Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion
Abstract
1. Introduction
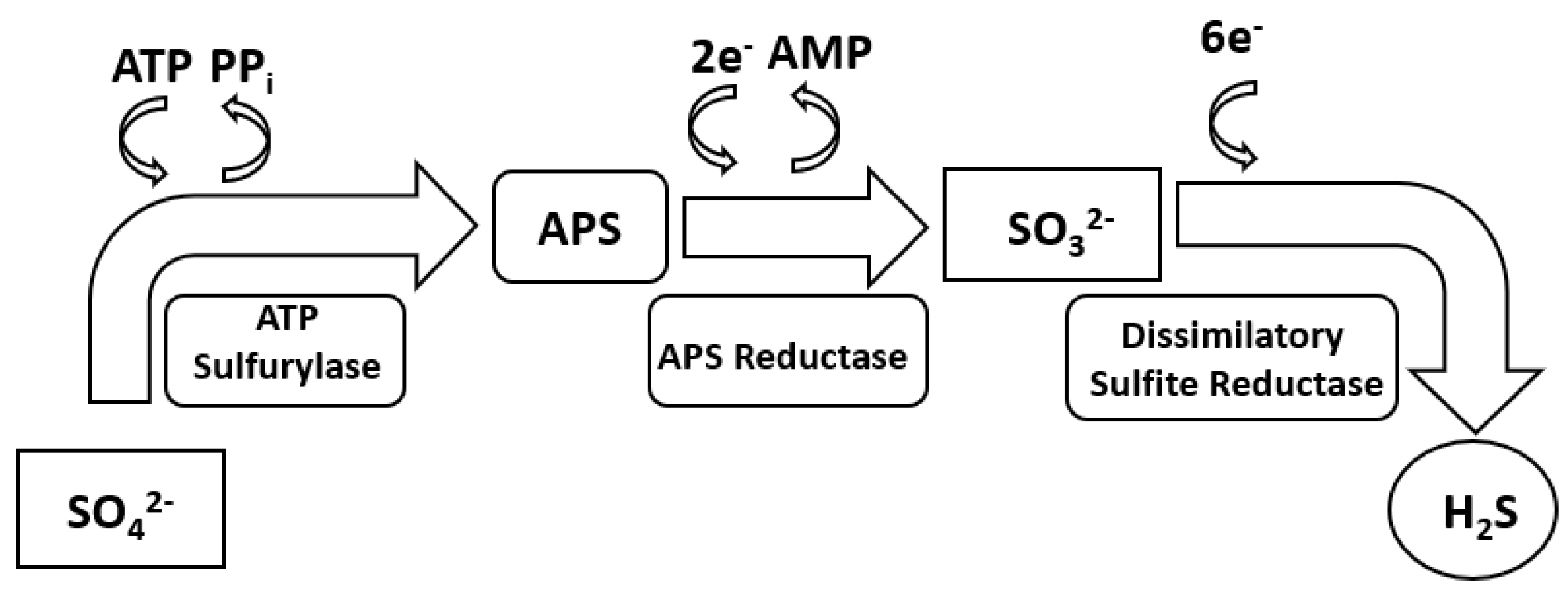
2. Sulphate Reduction Metabolism
3. SRB Activities in Acidic Environments
3.1. Response of SRB to Acidic Environments
3.1.1. The Maintenance of pH in Cytoplasm
Restriction of Proton Permeation
Increase in Pumping Proton Out of the Cytoplasm
Increase in Proton Consumption
3.1.2. Regulation of Protein Synthesis
3.1.3. Change in Metabolic Pathway
3.1.4. Other Factors Support Survival and Growth of SRB
3.2. Sulphate Reduction at Low pH
3.2.1. Proton Concentration
3.2.2. Organic Acid
3.2.3. Sulphide Concentration
3.2.4. Metals
4. SRB Activities in Alkaline Environment
4.1. Response of SRB to Alkaline Environment
4.1.1. pH Homeostasis
4.1.2. Cell Membrane Modification
4.1.3. Increase in Metabolic Production Change in Metabolic Pathway
4.1.4. Other Changes in Bacteria and Environment
4.2. Sulphate Reduction in Alkaline Environment
4.2.1. Environmental pH
4.2.2. Dissolved Sulphide Ion
4.2.3. Organic Matter
4.2.4. Metal/Mineral Precipitation
5. Microbial Corrosion by SRB
5.1. Microbial Corrosion in Acidic Environment
5.1.1. Environment Factors
5.1.2. Microbial Activities
5.2. Microbial Corrosion in Alkaline Environment
5.2.1. Environmental Factor
5.2.2. Microbial Activities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- John, N.; Vidyalakshmi, V.; Hatha, A.M. Effect of pH and salinity on the production of extracellular virulence factors by Aeromonas from food sources. J. Food Sci. 2019, 84, 2250–2255. [Google Scholar] [CrossRef]
- Leprince, F.; Quiquampoix, H. Extracellular enzyme activity in soil: Effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum. Eur. J. Soil Sci. 1996, 47, 511–522. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front. Environ. Sci 2018, 6, 21. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. pH as a primary control in environmental microbiology: 2. Kinetic perspective. Front. Environ. Sci 2018, 6, 101. [Google Scholar] [CrossRef]
- Widdel, F. Microbiology and ecology of sulfate-and sulfur-reducing bacteria. In Biology of Anaerobic Microorganisms, 99th ed.; Zehnder, A.J.B., Ed.; Wiley: California, CA, USA, 1988; Volume 1, pp. 469–585. [Google Scholar]
- Zagury, G.J.; Kulnieks, V.I.; Neculita, C.M. Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 2006, 64, 944–954. [Google Scholar] [CrossRef]
- Carlier, J.D.; Luís, A.T.; Alexandre, L.M.; Costa, M.C. Feasibility of Co-Treating Olive Mill Wastewater and Acid Mine Drainage. Mine. Water. Environ. 2020, 39, 1–22. [Google Scholar] [CrossRef]
- Abildgaard, L.; Nielsen, M.B.; Kjeldsen, K.U.; Ingvorsen, K. Desulfovibrio alkalitolerans sp. nov., a novel alkalitolerant, sulphate-reducing bacterium isolated from district heating water. Int. J. Syst. Evol. Microbiol. 2006, 56, 1019–1024. [Google Scholar] [CrossRef]
- Zhilina, T.N.; Zavarzin, G.A. Alkaliphilic anaerobic community at pH 10. Curr. Microbiol. 1994, 29, 109–112. [Google Scholar] [CrossRef]
- Ryzhmanova, Y.; Nepomnyashchaya, Y.; Abashina, T.; Ariskina, E.; Troshina, O.; Vainshtein, M.; Shcherbakova, V. New sulfate-reducing bacteria isolated from Buryatian alkaline brackish lakes: Description of Desulfonatronum buryatense sp. nov. Extremophiles 2013, 17, 851–859. [Google Scholar] [CrossRef]
- Sorokin, D.; Chernyh, N.; Poroshina, M. Desulfonatronobacter acetoxydans sp. nov.: A first acetate-oxidizing, extremely salt-tolerant alkaliphilic SRB from a hypersaline soda lake. Extremophiles 2015, 19, 899–907. [Google Scholar] [CrossRef]
- Sen, A.; Johnson, B. Acidophilic sulphate-reducing bacteria: Candidates for bioremediation of acid mine drainage. In Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 9, pp. 709–718. [Google Scholar]
- Johnson, D.B.; Ghauri, M.; McGinness, S. Biogeochemical cycling of iron and sulphur in leaching environments. EMS Microbiol. Rev. 1993, 11, 63–70. [Google Scholar] [CrossRef]
- Mori, K.; Kim, H.; Kakegawa, T.; Hanada, S. A novel lineage of sulfate-reducing microorganisms: Thermodesulfobiaceae fam. nov., Thermodesulfobium narugense, gen. nov., sp. nov., a new thermophilic isolate from a hot spring. Extremophiles 2003, 7, 283–290. [Google Scholar] [CrossRef]
- Thuy, T.T.; Krishnan, K.; Padovan, A.; Thennadil, S. Effect of pH regulation by sulphate reducing bacteria on corrosion behaviour of duplex stainless steel 2205 in acidic artificial seawater. R. Soc. Open Sci. 2020, 8, 1–13. [Google Scholar]
- Thuy, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. Effect of Alkaline Artificial Seawater Environment on the Corrosion Behaviour of Duplex Stainless Steel 2205. Appl. Sci. 2020, 10, 5043. [Google Scholar] [CrossRef]
- Jong, T.; Parry, D.L. Microbial sulfate reduction under sequentially acidic conditions in an upflow anaerobic packed bed bioreactor. Water. Res. 2006, 40, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.; Stams, A.J. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Cypionka, H. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 2000, 54, 827–848. [Google Scholar] [CrossRef]
- Bryant, M.; Campbell, L.L.; Reddy, C.; Crabill, M. Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl. Environ. Microbiol. 1977, 33, 1162–1169. [Google Scholar] [CrossRef]
- Koschorreck, M. Microbial sulphate reduction at a low pH. FEMS Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef]
- SATAKE, K.I. Microbial sulphate reduction in a volcanic acid lake having pH 1.8 to 2.0. Jpn. J. Limnnology 1977, 38, 33–35. [Google Scholar] [CrossRef]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, M.; Hou, B. Influence of sulfate-reducing bacteria on the corrosion behavior of high strength steel EQ70 under cathodic polarization. PLoS ONE 2016, 11, e0162315. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Zilberstein, D.; Schuldiner, S. pH homesstasis in bacteria. Biochim. Biophys. Acta. 1981, 650, 151–166. [Google Scholar] [CrossRef]
- Booth, I.R. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 1985, 49, 359. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta Biomembr 2005, 1717, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kang, D.-H. Survival mechanism of Escherichia coli O157: H7 against combined treatment with acetic acid and sodium chloride. Food Microbiol. 2016, 55, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Green, C.; Turner, J. Phosphatidylethanolamine distribution and fluidity in outer and inner membranes of the gram-negative bacterium Erwinia carotovora. Biochem. J. 1980, 188, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.A.; Cvitkovitch, D.G.; Bleiweis, A.S.; Kiriukhin, M.Y.; Debabov, D.V.; Neuhaus, F.C.; Hamilton, I.R. Defects in D-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 2000, 182, 6055–6065. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jiang, Z.; Lu, Y.; Yao, X.; Han, C.; Ouyang, Y.; Wang, H.; Guo, C.; Ling, F.; Dang, Z. Transcriptome Analysis of the Acid Stress Response of Desulfovibrio vulgaris ATCC 7757. Curr. Microbiol. 2020, 77, 2702–2712. [Google Scholar] [CrossRef]
- Amaro, A.; Chamorro, D.; Seeger, M.; Arredondo, R.; Peirano, I.; Jerez, C. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J. Bacteriol. 1991, 173, 910–915. [Google Scholar] [CrossRef]
- Kroll, R.; Booth, I. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem. J. 1981, 198, 691–698. [Google Scholar] [CrossRef]
- Zilberstein, D.; Agmon, V.; Schuldiner, S.; Padan, E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J. Biol. Chem. 1982, 257, 3687–3691. [Google Scholar] [CrossRef]
- Kobayashi, H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 1985, 260, 72–76. [Google Scholar] [CrossRef]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. Respiration and the F 1 Fo-ATPase enhance survival under acidic conditions in Escherichia coli. PLoS ONE 2012, 7, e52577. [Google Scholar] [CrossRef]
- Suzuki, T.; Murakami, T.; Iino, R.; Suzuki, J.; Ono, S.; Shirakihara, Y.; Yoshida, M. F0F1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of ϵ subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 2003, 278, 46840–46846. [Google Scholar] [CrossRef]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar] [CrossRef]
- Mols, M.; Abee, T. Bacillus cereus responses to acid stress. Environ. Microbiol. 2011, 13, 2835–2843. [Google Scholar] [CrossRef]
- Shimomura, H.; Hosoda, K.; Hayashi, S.; Yokota, K.; Hirai, Y. Phosphatidylethanolamine of Helicobacter pylori functions as a steroid-binding lipid in the assimilation of free cholesterol and 3β-hydroxl steroids into the bacterial cell membrane. J. Bacteriol. 2012, 194, 2658–2667. [Google Scholar] [CrossRef]
- Senouci-Rezkallah, K.; Schmitt, P.; Jobin, M.P. Amino acids improve acid tolerance and internal pH maintenance in Bacillus cereus ATCC14579 strain. Food Microbiol. 2011, 28, 364–372. [Google Scholar] [CrossRef]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef]
- Ryan, S.; Begley, M.; Gahan, C.G.; Hill, C. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: Regulation and role in acid tolerance. Environ. Microbiol. 2009, 11, 432–445. [Google Scholar] [CrossRef]
- Hersh, B.M.; Farooq, F.T.; Barstad, D.N.; Blankenhorn, D.L.; Slonczewski, J.L. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 1996, 178, 3978–3981. [Google Scholar] [CrossRef]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; McAuliffe, O.; Altermann, E.; Lick, S.; Russell, W.M.; Klaenhammer, T.R. Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl. Environ. Microbiol. 2005, 71, 5794–5804. [Google Scholar] [CrossRef]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef]
- Mates, A.K.; Sayed, A.K.; Foster, J.W. Products of the Escherichia coli acid fitness island attenuate metabolite stress at extremely low pH and mediate a cell density-dependent acid resistance. J. Bacteriol. 2007, 189, 2759–2768. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.J.; Castanheira, M.; Chopra, I. Characterization of global patterns and the genetics of fusidic acid resistance. Clin. Infect. Dis. 2011, 52, S487–S492. [Google Scholar] [CrossRef]
- Barton, L.L.; Fardeau, M.-L.; Fauque, G.D. Hydrogen sulfide: A toxic gas produced by dissimilatory sulfate and sulfur reduction and consumed by microbial oxidation. In The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment; Springer: Cham, Switerland, 2014; pp. 237–277. [Google Scholar]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef]
- Adhikari, S.; Curtis, P.D. DNA methyltransferases and epigenetic regulation in bacteria. FEMS Microbiol. Rev. 2016, 40, 575–591. [Google Scholar] [CrossRef]
- Fortin, D.; Davis, B.; Beveridge, T. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol. Ecol. 1996, 21, 11–24. [Google Scholar] [CrossRef]
- White, C.; Gadd, G. Copper accumulation by sulfate-reducing bacterial biofilms. FEMS Microbiol. Lett. 2000, 183, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Gadd, G. Heavy metal pollutants: Environmental and biotechnological aspects. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 321–334. [Google Scholar]
- Utgikar, V.P.; Harmon, S.M.; Chaudhary, N.; Tabak, H.H.; Govind, R.; Haines, J.R. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ. Toxicol. 2002, 17, 40–48. [Google Scholar] [CrossRef]
- Gu, T.; Zhao, K.; Nesic, S. A new mechanistic model for MIC based on a biocatalytic cathodic sulfate reduction theory. In Proceedings of the Corrosion Conference and Expo 2009, Atlanta, GA, USA, 22–26 March 2009. [Google Scholar]
- Bijmans, M.F.; Dopson, M.; Peeters, T.W.; Lens, P.N.; Buisman, C.J. Sulfate reduction at pH 5 in a high-rate membrane bioreactor: Reactor performance and microbial community analyses. J. Microbiol. Biotechnol. 2009, 19, 698–708. [Google Scholar] [CrossRef]
- Bayraktarov, E.; Price, R.E.; Ferdelman, T.G.; Finster, K. The pH and pCO2 dependence of sulfate reduction in shallow-sea hydrothermal CO2–venting sediments (Milos Island, Greece). Front. Microbiol. 2013, 4, 111. [Google Scholar] [CrossRef]
- Reis, M.; Almeida, J.; Lemos, P.; Carrondo, M. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Moosa, S.; Harrison, S. Product inhibition by sulphide species on biological sulphate reduction for the treatment of acid mine drainage. Hydrometallurgy 2006, 83, 214–222. [Google Scholar] [CrossRef]
- Banciu, H.L.; Sorokin, D.Y. Adaptation in haloalkaliphiles and natronophilic bacteria. In Polyextremophiles; Springer: Cham, Switerland, 2013; pp. 121–178. [Google Scholar]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Goeres, D.; Nielsen, P.H.; Smidt, H.; Frølund, B. The effect of alkaline pH conditions on a sulphate reducing consortium from a Danish district heating plant. Biofouling 1998, 12, 273–286. [Google Scholar] [CrossRef]
- Stolyar, S.; He, Q.; Joachimiak, M.P.; He, Z.; Yang, Z.K.; Borglin, S.E.; Joyner, D.C.; Huang, K.; Alm, E.; Hazen, T.C. Response of Desulfovibrio vulgaris to alkaline stress. J. Bacteriol. Res. 2007, 189, 8944–8952. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.; Glenn, A. Problems of Adverse pH and Bacterial Strategies to Combat It; Wiley: Chichester, UK, 1999. [Google Scholar]
- Skulachev, V.P.; Kobayashi, H.; Krulwich, T.; Schafer, G.; Fillingame, R.; Poole, R.; Cook, G.; Dimroth, M.; Konings, W.; Stock, J. Bacterial energetics at high pH: What happens to the H+ cycle when the extracellular H+ concentration decreases? In Novartis Foundation Symposium; Wiley: Chichester, UK, 1999; pp. 200–217. [Google Scholar]
- Saito, H.; Kobayashi, H. Bacterial responses to alkaline stress. Sci. Prog. 2003, 86, 271–282. [Google Scholar] [CrossRef]
- Hicks, D.B.; Liu, J.; Fujisawa, M.; Krulwich, T.A. F1F0-ATP synthases of alkaliphilic bacteria: Lessons from their adaptations. Biochim. Biophys. Acta 2010, 1797, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Mitchell, P. Molecule, group and electron translocation through natural membranes. Biochem. J. 1962, 83, 22. [Google Scholar]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–317. [Google Scholar]
- Vanhauteghem, D.; Janssens, G.P.J.; Lauwaerts, A.; Sys, S.; Boyen, F.; Cox, E.; Meyer, E. Exposure to the proton scavenger glycine under alkaline conditions induces Escherichia coli viability loss. PLoS ONE 2013, 8, e60328. [Google Scholar] [CrossRef]
- Mueller, E.A.; Egan, A.J.; Breukink, E.; Vollmer, W.; Levin, P.A. Plasticity of Escherichia coli cell wall metabolism promotes fitness and antibiotic resistance across environmental conditions. Elife 2019, 8, e40754. [Google Scholar] [CrossRef]
- Aono, R.; Ito, M.; Machida, T. Contribution of the cell wall component teichuronopeptide to pH homeostasis and alkaliphily in the alkaliphile Bacillus lentus C-125. J. Bacteriol. 1999, 181, 6600–6606. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, P.J.; Hodges, H.L.; Kraft, M.B.; Marando, V.M.; Kiessling, L.L. Bacterial cell wall modification with a glycolipid substrate. J. Am. Chem. Soc. 2019, 141, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Stancik, L.M.; Stancik, D.M.; Schmidt, B.; Barnhart, D.M.; Yoncheva, Y.N.; Slonczewski, J.L. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 2002, 184, 4246–4258. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. An acid/alkaline stress and the addition of amino acids induce a prolonged viability of Lactobacillus plantarum loaded into alginate gel. Int. J. Food Microbiol. 2010, 142, 242–246. [Google Scholar] [CrossRef]
- Al Shoffe, Y. Susceptibility and Expression of Chilling Injury. In Reference Module in Food Science; Elsiver: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Li, X.; Luo, Q.; Wofford, N.Q.; Keller, K.L.; McInerney, M.J.; Wall, J.D.; Krumholz, L.R. A molybdopterin oxidoreductase is involved in H2 oxidation in Desulfovibrio desulfuricans G20. J. Bacteriol. 2009, 191, 2675–2682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, W.; Mathews, A. Lactic acid production from lactose by Lactobacillus plantarum: Kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Rahmani, A.; Mathien, C.; Bidault, A.; Le Goïc, N.; Paillard, C.; Pichereau, V. External pH modulation during the growth of Vibrio tapetis, the etiological agent of Brown Ring Disease. J. Appl. Microbiol. 2020, 129, 3–16. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, G.; Mao, Z.; Fang, Z.; Yang, C. Precipitation of valuable metals from bioleaching solution by biogenic sulfides. Miner. Eng. 2009, 22, 289–295. [Google Scholar] [CrossRef]
- Berner, R. Sulphate reduction, organic matter decomposition and pyrite formation. Philos. Trans. Royal Soc. A 1985, 315, 25–38. [Google Scholar]
- Glombitza, C.; Stockhecke, M.; Schubert, C.J.; Vetter, A.; Kallmeyer, J. Sulfate reduction controlled by organic matter availability in deep sediment cores from the saline, alkaline Lake Van (Eastern Anatolia, Turkey). Front. Microbiol. 2013, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Campbell, C.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Curtin, D.; Peterson, M.E.; Anderson, C.R. pH-dependence of organic matter solubility: Base type effects on dissolved organic C, N, P, and S in soils with contrasting mineralogy. Geoderma 2016, 271, 161–172. [Google Scholar] [CrossRef]
- Jones, D.; Amy, P. A thermodynamic interpretation of microbiologically influenced corrosion. Corrosion 2002, 58, 638–645. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically Influenced Corrosion; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 3. [Google Scholar]
- Viles, H. Acid corrosion (of stone and metal). In Environmental Geology. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Panossian, Z.; de Almeida, N.L.; de Sousa, R.M.F.; de Souza Pimenta, G.; Marques, L.B.S. Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: A review. Corros. Sci. 2012, 58, 1–11. [Google Scholar] [CrossRef]
- Mehmeti, V.V.; Berisha, A.R. Corrosion study of mild steel in aqueous sulfuric acid solution using 4-methyl-4H-1, 2, 4-Triazole-3-Thiol and 2-mercaptonicotinic acid—an experimental and theoretical study. Front. Chem. 2017, 5, 61. [Google Scholar] [CrossRef]
- Damon, G.H. Acid corrosion of steel. J. Ind. Eng. Chem. 1941, 33, 67–69. [Google Scholar] [CrossRef]
- Ismail, M.; Noor, N.M.; Yahaya, N.; Abdullah, A.; Rasol, R.M.; Rashid, A.S.A. Effect of pH and temperature on corrosion of steel subject to sulphate-reducing bacteria. Environ. Sci. Technol. 2014, 7, 209–217. [Google Scholar] [CrossRef]
- Iliyasu, I.; Yawas, D.; Aku, S. Corrosion behavior of austenitic stainless steel in sulphuric acid at various concentrations. Adv. Appl. Sci. Res. 2012, 3, 3909–3915. [Google Scholar]
- Beech, I.B. Sulfate-reducing bacteria in biofilms on metallic materials and corrosion. Microbiol. Today 2003, 30, 115–117. [Google Scholar]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Zuo, R.; Kus, E.; Mansfeld, F.; Wood, T.K. The importance of live biofilms in corrosion protection. Corros. Sci. 2005, 47, 279–287. [Google Scholar] [CrossRef]
- Nagiub, A.; Mansfeld, F. Evaluation of microbiologically influenced corrosion inhibition (MICI) with EIS and ENA. Electrochim. Acta. 2002, 47, 2319–2333. [Google Scholar] [CrossRef]
- Jayaraman, A.; Earthman, J.; Wood, T.K. Corrosion inhibition by aerobic biofilms on SAE 1018 steel. Appl. Microbiol. Biotechnol. 1997, 47, 62–68. [Google Scholar] [CrossRef]
- Chongdar, S.; Gunasekaran, G.; Kumar, P. Corrosion inhibition of mild steel by aerobic biofilm. Electrochim. Acta. 2005, 50, 4655–4665. [Google Scholar] [CrossRef]
- Talukdar, A.; Rajaraman, P.V. Investigation of Acetic Acid Effect on Carbon Steel Corrosion in CO2–H2S Medium: Mechanistic Reaction Pathway and Kinetics. ACS Omega 2020, 5, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.-R.; Lee, H.-J.; Lee, H.-K.; Kim, Y.-K.; Oh, Y.-S.; Choi, S.-C. Involvement of organic acid during corrosion of iron coupon by Desulfovibrio desulfuricans. J. Microbiol. Biotechnol. 2003, 13, 937–941. [Google Scholar]
- Xin, L.; Xu, C.; Wuqi, S.; Jiaxing, Y.; Ming, W. Effect of pH Value on Microbial Corrosion Behavior of X70 Steel in a Sea Mud Extract Simulated Solution. J. Chin. Soc. Corros. Sci. Prot. 2019, 38, 565–572. [Google Scholar]
- Speller, F.; Texter, C. Effect of Alkaline Solutions on the Corrosion of Steel Immersed in Water. Ind. Eng. Chem. Res. 1924, 16, 393–397. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.; Li, X.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim. Acta. 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Tang, D.Z.; Du, Y.X.; Lu, M.X.; Liang, Y.; Jiang, Z.T.; Dong, L. Effect of pH value on corrosion of carbon steel under an applied alternating current. Mater. Corros. 2015, 66, 1467–1479. [Google Scholar] [CrossRef]
- Smart, N.; Rance, A.; Nixon, D.; Fennell, P.; Reddy, B.; Kursten, B. Summary of studies on the anaerobic corrosion of carbon steel in alkaline media in support of the Belgian supercontainer concept. Corros. Eng. Sci. Technol. 2017, 52, 217–226. [Google Scholar] [CrossRef]
- Senior, N.A.; Martino, T.; Diomidis, N. The anoxic corrosion behaviour of carbon steel in anoxic alkaline environments simulating a Swiss L/ILW repository environment. Mater. Corros. 2020, 72, 131–140. [Google Scholar] [CrossRef]
- King, F. Corrosion of Copper in Alkaline Chloride Environments; Swedish Nuclear Fuel and Waste Management Co.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Tabrizi, M.; Lyon, S.; Thompson, G.; Ferguson, J. The long-term corrosion of aluminium in alkaline media. Corros. Sci. 1991, 32, 733–742. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Giron, R.G.P.; Chen, X.; La Plante, E.C.; Gussev, M.N.; Leonard, K.J.; Sant, G. Revealing how alkali cations affect the surface reactivity of stainless steel in alkaline aqueous environments. ACS Omega 2018, 3, 14680–14688. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Hyökyvirta, O.; Saario, T. Effect of sulphide on the corrosion behaviour of AISI 316L stainless steel and its constituent elements in simulated Kraft digester conditions. Corros. Sci. 2010, 52, 1499–1507. [Google Scholar] [CrossRef]
- Wiener, M.S.; Salas, B.; Quintero-Nunez, M.; Zlatev, R. Effect of H2S on corrosion in polluted waters: A review. Corros. Eng. Sci. Technol. 2006, 41, 221–227. [Google Scholar] [CrossRef]
- Davoodi, A.; Pakshir, M.; Babaiee, M.; Ebrahimi, G.R. A comparative H2S corrosion study of 304L and 316L stainless steels in acidic media. Corros. Sci. 2011, 53, 399–408. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Starosvetsky, J.; Armon, R.; Ein-Eli, Y. A peculiar cathodic process during iron and steel corrosion in sulfate reducing bacteria (SRB) media. Corros. Sci. 2010, 52, 1536–1540. [Google Scholar] [CrossRef]
- Park, J.O.; Böhni, H. Local pH measurements during pitting corrosion at MnS inclusions on stainless steel. Electrochem. Solid. St. 2000, 3, 416–417. [Google Scholar] [CrossRef]
- Williams, D.E.; Kilburn, M.R.; Cliff, J.; Waterhouse, G. Composition changes around sulphide inclusions in stainless steels, and implications for the initiation of pitting corrosion. Corros. Sci. 2010, 52, 3702–3716. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Abdullah, A.; Yahaya, N.; Md Noor, N.; Mohd Rasol, R. Microbial corrosion of API 5L X-70 carbon steel by ATCC 7757 and consortium of sulfate-reducing bacteria. J. Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Musić, S.; Nowik, I.; Ristić, M.; Orehovec, Z.; Popović, S. The effect of bicarbonate/carbonate ions on the formation of iron rust. Croat. Chem. Acta. 2004, 77, 141–151. [Google Scholar]
- Troup, D.; Richardson, J. The Link Between the Corrosion and calcium carbonate scaling susceptibilities of heat transfer surfaces. Mater. Corros. 1978, 29, 312–320. [Google Scholar] [CrossRef]
- Ahmad, E.A.; Chang, H.-Y.; Al-Kindi, M.; Joshi, G.R.; Cooper, K.; Lindsay, R.; Harrison, N.M. Corrosion protection through naturally occurring films: New insights from iron carbonate. ACS Appl. Mater. Interfaces 2019, 11, 33435–33441. [Google Scholar] [CrossRef]
- Li, S.; Zeng, Z.; Harris, M.A.; Sánchez, L.J.; Cong, H. CO2 corrosion of low carbon steel under the joint effects of time-temperature-salt concentration. Front. Mater. 2019, 6, 10. [Google Scholar] [CrossRef]
- Schaller, R.F.; Jove-Colon, C.F.; Taylor, J.M.; Schindelholz, E.J. The controlling role of sodium and carbonate on the atmospheric corrosion rate of aluminum. NPJ Mater. Degrad. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Powell, S.T.; Bacon, H.; Lill, J. Corrosion prevention by controlled calcium carbonate scale. J. Ind. Eng. Chem. 1945, 37, 842–846. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Choi, Y.-S.; Young, D.; Nesic, S. Effect of Calcium on the Formation and Protectiveness of the Iron Carbonate Layer in CO2 Corrosion; Ohio University: Athens, OH, USA, 2013. [Google Scholar]

| Material Type | SRB Species | Solution | Corrosion Rate at Different pH (10−3 mm/Year) | Ref. | Note | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.0 | 5 | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | 9.0 | 9.5 | 10 | |||||
| DSS 2205 | D. vulgaris | Artificial sea water | 6.1 | 1.0 | - | 0.6 | - | - | 0.5 | - | - | - | - | - | [17] | Calculated from current density |
| DSS 2205 | D. vulgaris | Artificial sea water | - | - | - | - | - | - | 1.9 | 2.6 | - | 10.1 | - | 3.3 | [18] | Calculated from current density |
| CS API 5L X70 | D. vulgaris | Modified Baar’s medium | - | - | 13.1 | 8.5 | 6.8 | 12 | 7.5 | 9.4 | 11.4 | 13.1 | 14 | - | [102] | - |
| CS API 5L X70 | Not identified | Artificial sea mud extract | - | - | - | 18.4 | - | - | - | 26.8 | - | - | - | 21.3 | [112] | Calculated from current density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.T.; Kannoorpatti, K.; Padovan, A.; Thennadil, S. Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion. Appl. Sci. 2021, 11, 2201. https://doi.org/10.3390/app11052201
Tran TTT, Kannoorpatti K, Padovan A, Thennadil S. Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion. Applied Sciences. 2021; 11(5):2201. https://doi.org/10.3390/app11052201
Chicago/Turabian StyleTran, Thi Thuy Tien, Krishnan Kannoorpatti, Anna Padovan, and Suresh Thennadil. 2021. "Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion" Applied Sciences 11, no. 5: 2201. https://doi.org/10.3390/app11052201
APA StyleTran, T. T. T., Kannoorpatti, K., Padovan, A., & Thennadil, S. (2021). Sulphate-Reducing Bacteria’s Response to Extreme pH Environments and the Effect of Their Activities on Microbial Corrosion. Applied Sciences, 11(5), 2201. https://doi.org/10.3390/app11052201







