A New In Vitro Model to Evaluate Anti-Adhesive Effect against Fungal Nail Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bovine Membrane Production and Selection
2.2.1. Bovine Membrane Production
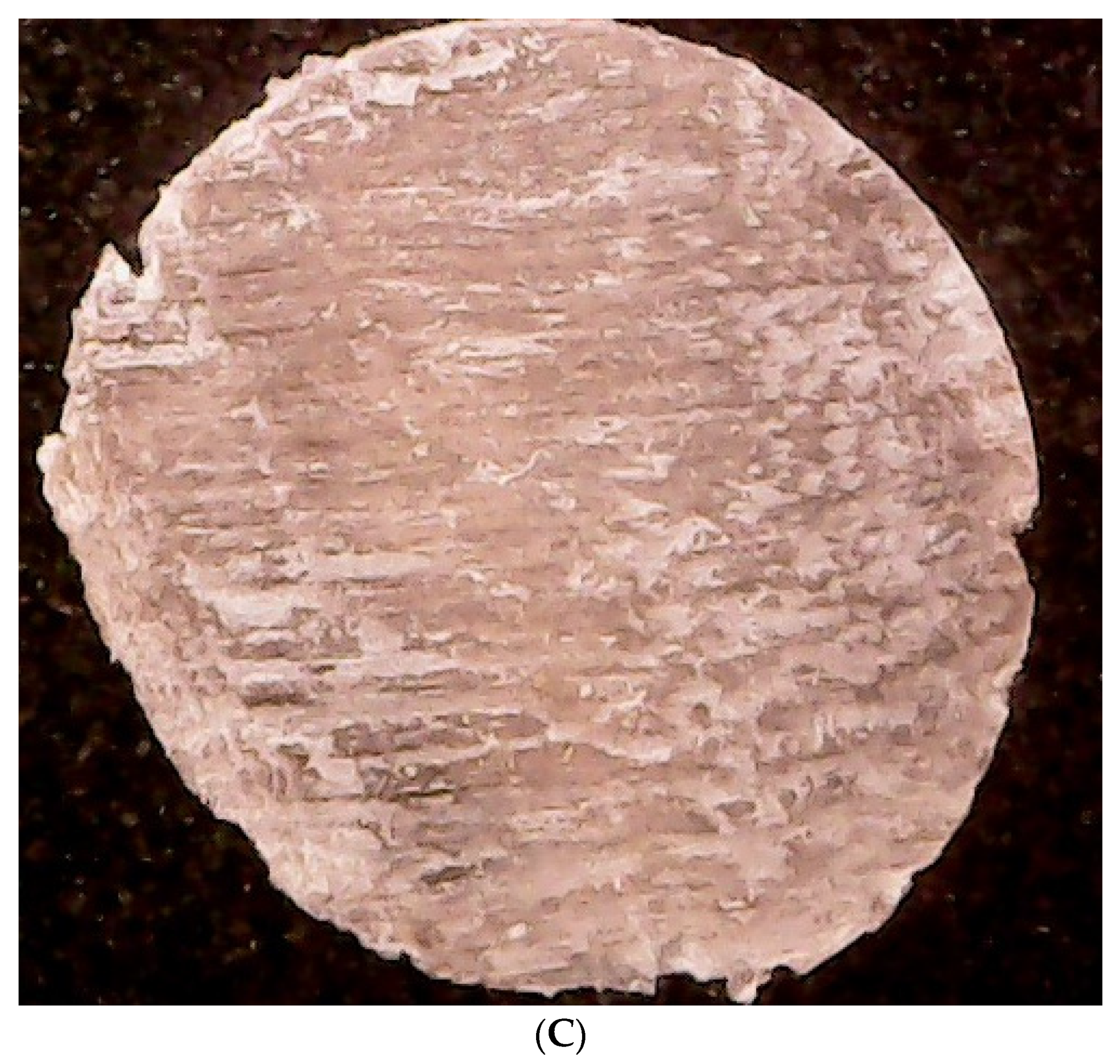
2.2.2. Bovine Membrane Morphology Evaluation
2.3. Preliminary Microbiological Experiment
2.3.1. Fungal Strains, Culture Conditions and Sample Preparation
2.3.2. Contamination of Membranes with the Chosen Microorganism
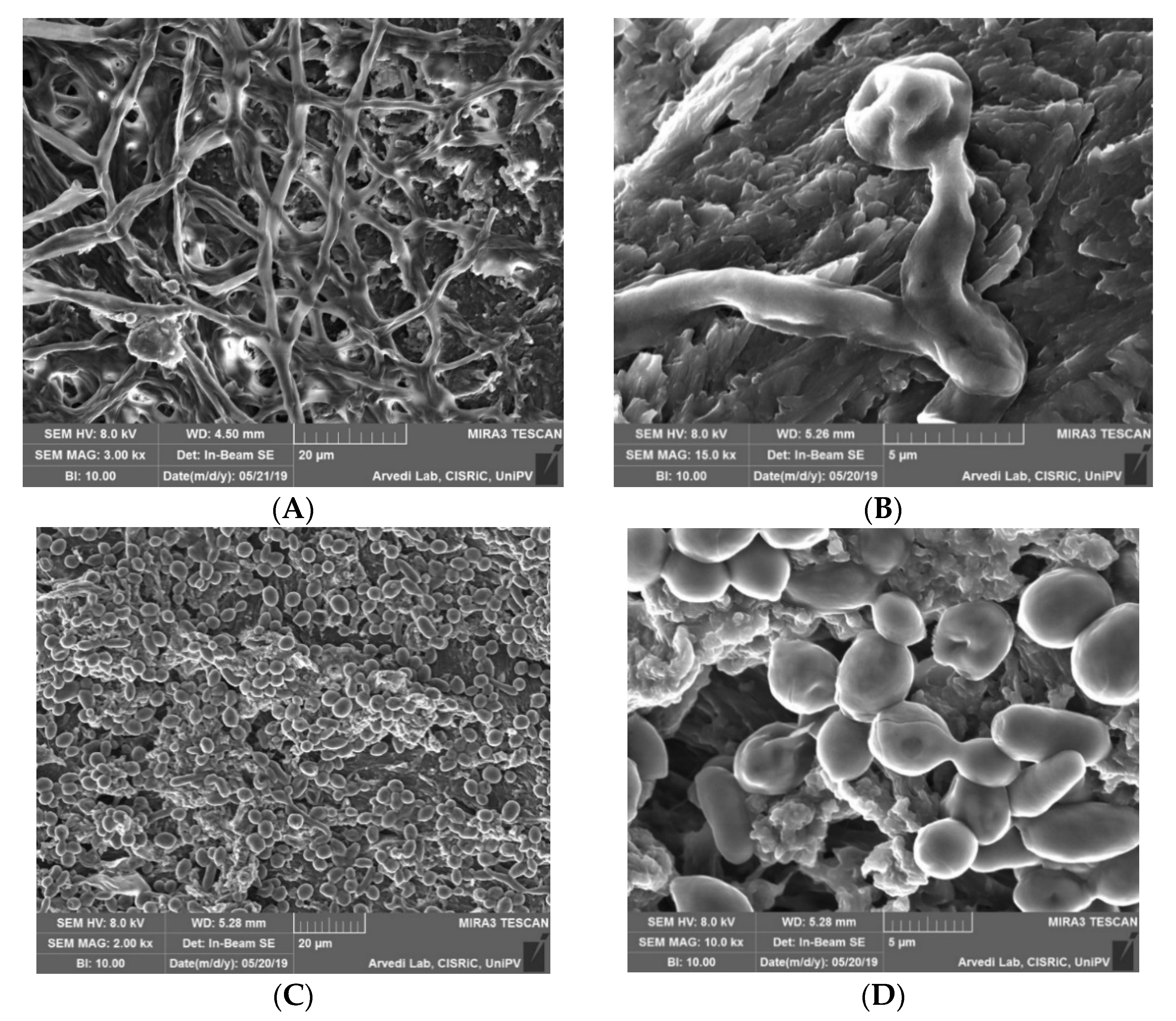
2.3.3. Morphological Analysis of Contaminated Membranes
2.4. In Vitro Evaluation of Reducing Fungal Adhesion to the Nail Plate
2.4.1. Sample Preparation
2.4.2. Test Procedure
2.4.3. Microbiological Assessment
2.4.4. Statistical Analyses
3. Results and Discussion
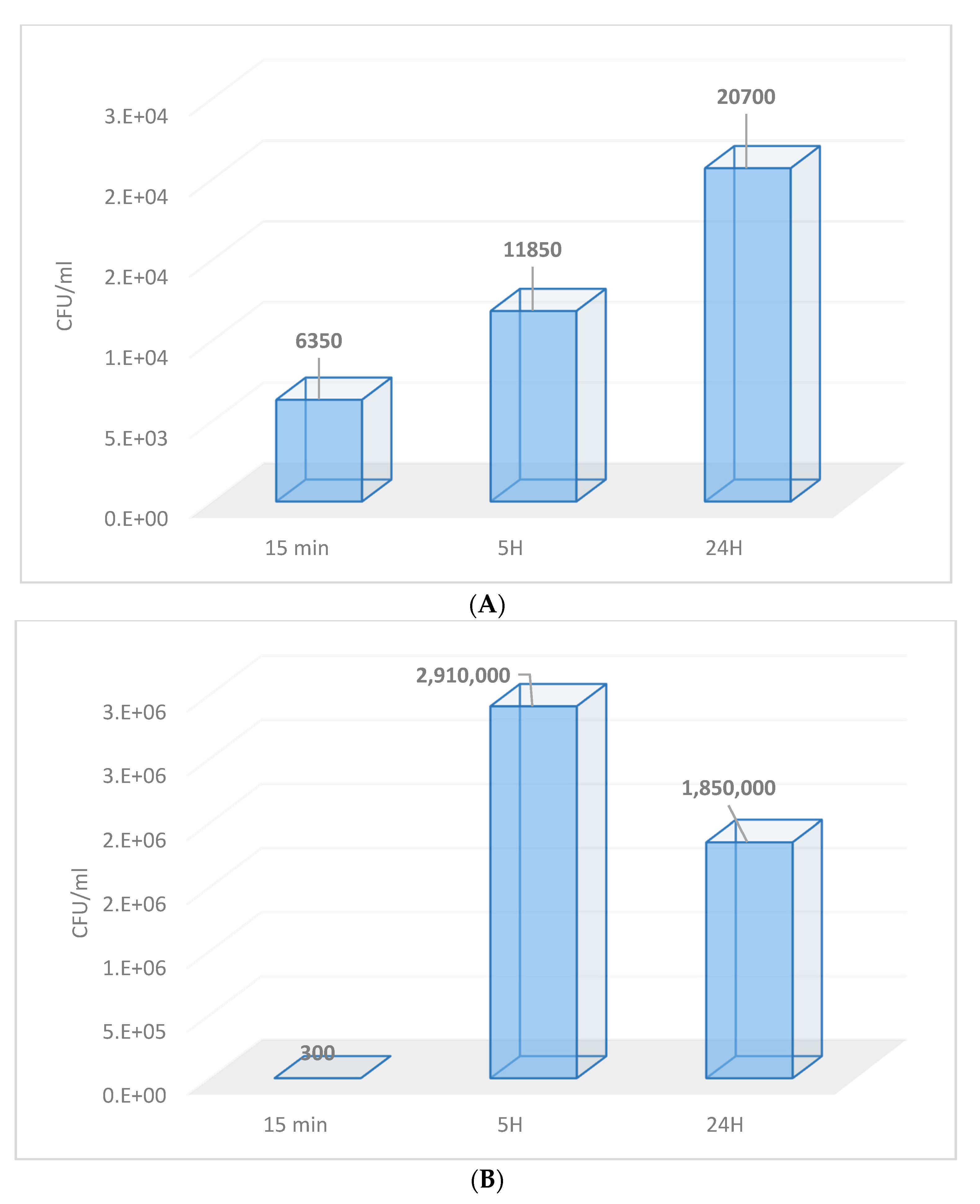
3.1. Contamination of Membranes by Fungi
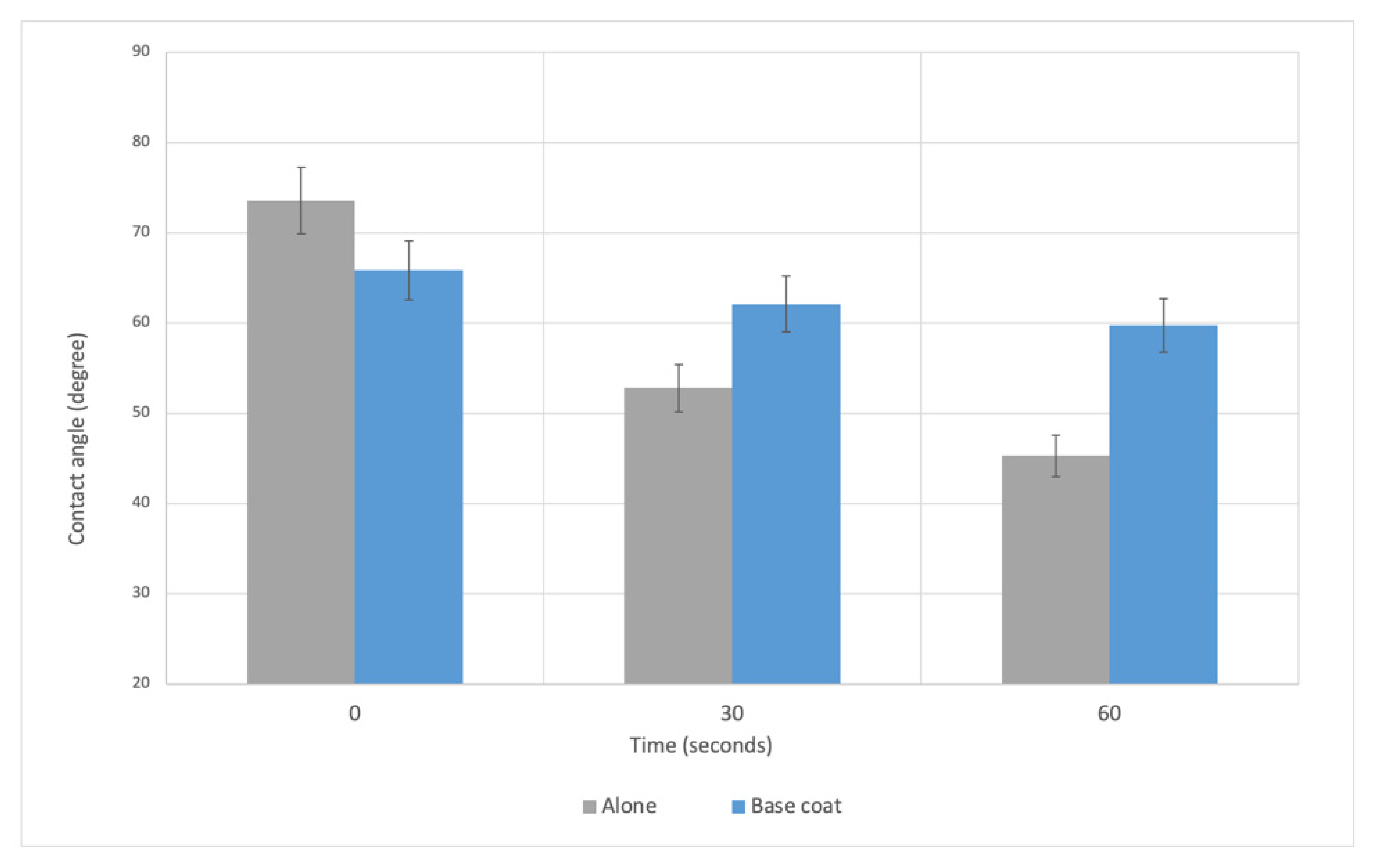
3.2. In Vitro Evaluation of Fungal Adhesion to the Nail Plate
3.2.1. Sample Preparation
3.2.2. Microbiological Assessment
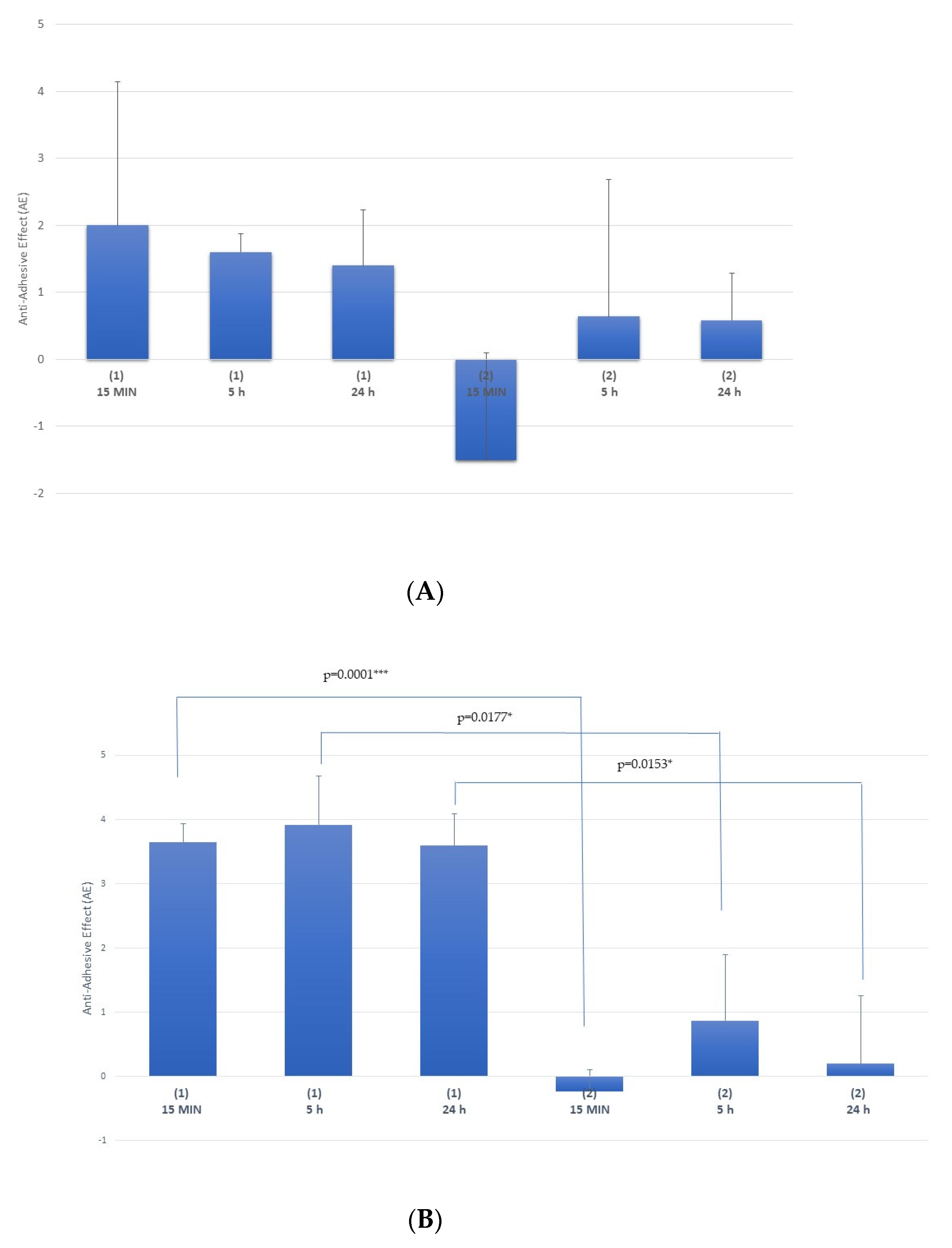
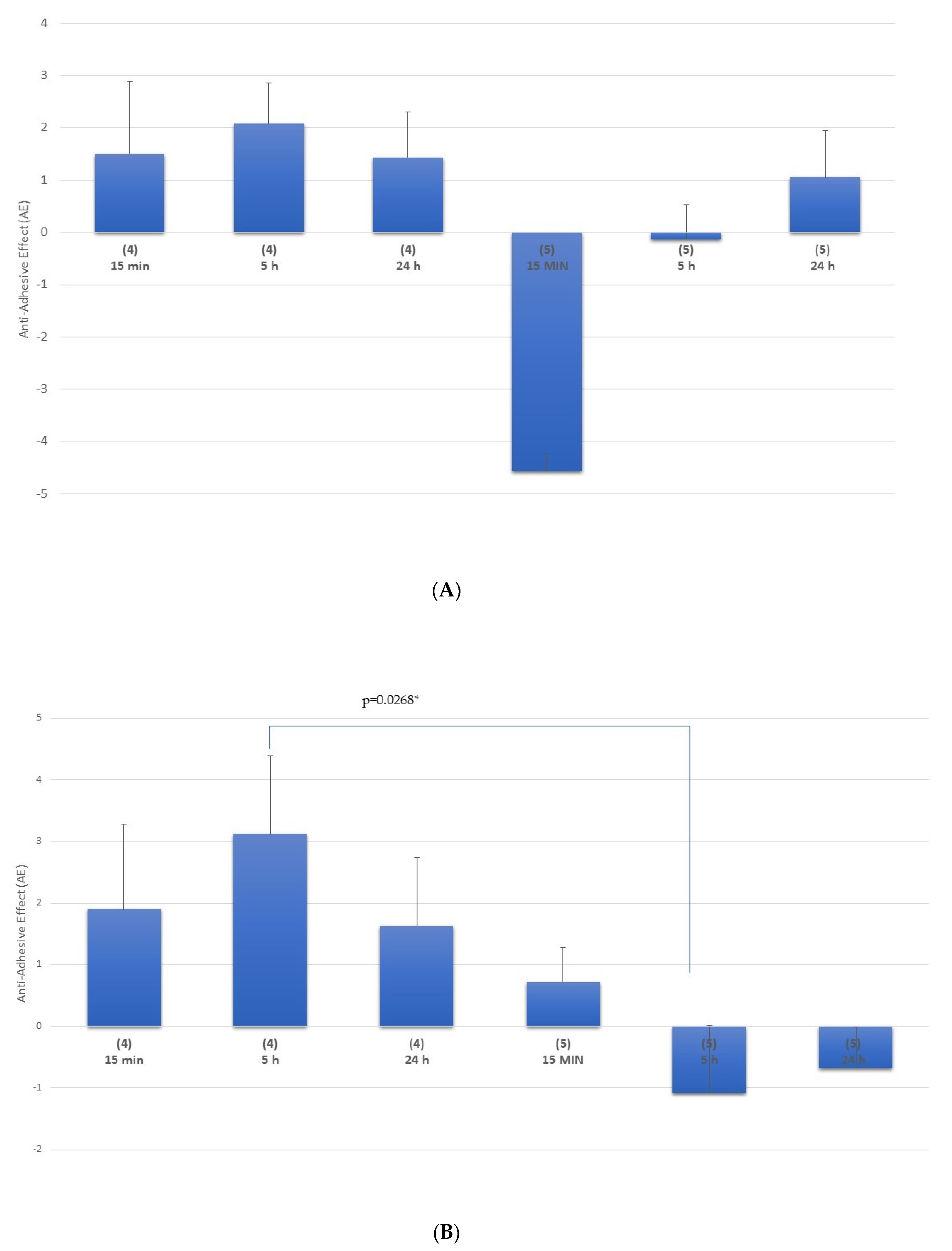
Multi-Treatment Procedure
Single-Treatment Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudela, J.L.R.; Denning, D.W. Recovery from serious fungal infections should be realisable for everyone. Lancet Infect. Dis. 2017, 17, 1111–1113. [Google Scholar] [CrossRef]
- Al-Khikhani, F.H. Dermatophytosis a Worldwide Contiguous Fungal Infection: Growing Challenge and Few Solutions. Biomed. Biotechnol. Res. J. 2020, 4, 117–122. [Google Scholar]
- Zhan, P.; Liu, W. The changing face of dermatophytic infections worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef]
- Hay, R. Fungal skin infections. Arch. Dis. Child. 1992, 67, 1065–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, R. Ecology and epidemiology of dermatophyte infections. J. Am. Acad. Dermatol. 1994, 31, 21–25. [Google Scholar] [CrossRef]
- Evans, E. Causative pathogens in onychomycosis and the possibility of treatment resistance: A review. J. Am. Acad. Dermatol. 1998, 38, 32–36. [Google Scholar] [CrossRef]
- Adeklandi, S.; Pal, S.; Sharma, N.; Juyal, D.; Sharma, M.; Dimri, D. Incidence and epidemiology of onychomycosis in patients visiting a tertiary care hospital in India. Cutis 2015, 95, E20–E25. [Google Scholar]
- Clayton, Y. Clinical and mycological diagnostic aspects of onychomycoses and dermatomycoses. Clin. Exp. Dermatol. 1992, 17, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Midgley, G.; Moore, M.K.; Cook, J.C.; Phan, Q.G. Mycology of nail disorders. J. Am. Acad. Dermatol. 1994, 31, 68–74. [Google Scholar] [CrossRef]
- Public Health England. UK standards for microbiology investigations B 39. In Investigations of Dermatological Specimens for Superificial Mycoses; Public Health English: London, UK, 2006; pp. 1–26. [Google Scholar]
- Elewsky, B.E. Large scale epidemiological study of the causal agents of onychomycosis: Mycological finding from the multicenter onychomycosis study of terbinafine. Arch. Dermatol. 1997, 133, 1317–1318. [Google Scholar] [CrossRef]
- Dompmartin, D.; Dompmartin, A.; Deluol, A.M.; Grosshans, E.; Coulaud, J.P. Onychomycosis and AIDS: Clinical laboratory findings in 62 patients. Int. J. Dermatol. 1990, 29, 337–339. [Google Scholar] [CrossRef]
- Elewsky, B.E. Onychomycosis: Pathogenesis, Diagnosis, and Management. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Scher, R.K.; Baran, R. Onychomycosis in clinical practice. Factors contributing to recurrence. Br. J. Dermatol. 2003, 149, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A. Update medical treatment of onychomycosis. Dermatol. Ther. 2012, 25, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.H.; Wong, T.E.; Yeung, K.C. Psychosocial perception of adults with onychomycosis: A blinded, controlled comparison of 1017 adult Hong Kong residents with or without onychomycosis. Biopsychosoc. Med. 2014, 15, 1–9. [Google Scholar]
- Tosti, A.; Elewski, B.E. Onychomycosis: Practical approaches to minimize relapse and recurrence. Skin Appendage Disord. 2016, 2, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Christenson, J.K.; Peterson, G.M.; Naunton, M.; Bushell, M.; Kosari, S.; Baby, K.E.; Thomas, J. Challenges and Opportunities in the Management of Onychomycosis. J. Fungi 2018, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Bonk, A.F.; Friedman, L.; Derbes, V.J. Experimental dermatophytosis. J. Investig. Derm. 1962, 39, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, J.H.; King, R.D.; Krebs, S.; Field, R. A quantitative dermatophyte infection model in the guinea pig—A parallel to the quantitated human infection model. J. Investig. Derm. 1967, 67, 704–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunte, D.M.; Hasselby, J.P.; Brillowska-Dabrowska, A.; Frimodt-Møller, N.; Svejgaard, E.L.; Linnemann, D.; Nielsen, S.S.; Haedersal, M.; Arendrup, M.C. Experimental guinea pig model of dermatophytosis: A simple and useful tool for the evaluation of new diagnostic. Med. Mycol. 2008, 46, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Klink, C.D.; Binnebosel, M.; Lambertz, A.; Alizai, H.P.; Roeth, A.; Otto, J.; Klinge, U.; Neumann, U.P.; Junge, K. In vitro and in vivo characteristics of gentamicin-supplemented polyvinylidenfluoride mesh materials. J. Biomed. Mater. Res. 2012, 100, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Moskowitz, J.S.; Veselinovic, J.; Rosario, R.A.; Timachova, K.; Blaisse, M.R.; Fuller, R.C.; Klibanov, A.M.; Hammond, P.T. Dual functional polyelectrolyte multilayer coatings for implants: Permanent microbicidal base with controlled release of therapeutic. J. Am. Chem. Soc. 2010, 132, 17840–17848. [Google Scholar] [CrossRef] [Green Version]
- Dijck, P.V.; Sjollema, J.; Cammue, B.P.A.; Lagrou, K.; Berman, J.; d’Enfert, C.; Andes, D.R.; Arendrup, M.C.; Brakhage, A.A.; Calderone, R.; et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb. Cell 2018, 5, 300–326. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Yokoo, M.; Senda, H.; Kakehi, K. Therapeutic efficacy of topically applied KP-103 against experimental tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 2002, 46, 3797–3801. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.; Borelli, C.; Berger, U.; Walker, B.; Schmidt, S.; Weindl, G.; Jackel, A. Susceptibility testing of amorolfine, bifonazole and ciclopiroxolamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med. Mycol. 2009, 47, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.; Mazzantini, D.; Tampucci, S.; Vecchione, A.; Celandroni, F.; Burgalassi, S. Ghelardi, Ciclopirox and Efinaconazole Transungual Permeation, Antifungal Activity, and Proficiency to Induce Resistance in Trichophyton rubrum. Antimicrob. Agents Chemother. 2019, 63, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueiras-Nieto, L. Hydration and N-acetyl-l-cysteine alter the microstructure of human nail and bovine hoof: Implication for drug delivery. J. Control. Release 2011, 156, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Sigurgeirsson, B.; Olafsson, J.H.; Steinsson, J.T.; Kerrouche, N.; Sidou, F. Efficacy of amorolfine nail lacquer for the prophylaxis of onychomycosis over 3 years. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Randall Lee, T. Contact Angle and Wetting Properties. Surf. Sci. Tech. Springer 2013, 51, 3–34. [Google Scholar]
- Russel, A.D.; Hugo, W.B.; Ayliffe, G.A.J. Principles and Practice of Disinfection, Preservation and Sterilization; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- 32 EN 13697:2019 Chemical Disinfectants and Antiseptics—Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements without Mechanical Action; CEN-CENELEC Management Centre: Brussels, Belgium, 2019.
- Guidance on the Biocidal Products Regulation Volume II Efficacy—Assessment and Evaluation (Parts B+C) Version 3.0; ECHA (European Chemical Agency): Helsinki, Finland, 2018.
- Li, R.; Wan, Z.; Wang, A.P.; Shen, Y.N.; Lu, C.M.; Li, M.; Xi, L.; Liu, W.D.; Zeng, F.K. In vitro susceptibility testing of amorolfine in pathogenic fungi isolated from dermatomycosis patients in China. Mycoses 2004, 47, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Jo Siu, W.; Tatsumi, Y.; Senda, H.; Pillai, R.; Nakamura, T.; Sone, D.; Fothergill, A. Comparison of In vitro Antifungal Activities of Efinaconazole and Currently Available Antifungal Agents against a Variety of Pathogenic Fungi Associated with Onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 1610–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciurea, C.N.; Kosovski, I.N.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]

| Multiple Comparisons Test against Tricophytum rubrum | ||||
| Comparison Groups | Mean Difference | 95.00% CI of Diff. | Significant | p Value |
| 1 vs. 4 | 1.503 | −0.2805 to 3.287 | No | 0.1018 |
| 1 vs. 2 | 3.440 | 1.656 to 5.224 | Yes | 0.0012 ** |
| 1 vs. 5 | 4.067 | 2.283 to 5.851 | Yes | 0.0004 *** |
| 4 vs. 2 | 1.937 | 0.1528 to 3.721 | Yes | 0.0341 * |
| 4 vs. 5 | 2.563 | 0.7795 to 4.347 | Yes | 0.0076 ** |
| 2 vs. 5 | 0.6267 | −1.157 to 2.411 | No | 0.8313 |
| Multiple Comparisons Test against Candida albicans | ||||
| Comparison Groups | Mean Difference | 95.00% CI of Diff. | Significant | p Value |
| 1 vs. 4 | −0.003333 | −4.240 to 4.233 | No | >0.9999 |
| 1 vs. 2 | 1.763 | −2.473 to 6.000 | No | 0.5695 |
| 1 vs. 5 | 2.883 | −1.353 to 7.120 | No | 0.2085 |
| 4 vs. 2 | 1.767 | −2.470 to 6.003 | No | 0.5682 |
| 4 vs. 5 | 2.887 | −1.350 to 7.123 | No | 0.2077 |
| 2 vs. 5 | 1.120 | −3.117 to 5.357 | No | 0.8313 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perugini, P.; Bonetti, M.; Guerini, M.; Musitelli, G.; Grisoli, P. A New In Vitro Model to Evaluate Anti-Adhesive Effect against Fungal Nail Infections. Appl. Sci. 2021, 11, 1977. https://doi.org/10.3390/app11051977
Perugini P, Bonetti M, Guerini M, Musitelli G, Grisoli P. A New In Vitro Model to Evaluate Anti-Adhesive Effect against Fungal Nail Infections. Applied Sciences. 2021; 11(5):1977. https://doi.org/10.3390/app11051977
Chicago/Turabian StylePerugini, Paola, Margherita Bonetti, Marta Guerini, Giorgio Musitelli, and Pietro Grisoli. 2021. "A New In Vitro Model to Evaluate Anti-Adhesive Effect against Fungal Nail Infections" Applied Sciences 11, no. 5: 1977. https://doi.org/10.3390/app11051977
APA StylePerugini, P., Bonetti, M., Guerini, M., Musitelli, G., & Grisoli, P. (2021). A New In Vitro Model to Evaluate Anti-Adhesive Effect against Fungal Nail Infections. Applied Sciences, 11(5), 1977. https://doi.org/10.3390/app11051977






