3.4. HAIC versus Sorafenib Based on Sarcopenia
Sarcopenia has been defined as the loss of skeletal muscle mass, physical performance (e.g., walking speed), and strength according to the European Working Group on Sarcopenia in older People and the Asian Working Group for Sarcopenia [45,46]. In contrast, the JSH proposed a diagnostic criterion for sarcopenia in patients with chronic liver disease of “loss of muscle mass plus low muscle strength” [47]. However, in previous reports, skeletal muscle depletion has been commonly used as the definition for sarcopenia in patients with HCC [48,49]. Previous studies analyzing HCC patients who received sorafenib demonstrated that skeletal muscle depletion was almost associated with poor prognosis [48,49,50,51,52,53]. Similarly, it has been reported that skeletal muscle depletion was a poor prognostic factor in patients with HCC treated with lenvatinib [54]. Furthermore, it is important to investigate skeletal muscle change during MTA use. The annual rates of skeletal muscle volume decline in cirrhotic patients without HCC were reported to be 1.3%, 3.5%, and 6.1% for Child–Pugh class A, B, and C, respectively [55]. Conversely, our previous study showed that skeletal muscle mass decreased by 5.5% at 3 months after starting sorafenib [53], and another report indicated that treatment with sorafenib or lenvatinib showed a significant depletion of skeletal muscle volume regardless of disease progression and hepatic reserve function [56]. In the era of MTAs, sequential therapy using MTAs may decrease skeletal muscle volume markedly higher than a first-line MTA therapy. Currently, there have been no reports regarding the relationship between sarcopenia and clinical outcomes in patients treated with atezolizumab plus bevacizumab and other MTAs, except for sorafenib and lenvatinib.
This is the first study demonstrating that skeletal muscle depletion is not associated with OS in patients with HCC treated with HAIC compared with sorafenib [57]. As there have been no similar reports, this finding will need to be validated. Thus, the different results related to skeletal muscle mass between HAIC and sorafenib may be worthy of notice when considering the use of treatment modalities for advanced HCC.
3.5. Sequential Therapy: HAIC Followed by Sorafenib versus Sorafenib Followed by HAIC
The RESORCE study and a sub-analysis of the REFLECT study demonstrated that sequential therapy improved survival in patients who were refractory to the first-line therapy [5,58]. Post-progression survival (PPS) is an important factor for prolonging OS. Our previous reports showed that post-treatment after HAIC failure was a significant independent predictor of OS before the development of MTAs [59,60]. Retrospective cohort studies, including the present study, demonstrated that conversion to sorafenib after HAIC failure was a significant prognostic factor [57,61]. However, as these studies had small sample sizes, large comparative studies are necessary to confirm the survival benefit of this sequential therapy.
In contrast, it has been reported that subsequent therapy, including TACE and HAIC, contributed to prolonging PPS after sorafenib failure [62,63,64]. However, the sequential therapies administered after sorafenib failure were heterogeneous. To our knowledge, there have been no reports comparing between patients who received only HAIC and those who did not receive any subsequent therapy. As it is difficult to perform a prospective study of subsequent therapy using HAIC versus no therapy, propensity score matching will be needed to evaluate this finding.
4. Clinical Benefits and Disadvantages of HAIC
The clinical benefits of HAIC for advanced HCC are as follows: (1) even a patient with Child–Pugh B HCC (7 or 8 points) is a candidate for HAIC [43], (2) Child–Pugh scores barely decline after HAIC [42,44], (3) HAIC is highly effective in patients with vascular invasion compared with sorafenib [11,35], and 4) survival in patients receiving HAIC may not be associated with skeletal muscle volume [57]. In contrast, the disadvantages of HAIC for advanced HCC are as follows: (1) a highly technical procedure is needed to implant a catheter with a reservoir port; (2) hospitalization is needed to continue HAIC treatments; (3) patients have to return for follow-up visits every 2 weeks to maintain the reservoir system; and (4) adverse events related to the reservoir system, such as port migration, catheter dislocation, arterial occlusion, reservoir system occlusion, subcutaneous hematomas, or infection [65].
Atezolizumab combined with bevacizumab was recently approved, and this combination will be recommended as the first-line therapy for advanced HCC. However, a comparison between atezolizumab plus bevacizumab and HAIC has not been performed. Patients with macrovascular invasion, including an invasion of the main portal trunk, accounted for 38% of those in the atezolizumab plus bevacizumab group; however, the details were not shown [9]. Therefore, as there has been no information regarding this combination therapy in real-world practice, further studies are required.
We present a draft of the treatment proposal for HAIC for advanced HCC in Figure 1. The combination of atezolizumab and bevacizumab will be shifted to the first-line therapy in patients with Child–Pugh A HCC, regardless of EHM, and currently used MTAs will be shifted to later lines of therapy [10]. HAIC may be an optional treatment in patients with Child–Pugh A HCC and vascular invasion, especially Vp3 or Vp4, without EHM [11,35]. MTAs are generally used in patients with Child–Pugh A HCC, whereas the use of MTAs in patients with Child–Pugh B HCC remains controversial. Some Asian guidelines recommended that sorafenib is considered in selected patients with Child–Pugh B (e.g., score, 7 points) [18,33,34,66,67], although sorafenib treatment significantly worsened survival in patients with Child–Pugh B HCC compared to those with Child–Pugh A HCC [68]. In contrast, patients with Child–Pugh B HCC (score 7 or 8 points) are candidates for HAIC [43]. The medical needs of patients receiving second-line therapy for Child–Pugh B HCC without EHM and those who have EHM with Child–Pugh B HCC, are yet to be met. However, HAIC may be considered in a subpopulation of both Child–Pugh B HCC and EHM patients if the intrahepatic tumor is directly linked to prognosis. Therefore, patients in clinical trials who can tolerate deteriorated liver function would be candidates for the novel therapy [69,70]. We have reported the efficacy of arterial infusion of an iron chelator, deferoxamine, which is not an anti-cancer drug but is used for treating iron overload disease in advanced HCC patients, including Child–Pugh B or C patients [69]. However, deferasirox, an oral iron chelator, has limited efficacy due to associated adverse effects, especially renal dysfunction [70]. In the future, systemic therapeutic agents would be expected to be developed for the unmet medical needs of patients undergoing advanced HCC treatment.
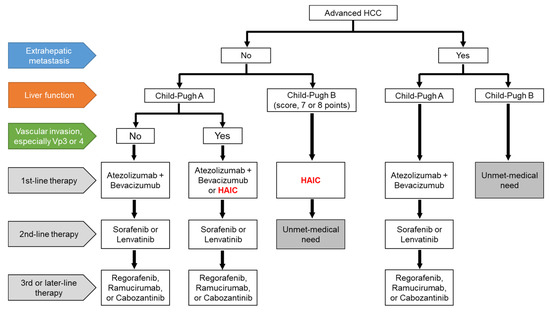
Figure 1.
A draft of the treatment proposal for advanced HCC. HCC, hepatocellular carcinoma; HAIC, hepatic arterial infusion chemotherapy; Vp3, primary branch portal vein invasion; Vp4, main portal vein invasion.
5. Conclusions
Although HAIC was not recommended as a treatment option for advanced HCC by various guidelines, several studies demonstrated that HAIC has clinical benefits in a subpopulation of patients with advanced HCC, such as Child–Pugh A HCC with primary branch/main portal vein invasion without EHM and Child–Pugh B HCC without EHM. In fact, HAIC is currently the only treatment option to address the unmet medical needs of Child–Pugh B HCC patients. In the future, HAIC may be recommended as a treatment for advanced HCC if it is widely adopted and a large body of supporting evidence is generated.
Author Contributions
T.Y., I.S. (Issei Saeki), Y.K.-Y., R.S., N.T., T.O., T.M. (Takashi Matsuda), T.H., T.M. (Toshihiko Matsumoto), I.H. and T.I. analyzed the literature. T.Y. and I.S. (Issei Saeki) were involved in writing the manuscript. T.T. and Y.S. were involved editing the manuscript. I.S. (Isao Sakaida) was involved in critical editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant numbers, 16H05287 and 20K08332.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, T.H.; Yau, T.; Hsu, C. Novel systemic therapy for hepatocellular carcinoma. Hepatol. Int. 2020, 14, 638–651. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. A Paradigm Change in the Treatment Strategy for Hepatocellular Carcinoma. Liver Cancer 2020, 9, 367–377. [Google Scholar] [CrossRef]
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Chung, W.J.; Bae, S.H.; Song, D.S.; Song, M.J.; Kim, Y.S.; Yim, H.J.; Jung, Y.K.; Suh, S.J.; Park, J.Y.; et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2018, 82, 469–478. [Google Scholar] [CrossRef]
- Kang, M.K.; Park, J.G.; Lee, H.J. Comparison of clinical outcomes between sorafenib and hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma: A STROBE-compliant article. Medicine 2018, 97, e0611. [Google Scholar] [CrossRef]
- Moriguchi, M.; Aramaki, T.; Nishiofuku, H.; Sato, R.; Asakura, K.; Yamaguchi, K.; Tanaka, T.; Endo, M.; Itoh, Y. Sorafenib versus Hepatic Arterial Infusion Chemotherapy as Initial Treatment for Hepatocellular Carcinoma with Advanced Portal Vein Tumor Thrombosis. Liver Cancer 2017, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; Song, M.J.; Bae, S.H.; Chung, W.J.; Jang, J.Y.; Kim, Y.S.; Lee, S.H.; Park, J.Y.; Yim, H.J.; Cho, S.B.; et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroenterol. 2015, 50, 445–454. [Google Scholar] [CrossRef]
- Kawaoka, T.; Aikata, H.; Hyogo, H.; Morio, R.; Morio, K.; Hatooka, M.; Fukuhara, T.; Kobayashi, T.; Naeshiro, N.; Miyaki, D.; et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J. Dig. Dis. 2015, 16, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fukubayashi, K.; Tanaka, M.; Izumi, K.; Watanabe, T.; Fujie, S.; Kawasaki, T.; Yoshimaru, Y.; Tateyama, M.; Setoyama, H.; Naoe, H.; et al. Evaluation of sorafenib treatment and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparative study using the propensity score matching method. Cancer Med. 2015, 4, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electric address: Easloffice@easloffice.eu Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Saeki, I.; Yamasaki, T.; Tanabe, N.; Iwamoto, T.; Matsumoto, T.; Urata, Y.; Hidaka, I.; Ishikawa, T.; Takami, T.; Yamamoto, N.; et al. A new therapeutic assessment score for advanced hepatocellular carcinoma patients receiving hepatic arterial infusion chemotherapy. PLoS ONE 2015, 10, e0126649. [Google Scholar] [CrossRef]
- Nouso, K.; Miyahara, K.; Uchida, D.; Kuwaki, K.; Izumi, N.; Omata, M.; Ichida, T.; Kudo, M.; Ku, Y.; Kokudo, N.; et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br. J. Cancer 2013, 109, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology 2010, 78, 148–153. [Google Scholar] [CrossRef]
- Monden, M.; Sakon, M.; Sakata, Y.; Ueda, Y.; Hashimura, E. FAIT Research Group. 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: A multicenter, randomized, phase II study. Hepatol. Res. 2012, 42, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Terashima, T.; Mizukoshi, E.; Sakai, A.; Nakamoto, Y.; Honda, M.; Kaneko, S. Randomized, phase II study comparing interferon combined with hepatic arterial infusion of fluorouracil plus cisplatin and fluorouracil alone in patients with advanced hepatocellular carcinoma. Oncology 2011, 81, 281–290. [Google Scholar] [CrossRef]
- Nagano, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Tomimaru, Y.; Osuga, K.; Umeshita, K.; Doki, Y.; et al. Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology 2011, 80, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Obi, S.; Yoshida, H.; Toune, R.; Unuma, T.; Kanda, M.; Sato, S.; Tateishi, R.; Teratani, T.; Shiina, S.; Omata, M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2006, 106, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Okusaka, T.; Furuse, J.; Mitsunaga, S.; Ueno, H.; Yamaura, H.; Inaba, Y.; Takeuchi, Y.; Satake, M.; Arai, Y. A multi-institutional phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2013, 72, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Park, J.Y.; Choi, H.J.; Kim, d.Y.; Ahn, S.H.; Kim, J.K.; Lee, D.Y.; Lee, K.H.; Han, K.H. Long-term clinical outcomes of hepatic arterial infusion chemotherapy with cisplatin with or without 5-fluorouracil in locally advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2011, 137, 659–667. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ono, N.; Yodono, H.; Ichida, T.; Nakamura, H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol. Res. 2008, 38, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 21th Nationwide follow-up survey of primary liver cancer in Japan (2010–2011). Hepatol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Group; Diagnosis Group; Staging Group; Surgery Group; Local Ablation Group; TACE/TARE/HAI Group; Target Therapy/Systemic Therapy Group; Radiotherapy Group; Prevention Group; Drafting Group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2018, 117, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, J.; Mou, T.; Wang, Y.; Wu, Z.; Shen, A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroenterol. Hepatol. 2020, 35, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Hatooka, M.; Kawaoka, T.; Aikata, H.; Morio, K.; Kobayashi, T.; Hiramatsu, A.; Imamura, M.; Kawakami, Y.; Murakami, E.; Waki, K.; et al. Comparison of Outcome of Hepatic Arterial Infusion Chemotherapy and Sorafenib in Patients with Hepatocellular Carcinoma Refractory to Transcatheter Arterial Chemoembolization. Anticancer Res. 2016, 36, 3523–3529. [Google Scholar] [PubMed]
- Rimola, J.; Díaz-González, Á.; Darnell, A.; Varela, M.; Pons, F.; Hernandez-Guerra, M.; Delgado, M.; Castroagudin, J.; Matilla, A.; Sangro, B.; et al. Complete response under sorafenib in patients with hepatocellular carcinoma: Relationship with dermatologic adverse events. Hepatology 2018, 67, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Fujisawa, K.; Matsumoto, T.; Hidaka, I.; Marumoto, Y.; Ishikawa, T.; et al. Treatment strategies for advanced hepatocellular carcinoma: Sorafenib vs. hepatic arterial infusion chemotherapy. World J. Hepatol. 2018, 10, 571–584. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs. Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Ikeda, M.; Shimizu, S.; Sato, T.; Morimoto, M.; Kojima, Y.; Inaba, Y.; Hagihara, A.; Kudo, M.; Nakamori, S.; Kaneko, S.; et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann. Oncol. 2016, 27, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Beneficial Effect of Maintaining Hepatic Reserve during Chemotherapy on the Outcomes of Patients with Hepatocellular Carcinoma. Liver Cancer 2017, 6, 236–249. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Response to chemotherapy improves hepatic reserve for patients with hepatocellular carcinoma and Child-Pugh B cirrhosis. Cancer Sci. 2016, 107, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Evaluation of the “assessment for continuous treatment with hepatic arterial infusion chemotherapy” scoring system in patients with advanced hepatocellular carcinoma. Hepatol. Res. 2018, 48, E87–E97. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Kochi, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int. J. Mol. Sci. 2015, 16, 9612–9624. [Google Scholar] [CrossRef]
- Hiraoka, A.; Hirooka, M.; Koizumi, Y.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Tomida, H.; Miyamoto, Y.; et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2017, 47, 558–565. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Kawano, R.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; et al. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Biomarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 359–371. [Google Scholar] [CrossRef]
- Uojima, H.; Chuma, M.; Tanaka, Y.; Hidaka, H.; Nakazawa, T.; Iwabuchi, S.; Kobayashi, S.; Hattori, N.; Ogushi, K.; Morimoto, M.; et al. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer 2020, 9, 193–206. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 743–751. [Google Scholar] [CrossRef]
- Uchikawa, S.; Kawaoka, T.; Namba, M.; Kodama, K.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Tsuge, M.; Hiramatsu, A.; et al. Skeletal Muscle Loss during Tyrosine Kinase Inhibitor Treatment for Advanced Hepatocellular Carcinoma Patients. Liver Cancer 2020, 9, 148–155. [Google Scholar] [CrossRef]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Effect of body composition on survival benefit of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparison with sorafenib therapy. PLoS ONE 2019, 14, e0218136. [Google Scholar] [CrossRef]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.L.; Tak, W.Y.; Ryoo, B.-Y.; Evans, T.R.J.; López López, C.; Daniele, B.; Misir, S.; et al. Effects of Subsequent Systemic Anticancer Medication Following First-Line Lenvatinib: A Post Hoc Responder Analysis from the Phase 3 REFLECT Study in Unresectable Hepatocellular Carcinoma. Liver Cancer 2020, 9, 93–104. [Google Scholar] [CrossRef]
- Urayama, N.; Yamasaki, T.; Harima, Y.; Saeki, I.; Zaitsu, J.; Hamabe, S.; Harano, M.; Takami, T.; Kaino, S.; Uchida, K.; et al. Hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma: Analysis of 114 cases. Kanzo 2011, 52, 499–560. [Google Scholar] [CrossRef]
- Yamasaki, T.; Sakaida, I. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma and future treatments for the poor responders. Hepatol. Res. 2012, 42, 340–348. [Google Scholar] [CrossRef]
- Miyaki, D.; Aikata, H.; Kan, H.; Fujino, H.; Urabe, A.; Masaki, K.; Fukuhara, T.; Kobayashi, T.; Naeshiro, N.; Nakahara, T.; et al. Clinical outcome of sorafenib treatment in patients with advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy. J. Gastroenterol. Hepatol. 2013, 28, 1834–1841. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Kitahara, M.; Nakagawa, H.; Kagaya, T.; Mizukoshi, E.; Honda, M.; Kaneko, S. Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol. Res. 2014, 44, 1179–1185. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Horii, R.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; et al. Potential efficacy of therapies targeting intrahepatic lesions after sorafenib treatment of patients with hepatocellular carcinoma. BMC Cancer 2016, 16, 338. [Google Scholar] [CrossRef]
- Kondo, M.; Numata, K.; Hara, K.; Nozaki, A.; Fukuda, H.; Chuma, M.; Maeda, S.; Tanaka, K. Treatment of Advanced Hepatocellular Carcinoma after Failure of Sorafenib Treatment: Subsequent or Additional Treatment Interventions Contribute to Prolonged Survival Postprogression. Gastroenterol. Res. Pract. 2017, 2017, 5728946. [Google Scholar] [CrossRef] [PubMed]
- Obi, S.; Sato, S.; Kawai, T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer 2015, 4, 188–199. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Wang, S.Y.; Lin, S.M.; Diagnosis Group; Systemic Therapy Group. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Hollebecque, A.; Cattan, S.; Romano, O.; Sergent, G.; Mourad, A.; Louvet, A.; Dharancy, S.; Boleslawski, E.; Truant, S.; Pruvot, F.R.; et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: The impact of the Child-Pugh score. Aliment. Pharmacol. Ther. 2011, 34, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Terai, S.; Sakaida, I. Deferoxamine for advanced hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamamoto, N.; Yamasaki, T.; Takami, T.; Maeda, M.; Fujisawa, K.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 8967–8977. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).