Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma-Activated Water Preparation and Physicochemical Analysis
2.2. Plant Material and Growth Conditions
2.3. Morphological Analysis
2.4. Microscopy
2.5. RNA Extraction and Real-Time Polymerase Chain Reaction (PCR) Analysis
3. Results
3.1. Plasma Diagnostics and Plasma-Activated Water (PAW) Characteristics
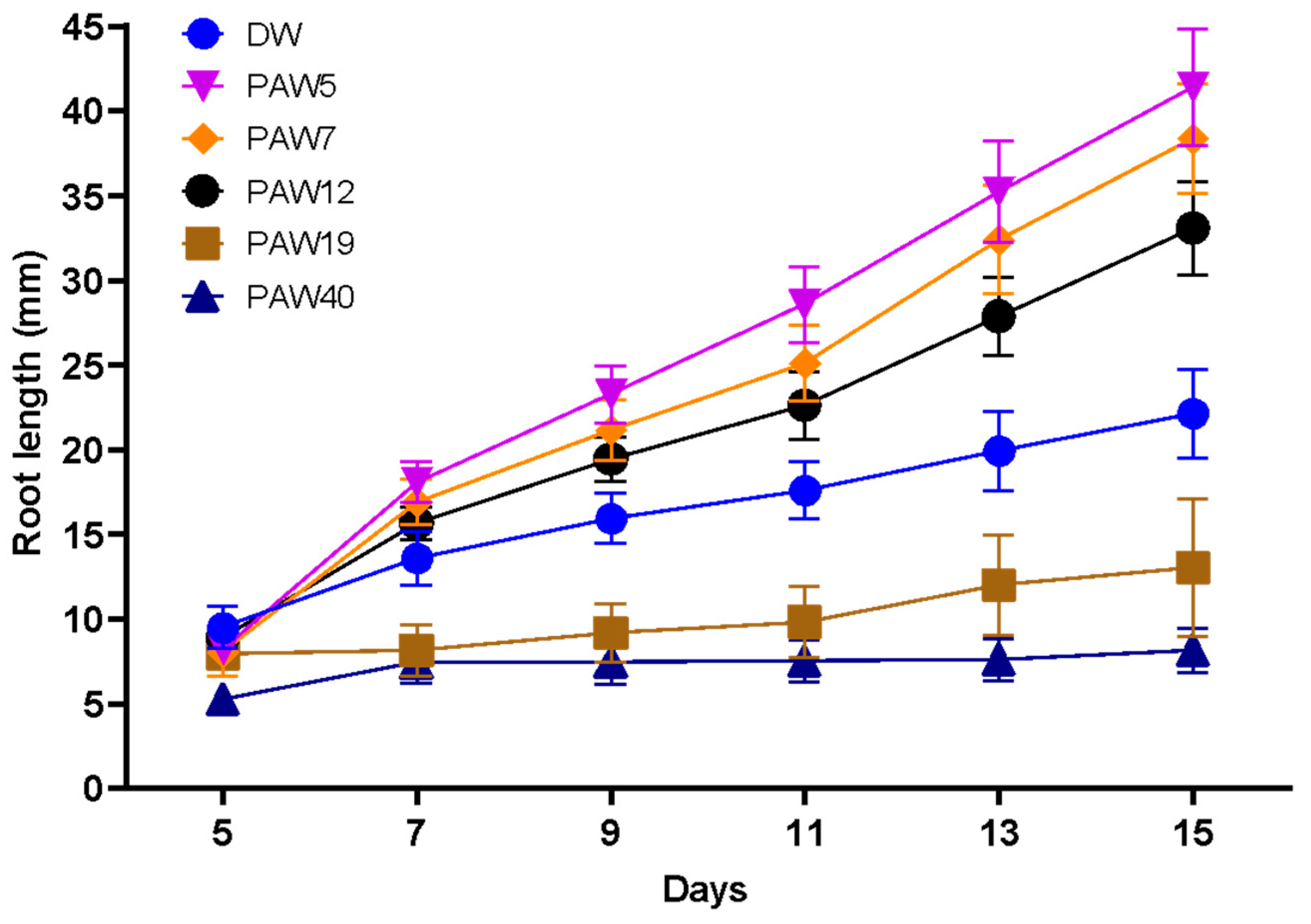
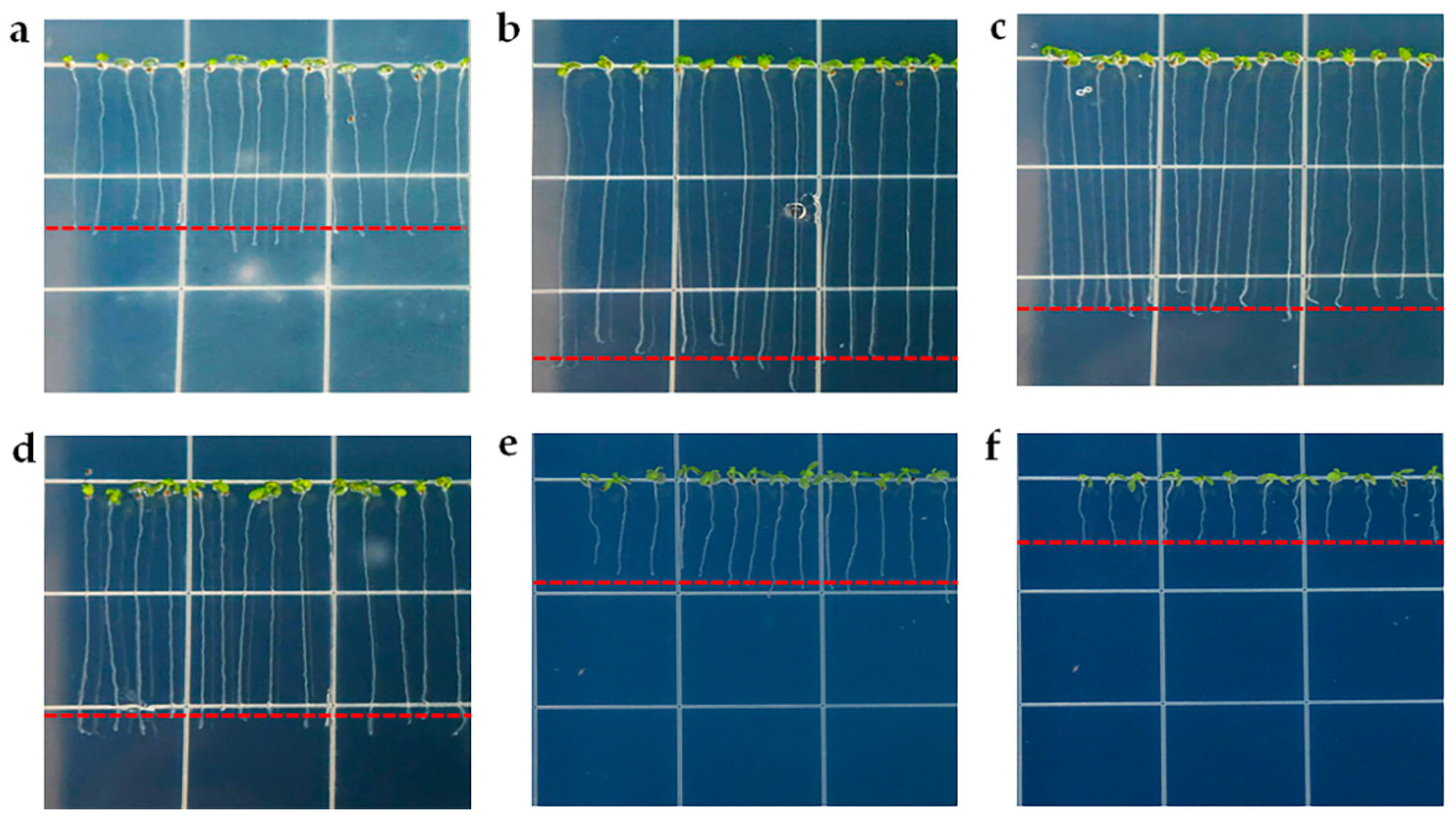
3.2. PAW Increases the Total Root Length and Root Hair Number in the Arabidopsis Root
3.3. PAW Treatment Increases the Cotyledon Size Dependent on Palisade Cell Enlargement
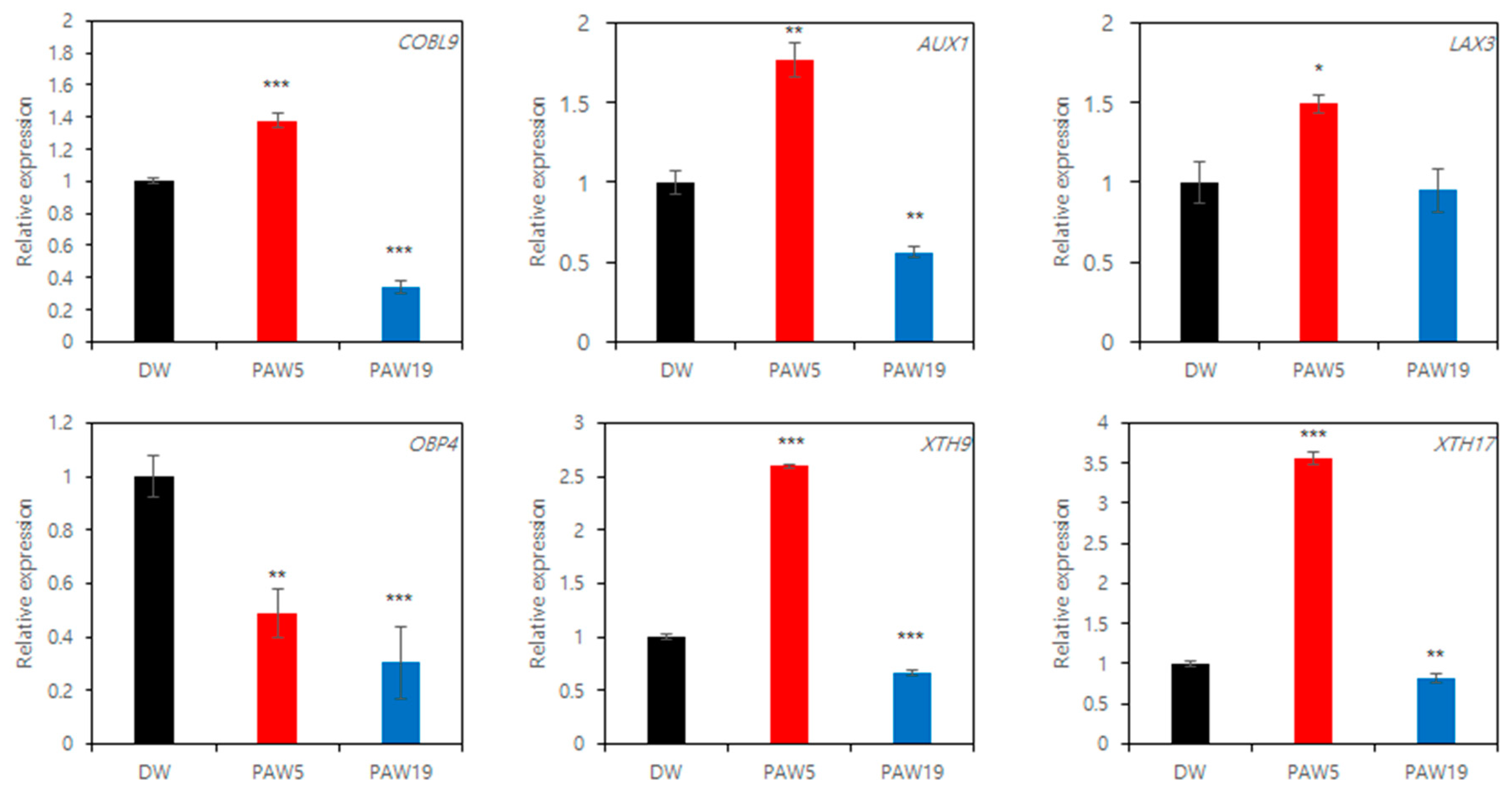
3.4. PAW Modulates the Root Hair Density through Root Developmental Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Ito, M.; Ohta, T.; Hori, M. Plasma Agriculture. J. Korean Phys. Soc. 2012, 60, 937–943. [Google Scholar] [CrossRef]
- Ohta, T. Plasma in Agriculture. In Cold Plasma in Food and Agriculture; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–221. ISBN 978-0-12-801365-6. [Google Scholar]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene Expression in Tomato Seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of Plasma-Activated Water Treatment on Seed Germination and Growth of Mung Bean Sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of Low Temperature Plasmas and Plasma Activated Waters on Arabidopsis Thaliana Germination and Growth. PLoS ONE 2018, 13, e0195512. [Google Scholar] [CrossRef] [PubMed]
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-Term Antibacterial Efficacy of Air Plasma-Activated Water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-prochnow, A.; Lu, X.P.; Cullen, P.; Ostrikov, K. (Ken); Bazaka, K. Plasma Activated Water (PAW): Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting Lentil Germination and Stem Growth by Plasma Activated Tap Water, Demineralized Water and Liquid Fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.B.; Bakken, L.B.; Jensen, M.B.; Ingels, R. Plasma Activated Organic Fertilizer. Plasma Chem. Plasma Process. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; Liseron-Monfils, C.; Bagman, A.M.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.; et al. Transcriptional Regulation of Nitrogen-Associated Metabolism and Growth. Nature 2018, 563, 259–264. [Google Scholar] [CrossRef]
- Ueda, Y.; Yanagisawa, S. Engineering Nitrogen Utilization in Crop. Plants; Springer International Publishing: Basingstoke, UK, 2018; ISBN 978-3-319-92957-6. [Google Scholar]
- Chandran, A.K.N.; Priatama, R.A.; Kumar, V.; Xuan, Y.; Je, B.I.; Kim, C.M.; Jung, K.H.; Han, C.D. Genome-Wide Transcriptome Analysis of Expression in Rice Seedling Roots in Response to Supplemental Nitrogen. J. Plant Physiol. 2016, 200, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Sarinont, T.; Katayama, R.; Wada, Y.; Koga, K.; Shiratani, M. Plant Growth Enhancement of Seeds Immersed in Plasma Activated Water. MRS Adv. 2017, 2, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Sivachandiran, L.; Khacef, A. Enhanced Seed Germination and Plant Growth by Atmospheric Pressure Cold Air Plasma: Combined Effect of Seed and Water Treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-S.; Lee, M.J.; Ra, J.E.; Lee, K.S.; Eom, S.; Ham, H.M.; Kim, H.Y.; Kim, S.B.; Lim, J. Growth and Bioactive Phytochemicals in Barley (Hordeum Vulgare L.) Sprouts Affected by Atmospheric Pressure Plasma during Seed Germination. J. Phys. Appl. Phys. 2020, 53, 314002. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold Plasma Seed Priming Modulates Growth, Redox Homeostasis and Stress Response by Inducing Reactive Species in Tomato (Solanum Lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C. Cold Plasma: A Novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Meinke, D.W.; Cherry, J.M.; Dean, C.; Rounsley, S.D.; Koornneef, M. Arabidopsis Thaliana: A Model Plant for Genome Analysis. Science 1998, 282, 662–682. [Google Scholar] [CrossRef] [Green Version]
- The Arabidopsis Genome Initiative. Analysis of the Genome Sequence of the Flowering Plant Arabidopsis Thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Lim, J.; Hong, E.J.; Kim, S.B. Plasma-Activated Water Regulates Root Hairs and Cotyledon Size Dependent on Cell Elongation in Nicotiana Tabacum L. Plant Biotechnol. Rep. 2020, 14, 663–672. [Google Scholar] [CrossRef]
- Lee, M.M.; Schiefelbein, J. Cell Pattern in the Arabidopsis Root Epidermis Determined by Lateral Inhibition with Feedback. Plant Cell 2002, 14, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Bruex, A.; Kainkaryam, R.M.; Wieckowski, Y.; Kang, Y.H.; Bernhardt, C.; Xia, Y.; Zheng, X.; Wang, J.Y.; Lee, M.M.; Benfey, P.; et al. A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis. PLoS Genet. 2012, 8, e1002446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, G.; Ferjani, A.; Fujikura, U.; Tsukaya, H. Coordination of Cell Proliferation and Cell Expansion in the Control of Leaf Size in Arabidopsis Thaliana. J. Plant Res. 2006, 119, 37–42. [Google Scholar] [CrossRef]
- Aiken, R.M.; Smucker, A.J.M. Root System Regulation of Whole Plant Growth. Annu. Rev. Phytopathol. 1996, 34, 325–346. [Google Scholar] [CrossRef] [Green Version]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root Hairs. Arab. Book 2014, 12, e0172. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Kim, C.M.; Pernas, M.; Pires, N.D.; Proust, H.; Tam, T.; Vijayakumar, P.; Dolan, L. Root Hairs: Development, Growth and Evolution at the Plant-Soil Interface. Plant Soil 2011, 346, 1–14. [Google Scholar] [CrossRef]
- Julkowska, M. Releasing the Cytokinin Brakes on Root Growth. Plant Physiol. 2018, 177, 865–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, G.; Marchant, A.; May, S.; Swarup, R.; Swarup, K.; James, N.; Graham, N.; Allen, T.; Martucci, T.; Yemm, A.; et al. Quick on the Uptake: Characterization of a Family of Plant Auxin Influx Carriers. J. Plant Growth Regul. 2001, 20, 217–225. [Google Scholar] [CrossRef]
- Schindelman, G. COBRA Encodes a Putative GPI-Anchored Protein, Which Is Polarly Localized and Necessary for Oriented Cell Expansion in Arabidopsis. Genes Dev. 2001, 15, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Swarup, R.; Bhosale, R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Cai, W. Nitrate-Responsive OBP4-XTH9 Regulatory Module Controls Lateral Root Development in Arabidopsis Thaliana. PLoS Genet. 2019, 15, e1008465. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Rhee, J.Y.; Lee, S.H.; Chung, G.C.; Park, S.J.; Segami, S.; Maeshima, M.; Choi, G. Functionally Redundant LNG3 and LNG4 Genes Regulate Turgor-Driven Polar Cell Elongation through Activation of XTH17 and XTH24. Plant Mol. Biol. 2018, 97, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Maris, A.; Suslov, D.; Fry, S.C.; Verbelen, J.-P.; Vissenberg, K. Enzymic Characterization of Two Recombinant Xyloglucan Endotransglucosylase/Hydrolase (XTH) Proteins of Arabidopsis and Their Effect on Root Growth and Cell Wall Extension. J. Exp. Bot. 2009, 60, 3959–3972. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.; Gill, J.; Ruzic, D.N. Growth of Plasma-Treated Corn Seeds under Realistic Conditions. Sci. Rep. 2019, 9, 4355. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Sajib, S.A.; Rahi, M.S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, M.A.; Talukder, M.R.; Kabir, A.H. Mechanisms and Signaling Associated with LPDBD Plasma Mediated Growth Improvement in Wheat. Sci. Rep. 2018, 8, 10498. [Google Scholar] [CrossRef] [Green Version]
- del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Réthoré, E.; d’Andrea, S.; Benamar, A.; Cukier, C.; Tolleter, D.; Limami, A.M.; Avelange-Macherel, M.-H.; Macherel, D. Arabidopsis Seedlings Display a Remarkable Resilience under Severe Mineral Starvation Using Their Metabolic Plasticity to Remain Self-Sufficient for Weeks. Plant J. 2019, 99, 302–315. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and Sink Mechanisms of Nitrogen Transport and Use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Zhao, M.; Gu, S.; Sun, J.; Ma, Z.; Wang, L.; Zheng, W.; Xu, Z. DEP1 Is Involved in Regulating the Carbon–Nitrogen Metabolic Balance to Affect Grain Yield and Quality in Rice (Oriza Sativa L.). PLoS ONE 2019, 14, e0213504. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kim, S.H.; Priatama, R.A.; Jeong, J.H.; Adnan, M.R.; Saputra, B.A.; Kim, C.M.; Je, B.I.; Park, S.J.; Jung, K.H.; et al. NH4+ Suppresses NO3–-Dependent Lateral Root Growth and Alters Gene Expression and Gravity Response in OsAMT1 RNAi Mutants of Rice (Oryza Sativa). J. Plant Biol. 2020, 63, 391–407. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Priatama, R.A.; Huang, J.; Je, B.I.; Liu, J.M.; Park, S.J.; Piao, H.L.; Son, D.Y.; Lee, J.J.; Park, S.H.; et al. Indeterminate Domain 10 Regulates Ammonium-Mediated Gene Expression in Rice Roots. New Phytol. 2013, 197, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.D.; Santos Teixeira, J.; Ten Tusscher, K.H. Modeling of Root Nitrate Responses Suggests Preferential Foraging Arises From the Integration of Demand, Supply and Local Presence Signals. Front. Plant Sci. 2020, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Regulation of Arabidopsis Root Development by Nitrate Availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Canales, J.; Contreras-López, O.; Álvarez, J.M.; Gutiérrez, R.A. Nitrate Induction of Root Hair Density Is Mediated by TGA1/TGA4 and CPC Transcription Factors in Arabidopsis Thaliana. Plant J. 2017, 92, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Quint, M.; Gray, W.M. Auxin Signaling. Curr. Opin. Plant Biol. 2006, 9, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Ullah, Z.; Xu, F.; An, L.; Aluko, O.O.; Wang, Q.; Liu, H. Nitrate Signaling, Functions, and Regulation of Root System Architecture: Insights from Arabidopsis Thaliana. Genes 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Henao, J.E.; Vélez-Bermúdez, I.C.; Schmidt, W. The Regulation and Plasticity of Root Hair Patterning and Morphogenesis. Development 2016, 143, 1848–1858. [Google Scholar] [CrossRef] [Green Version]
- Itoh, J.-I.; Hibara, K.-I.; Sato, Y.; Nagato, Y. Developmental Role and Auxin Responsiveness of Class III Homeodomain Leucine Zipper Gene Family Members in Rice. Plant Physiol. 2008, 147, 1960–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
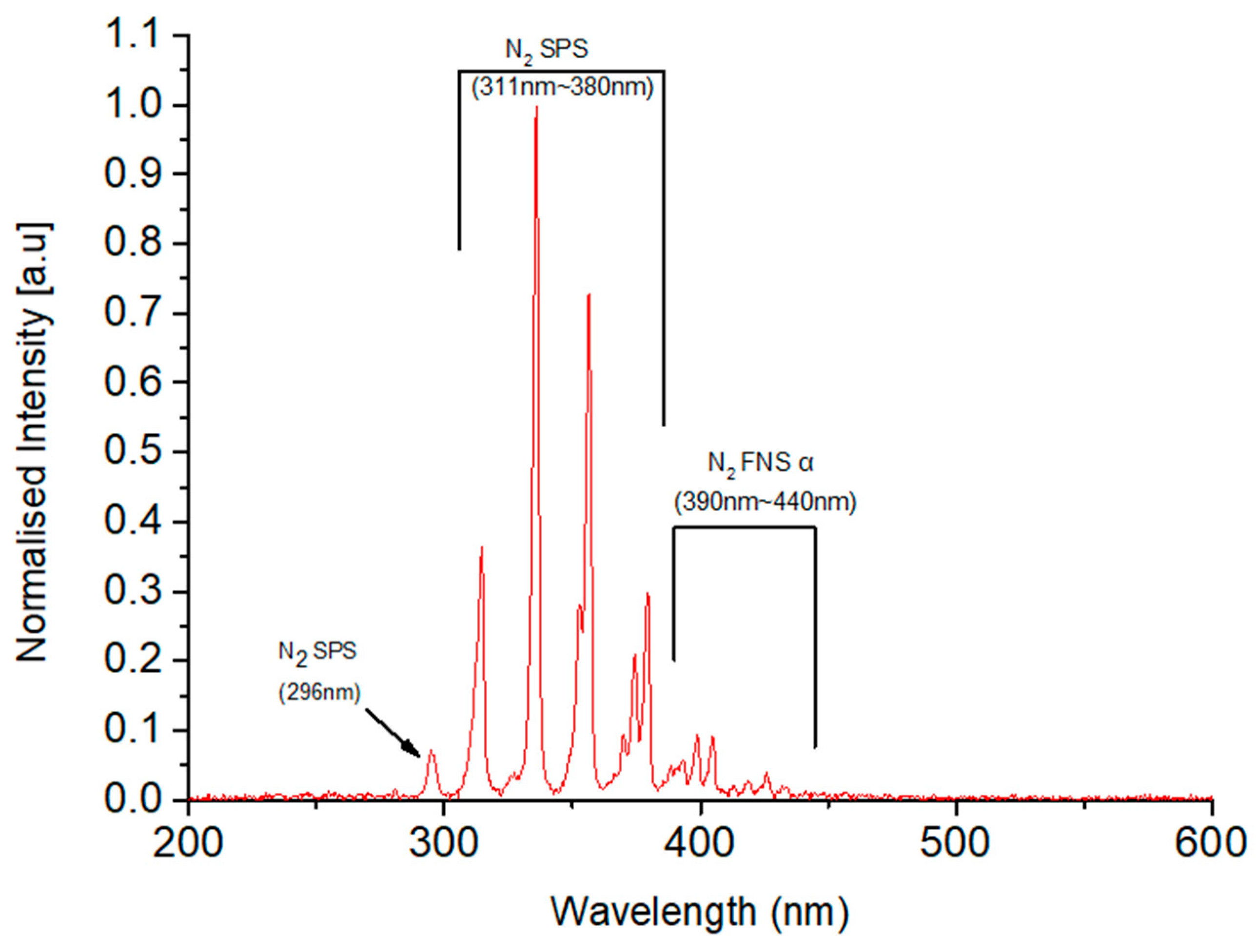


| pH | Nitrite NO2− (mg/L) | H2O2 (mg/L) | Nitrate NO3− (mg/L) | Conductivity (µs/cm) | |
|---|---|---|---|---|---|
| Deionized Water(DW) | 5.74 ± 0.06 | 0.00 | 0.00 | 0.00 | 1.53 ± 0.13 |
| PAW5 min(PAW5) | 3.62 ± 0.02 | 1.09 ± 0.11 | 0.09 ± 0.01 | 25.29 ± 2.88 | 118.10 ± 2.26 |
| PAW7 min(PAW7) | 3.34 ± 0.03 | 1.24 ± 0.12 | 0.14 ± 0.01 | 49.05 ± 2.61 | 218.50 ± 9.64 |
| PAW12 min(PAW12) | 2.94 ± 0.08 | 1.85 ± 0.07 | 0.27 ± 0.02 | 102.67 ± 6.30 | 460.33 ± 15.25 |
| PAW19 min(PAW19) | 2.62 ± 0.07 | 3.68 ± 0.12 | 0.88 ± 0.04 | 204.87 ± 8.74 | 972.93 ± 32.41 |
| PAW40 min(PAW40) | 2.37 ± 0.04 | 5.17 ± 0.16 | 1.31 ± 0.04 | 389.08 ± 12.24 | 1847.00 ± 70.19 |
| DW | PAW5 | PAW7 | PAW12 | PAW19 | |
|---|---|---|---|---|---|
| Root length (mm) | 9.91 ± 0.85 (n = 15) | 8.19 ± 0.68 ns (n = 15) | 8.04 ± 1.40 ns (n = 15) | 8.92 ± 0.82 ns (n = 15) | 7.95 ± 0.77 ns (n = 15) |
| Root hair number | 84.83 ± 9.21 (n = 6) | 112.00 ± 5.25 *** (n = 6) | 107.83 ± 5.74 *** (n = 6) | 100.50 ± 8.11 ** (n = 6) | 92.66 ± 6.18 ns (n = 6) |
| Root hair length (μm) | 550.01 ± 138.61 (N = 100) | 284.06 ± 63.12 *** (N = 100) | 193.20 ± 33.45 *** (N = 100) | 188.12 ± 46.67 *** (N = 100) | 161.50 ± 38.05 *** (N = 100) |
| DW | PAW5 | PAW7 | PAW12 | PAW19 | PAW40 | |
|---|---|---|---|---|---|---|
| Cotyledon size in the leaf length direction (mm) | 0.60 ± 0.03 (n = 30) | 0.77 ± 0.09 *** (n = 21) | 0.81 ± 0.09 *** (n = 25) | 0.84 ± 0.09 *** (n = 30) | 0.88 ± 0.10 *** (n = 15) | 0.96 ± 0.14 *** (n = 23) |
| Cotyledon size in the leaf width direction (mm) | 0.46 ± 0.03 (n = 30) | 0.62 ± 0.07 *** (n = 21) | 0.64 ± 0.06 ** (n = 25) | 0.67 ± 0.07 *** (n = 30) | 0.63 ± 0.05 *** (n = 25) | 0.76 ± 0.08 *** (n = 23) |
| Palisade cell number | 48.20 ± 6.83 (n = 5) | 43.20 ± 8.19 ns (n = 5) | 45.40 ± 5.59 ns (n = 5) | 47.40 ± 5.85 ns (n = 5) | 48.40 ± 5.59 ns (n = 5) | 40.40 ± 5.31 ns (n = 5) |
| Palisade cell size (μm) | 15.47 ± 1.50 (N = 300) | 24.63 ± 2.34 *** (N = 300) | 25.07 ± 2.39 ** (N = 300) | 25.59 ± 2.00 *** (N = 300) | 31.35 ± 3.93 *** (N = 300) | 42.88 ± 3.36 *** (N = 300) |
| DW | PAW5 | PAW7 | PAW12 | PAW19 | |
|---|---|---|---|---|---|
| Root length (mm) | 9.75 ± 0.96 (n = 15) | 10.07 ± 0.75 ns (n = 15) | 10.10 ± 0.63 ns (n = 15) | 9.78 ± 0.95 ns (n = 15) | 10.08 ± 0.66 ns (n = 15) |
| Root hair number | 14.50 ± 1.37 (n = 6) | 40.66 ± 5.71 *** (n = 6) | 33.16 ± 3.6 *** (n = 6) | 31.00 ± 3.84 ** (n = 6) | 27.50 ± 5.54 ns (n = 6) |
| Root hair length (μm) | 261.21 ± 100.37 (N = 60) | 138.35 ± 11.11 *** (N = 60) | 108.25 ± 11.54 *** (N = 60) | 99.50 ± 13.65 *** (N = 60) | 85.05 ± 11.65 *** (N = 60) |
| DW | PAW5 | PAW19 | |
|---|---|---|---|
| Root hair number | 15.14 ± 2.47 (n = 7) | 24.57 ± 3.10 *** (n = 7) | 26.85 ± 5.33 *** (n = 7) |
| Root hair length (μm) | 162.92 ± 29.10 (N = 50) | 77.40 ± 7.21 *** (N = 50) | 54.33 ± 7.83 *** (N = 50) |
| Root epidermal cell (μm) | 133.01 ± 12.67 (N = 50) | 141.33 ± 11.45 *** (N = 50) | 110.46 ± 9.38 *** (N = 50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ka, D.H.; Priatama, R.A.; Park, J.Y.; Park, S.J.; Kim, S.B.; Lee, I.A.; Lee, Y.K. Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L. Appl. Sci. 2021, 11, 2240. https://doi.org/10.3390/app11052240
Ka DH, Priatama RA, Park JY, Park SJ, Kim SB, Lee IA, Lee YK. Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L. Applied Sciences. 2021; 11(5):2240. https://doi.org/10.3390/app11052240
Chicago/Turabian StyleKa, Dong Hyeun, Ryza Aditya Priatama, Joo Young Park, Soon Ju Park, Seong Bong Kim, In Ah Lee, and Young Koung Lee. 2021. "Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L." Applied Sciences 11, no. 5: 2240. https://doi.org/10.3390/app11052240






