The Influence of Various Preparation Parameters on the Histological Image of Bone Tissue during Implant Bed Preparation—An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
- 0
- —smooth drill hole surfaces, even edges, no compression, no charring, no cracks or damage to adjacent tissues—positive—OK
- 1
- —surface smoothness (jagged drill holes, uneven edges, presence of tissue residues)—not positive—NOK
- 2
- —the presence of compressed (pressed) tissues—not positive—NOK
- 3
- —presence of carbonization (char)—not positive—NOK
- 4
- —cracking and damage to neighboring tissues—not positive—NOK
Statistical Analysis
3. Results
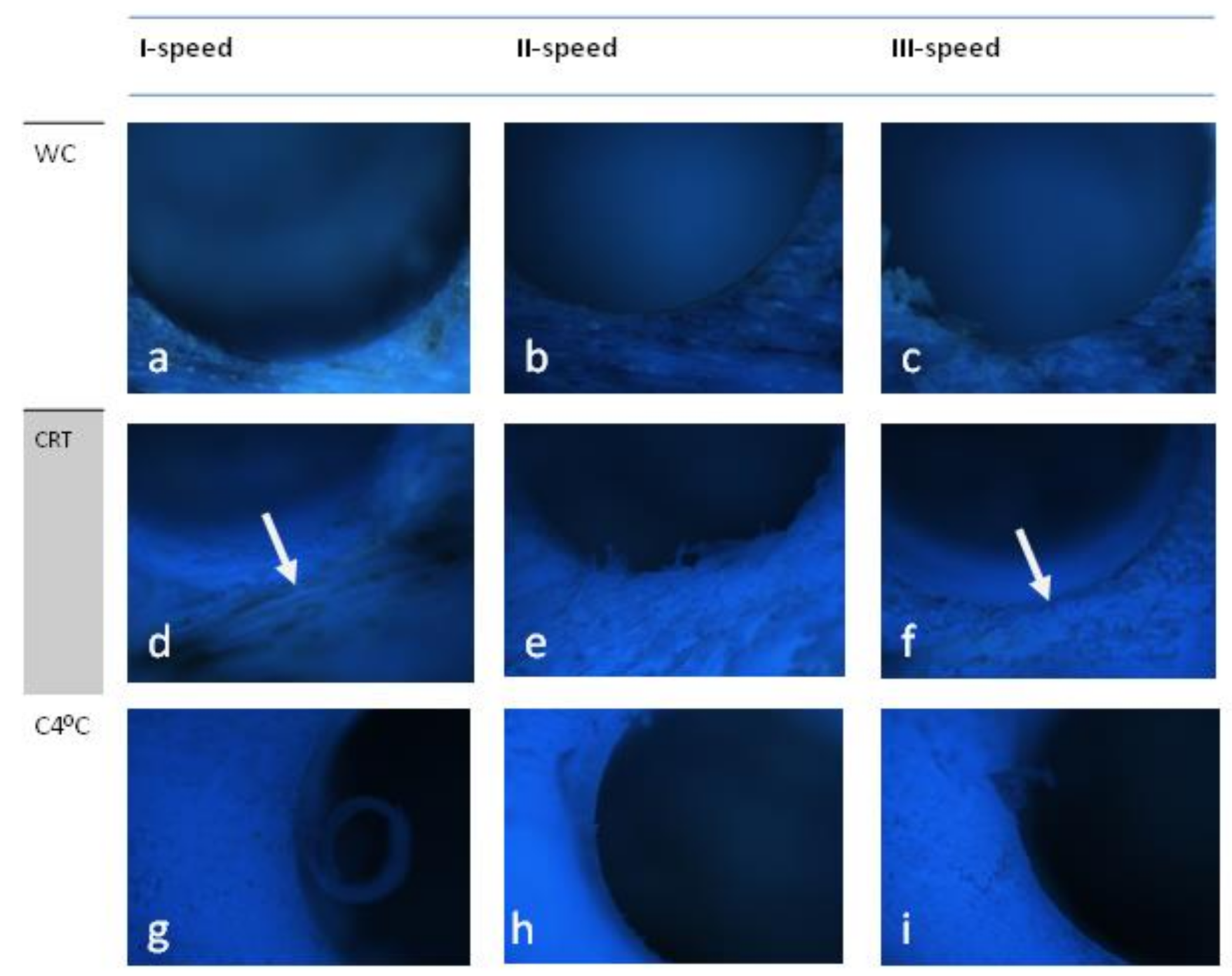
3.1. Osstem (D1)
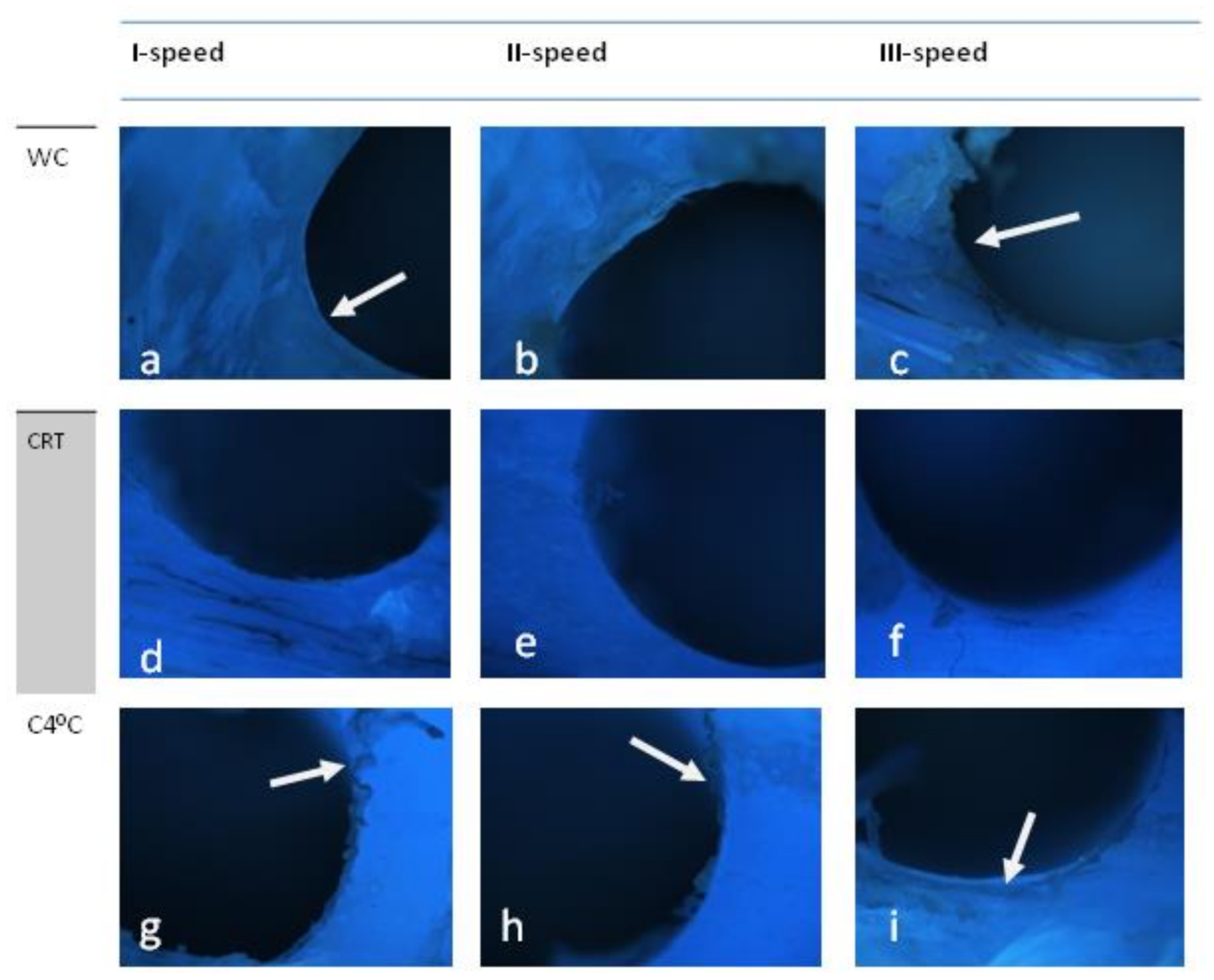
3.2. Straumann (D2)
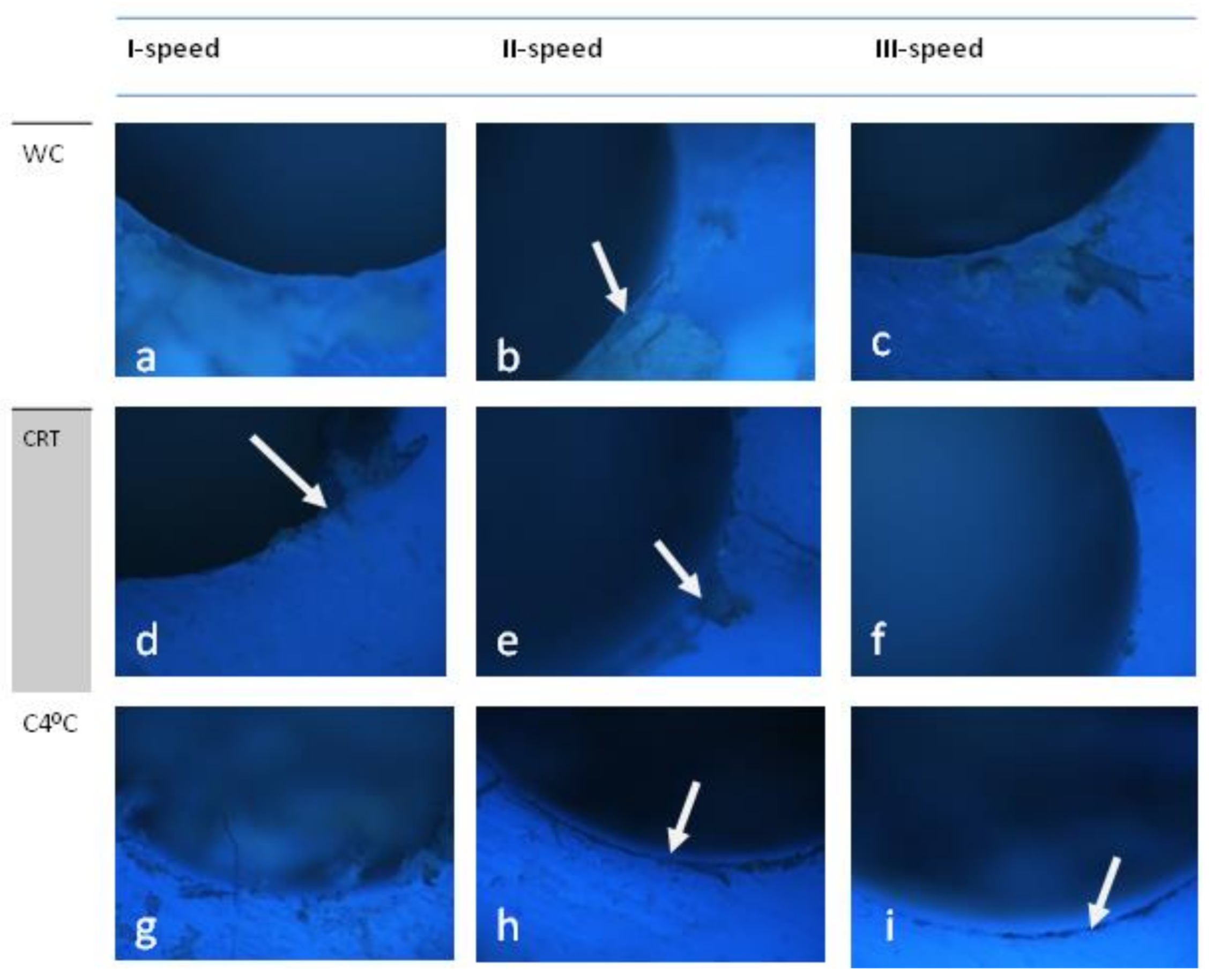
3.3. S-Wide (Neobiotech S-Wide)
4. Scoring
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marheineke, N.; Scherer, U.; Rücker, M.; Von See, C.; Rahlf, B.; Stoetzer, M.; Gellrich, N.-C. Evaluation of accuracy in implant site preparation performed in single- or multi-step drilling procedures. Clin. Oral Investig. 2017, 22, 2057–2067. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Jinno, Y.; Cecchinato, F.; Becktor, J.P.; Naito, Y.; Halldin, A.; Jimbo, R. Influence of different drilling preparation on cortical bone: A biomechanical, histological, and micro-CT study on sheep. Clin. Oral Implant. Res. 2018, 29, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favero, V.; Sakuma, S.; Alccayhuaman, K.A.A.; Benedetto, G.A.; Bengazi, F.; Botticelli, D. Healing at sites prepared using different drilling protocols. An experimental study in the tibiae of sheep. PLoS ONE 2018, 13, e0202957. [Google Scholar] [CrossRef]
- Di Fiore, A.; Sivolella, S.; Stocco, E.; Favero, V.; Stellini, E. Experimental Analysis of Temperature Differences during Implant Site Preparation: Continuous Drilling Technique Versus Intermittent Drilling Technique. J. Oral Implant. 2018, 44, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Effect of Surgical Drill Guide and Irrigans Temperature on Thermal Bone Changes during Drilling Implant Sites—Thermographic Analysis on Bovine Ribs. Available online: https://pubmed.ncbi.nlm.nih.gov/29328609/ (accessed on 21 December 2020).
- Sannino, G.; Capparé, P.; Gherlone, E.F.; Barlattani, A. Influence of the Implant Drill Design and Sequence on Temperature Changes during Site Preparation. Int. J. Oral Maxillofac. Implant. 2015, 30, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavropoulos, A.; Cochran, D.; Obrecht, M.; Pippenger, B.; Dard, M. Effect of Osteotomy Preparation on Osseointegration of Immediately Loaded, Tapered Dental Implants. Adv. Dent. Res. 2016, 28, 34–41. [Google Scholar] [CrossRef]
- Folkman, M.; Becker, A.; Meinster, I.; Masri, M.; Ormianer, Z. Comparison of bone-to-implant contact and bone volume around implants placed with or without site preparation: A histomorphometric study in rabbits. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.; Anand, P.S.; Anil, S. Undersized Implant Site Preparation to Enhance Primary Implant Stability in Poor Bone Density: A Prospective Clinical Study. J. Oral Maxillofac. Surg. 2011, 69, e506–e512. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Walboomers, X.F.; Jansen, J.A. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin. Oral Implant. Res. 2013, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.; Lang, N.P.; Ricci, S.; Mintrone, F.; González, G.G.; Botticelli, D. Healing of implants installed in over- or under-prepared sites—An experimental study in dogs. Clin. Oral Implant. Res. 2014, 26, 442–446. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Influence of implant taper on the primary and secondary stability of osseointegrated titanium implants. Clin. Oral Implant. Res. 2004, 15, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Sennerby, L.; Bedrossian, E.; Becker, B.E.; Lucchini, J.P. Implant Stability Measurements for Implants Placed at the Time of Extraction: A Cohort, Prospective Clinical Trial. J. Periodontol. 2005, 76, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Osseodensification Drilling vs. Standard Protocol of Implant Site Preparation: An In Vitro Study on Polyurethane Foam Sheets. Available online: https://www.researchgate.net/publication/341808179_Osseodensification_Drilling_vs_Standard_Protocol_of_Implant_Site_Preparation_An_In_Vitro_Study_on_Polyurethane_Foam_Sheets (accessed on 23 January 2021).
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the Thermal Treatment to Address a Better Osseointegration of Ti6Al4V Dental Implants: Histological and Histomorphometrical Study in a Rabbit Model. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kirstein, K.; Dobrzyński, M.; Kosior, P.; Chrószcz, A.; Dudek, K.; Fita, K.; Parulska, O.; Rybak, Z.; Skalec, A.; Szklarz, M.; et al. Infrared Thermographic Assessment of Cooling Effectiveness in Selected Dental Implant Systems. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Möhlhenrich, S.; Modabber, A.; Steiner, T.; Mitchell, D.; Hölzle, F. Heat generation and drill wear during dental implant site preparation: Systematic review. Br. J. Oral Maxillofac. Surg. 2015, 53, 679–689. [Google Scholar] [CrossRef]
- Alam, K.; Silberschmidt, V.V. Analysis of temperature in conventional and ultrasonically-assisted drilling of cortical bone with infrared thermography. Technol. Health Care 2014, 22, 243–252. [Google Scholar] [CrossRef]
- Ihde, S.; Pałka, Ł.; Janeczek, M.; Kosior, P.; Kiryk, J.; Dobrzynski, M. Bite Reconstruction in the Aesthetic Zone Using One-Piece Bicortical Screw Implants. Case Rep. Dent. 2018, 2018, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chana, H.; Smith, G.; Bansal, H.; Zahra, D. A Retrospective Cohort Study of the Survival Rate of 88 Zygomatic Implants Placed Over an 18-year Period. Int. J. Oral Maxillofac. Implant. 2019, 34, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Osseointegration of Zirconium Dental Implants Three Months after Insertion in Rabbit Femur. Histopathological Study. Available online: https://pubmed.ncbi.nlm.nih.gov/30534817/ (accessed on 21 December 2020).
- Sarendranath, A.; Khan, R.; Tovar, N.; Marin, C.; Yoo, D.; Redisch, J.; Jimbo, R.; Coelho, P. Effect of low speed drilling on osseointegration using simplified drilling procedures. Br. J. Oral Maxillofac. Surg. 2015, 53, 550–556. [Google Scholar] [CrossRef]
- Implant Materials, Designs, and Surface Topographies: Their Effect on Osseointegration. A Literature Review. Available online: https://pubmed.ncbi.nlm.nih.gov/11055135/ (accessed on 25 January 2021).
- Saini, M. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Histologic Evidence of a Connective Tissue Attachment to Laser Microgrooved Abutments: A Canine Study. Available online: https://pubmed.ncbi.nlm.nih.gov/20386781/ (accessed on 25 January 2021).
- Collaert, B.; Wijnen, L.; De Bruyn, H. A 2-year prospective study on immediate loading with fluoride-modified implants in the edentulous mandible. Clin. Oral Implant. Res. 2011, 22, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Johansson, C.B.; Röser, K.; Albrektsson, T. Qualitative and quantitative observations of bone tissue reactions to anodised implants. Biomater. 2002, 23, 1809–1817. [Google Scholar] [CrossRef]
- Yeniyol, S.; Jimbo, R.; Marin, C.; Tovar, N.; Janal, M.N.; Coelho, P.G. The effect of drilling speed on early bone healing to oral implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 550–555. [Google Scholar] [CrossRef]
- Slete, F.B.; Olin, P.; Prasad, H. Histomorphometric Comparison of 3 Osteotomy Techniques. Implant. Dent. 2018, 27, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P.; Masciotra, L. Effect of 50 to 60°C Heating on Osseointegration of Dental Implants in Dense Bone. Implant. Dent. 2014, 23, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.; Jimbo, R.; Tovar, N.; Yoo, D.Y.; Anchieta, R.B.; Yamaguchi, S.; Coelho, P.G. The effect of osteotomy dimension on osseointegration to resorbable media-treated implants: A study in the sheep. J. Biomater. Appl. 2014, 29, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Osseointegrated Dental Implants. Available online: https://pubmed.ncbi.nlm.nih.gov/3514290/ (accessed on 21 December 2020).
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implant. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Brunski, J.B. Biomechanical factors affecting the bone-dental implant interface. Clin. Mater. 1992, 10, 153–201. [Google Scholar] [CrossRef]
- Boustany, C.M.; Reed, H.; Cunningham, G.; Richards, M.; Kanawati, A. Effect of a Modified Stepped Osteotomy on the Primary Stability of Dental Implants in Low-Density Bone: A Cadaver Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Gröndahl, K.; Bergström, C.; Bäck, T.; Lekholm, U. On cutting torque measurements during implant placement: A 3-year clinical prospective study. Clin. Implant. Dent. Relat. Res. 1999, 1, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Stocchero, M. On Influence of an Undersized Implant Site on Implant Stability and Osseointegration. Ph.D. Thesis, Malmö University, Malmo, Sweden, 2018. [Google Scholar]

| X1 Systems | X2 vc (m/s) | X3 Environmental Conditions | Quality of the Drill Hole Surface (Score) | Y |
|---|---|---|---|---|
| 1—Osstem | 0.147 | 1—without cooling | 0 | OK |
| 1—Osstem | 0.220 | 1—without cooling | 0 | OK |
| 1—Osstem | 0.275 | 1—without cooling | 1 | NOK |
| 1—Osstem | 0.147 | 2—with cooling at room temperature | 2 | NOK |
| 1—Osstem | 0.220 | 2—with cooling at room temperature | 2 | NOK |
| 1—Osstem | 0.275 | 2—with cooling at room temperature | 2 | NOK |
| 1—Osstem | 0.147 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 1—Osstem | 0.220 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 1—Osstem | 0.275 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 2—Strauman | 0.147 | 1—without cooling | 0 | OK |
| 2—Strauman | 0.220 | 1—without cooling | 0 | OK |
| 2—Strauman | 0.275 | 1—without cooling | 1 | NOK |
| 2—Strauman | 0.147 | 2—with cooling at room temperature | 0 | OK |
| 2—Strauman | 0.220 | 2—with cooling at room temperature | 0 | OK |
| 2—Strauman | 0.275 | 2—with cooling at room temperature | 1 | NOK |
| 2—Strauman | 0.147 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 2—Strauman | 0.220 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 2—Strauman | 0.275 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 3—Neobiotech S-Wide | 0.230 | 1—without cooling | 1 | NOK |
| 3—Neobiotech S-Wide | 0.346 | 1—without cooling | 1 | NOK |
| 3—Neobiotech S-Wide | 0.432 | 1—without cooling | 1 | NOK |
| 3—Neobiotech S-Wide | 0.230 | 2—with cooling at room temperature | 1 | NOK |
| 3—Neobiotech S-Wide | 0.346 | 2—with cooling at room temperature | 1 | NOK |
| 3—Neobiotech S-Wide | 0.432 | 2—with cooling at room temperature | 1 | NOK |
| 3—Neobiotech S-Wide | 0.230 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 3—Neobiotech S-Wide | 0.346 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| 3—Neobiotech S-Wide | 0.432 | 3—with cooling with normal saline at 4°C | 1 | NOK |
| Parameters | The Quality of the Drill Hole Surface | Fischer’s Exact Test p-Value | |||
|---|---|---|---|---|---|
| OK | NOK | ||||
| n | % | n | % | ||
| System: | 0.076 * | ||||
| Osstem | 2 | 33.3% | 7 | 33.3% | |
| Straumann | 4 | 66.7% | 5 | 23.8% | |
| Neobiotech S-Wide | 0 | 0.0% | 9 | 42.9% | |
| Environmental conditions: | 0.076 * | ||||
| Without cooling | 4 | 66.7% | 5 | 23.8% | |
| With cooling at room temperature | 2 | 33.3% | 7 | 33.3% | |
| With cooling with normal saline at 4 °C | 0 | 0.0% | 9 | 42.9% | |
| Cutting speed vc(m/s) | 0.003 ** | ||||
| <0.22 | 6 | 100.0% | 6 | 28.6% | |
| >0.22 | 0 | 0.0% | 15 | 71.4% | |
| System and Cooling | High Quality | OR [95% CI] | |
|---|---|---|---|
| OK | NOK | ||
| For: Straumann | 4 | 5 | 6.40 [0.89–46.00] |
| For: Osstem or Neobiotech S-Wide | 2 | 16 | 0.16 [0.02–1.12] |
| For: without cooling | 4 | 5 | 6.40 [0.89–46.00] |
| For: with cooling | 2 | 16 | 0.16 [0.02–1.12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosior, P.; Kuropka, P.; Janeczek, M.; Mikulewicz, M.; Zakrzewski, W.; Dobrzyński, M. The Influence of Various Preparation Parameters on the Histological Image of Bone Tissue during Implant Bed Preparation—An In Vitro Study. Appl. Sci. 2021, 11, 1916. https://doi.org/10.3390/app11041916
Kosior P, Kuropka P, Janeczek M, Mikulewicz M, Zakrzewski W, Dobrzyński M. The Influence of Various Preparation Parameters on the Histological Image of Bone Tissue during Implant Bed Preparation—An In Vitro Study. Applied Sciences. 2021; 11(4):1916. https://doi.org/10.3390/app11041916
Chicago/Turabian StyleKosior, Piotr, Piotr Kuropka, Maciej Janeczek, Marcin Mikulewicz, Wojciech Zakrzewski, and Maciej Dobrzyński. 2021. "The Influence of Various Preparation Parameters on the Histological Image of Bone Tissue during Implant Bed Preparation—An In Vitro Study" Applied Sciences 11, no. 4: 1916. https://doi.org/10.3390/app11041916









