High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Static Corrosion Cells
2.2. Preparation of Selected Materials and Specimens
2.3. Test Procedures and Specimen Post-Processing
3. Results
3.1. Oxygen Concentration and Temperature Monitoring
- Oxygen concentration increase due to excess hydrogen gas;
- Oxygen concentration decrease due to aeration and decreased temperature;
- Power blackout;
- Change of gas cylinders and water tanks in the oxygen control system;
- End of test.
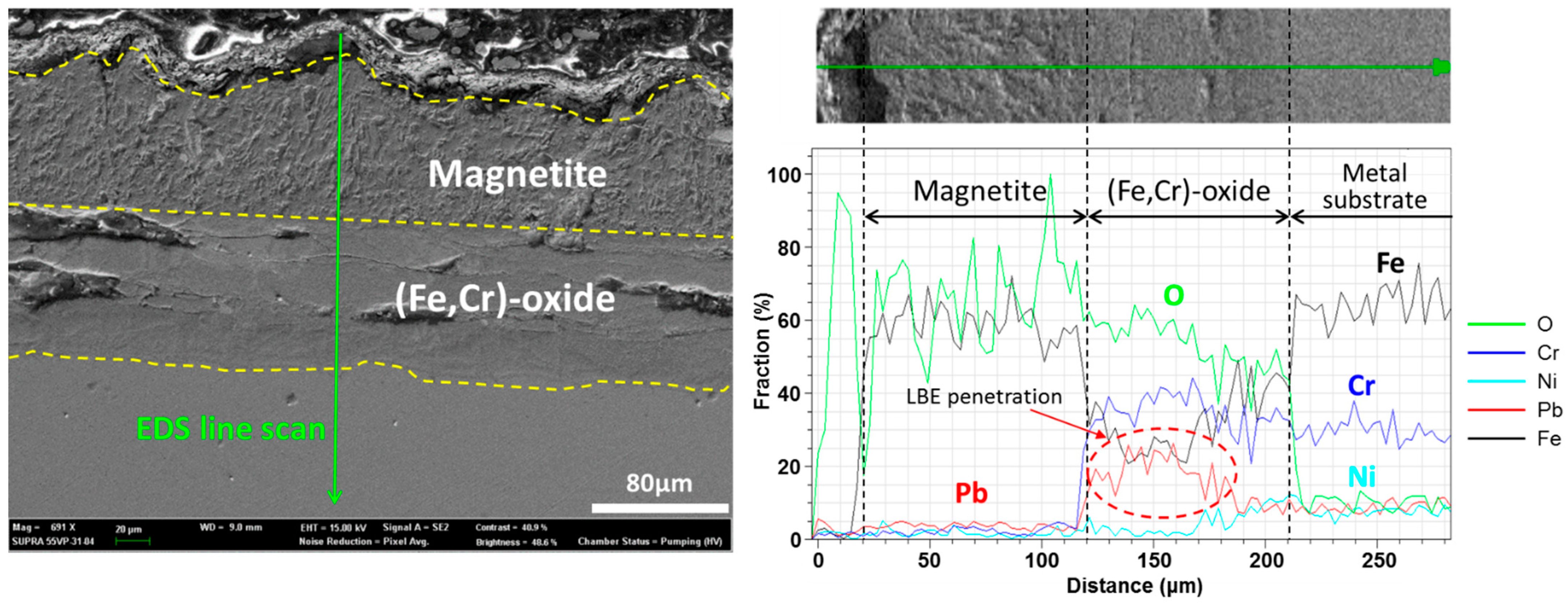
3.2. T91
3.3. HT9
3.4. SS316L
4. Discussion
4.1. Corrosion of Cr–Oxide-Forming Alloys in LBE
4.2. Oxygen Concentration Control in LBE
4.3. Prediction of Oxide Layer Thickness in High-Temperature LBE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Temperature (°C) | Reference | (Time (h), Corrosion Depth (μm)) | ||||
|---|---|---|---|---|---|---|
| 350 | [60] | (1000, 2) | ||||
| [65] | (1500, 6) | (3000, 6) | ||||
| 450 | [60] | (550, 10) | (1000, 12) | (2000, −3) | ||
| [59] | (3000, 12) | (3000, 30) | ||||
| [54] | (500, 6) | (1500, 5) | ||||
| 470 | [15] | (1116, 11) | (2000, 14) | (3116, 16) | ||
| [55] | (2300, 16) | (3800, 19) | (4500, 24) | (6300, 25) | (7800, 30) | |
| 535 | [66] | (500, 18) | (3000, −26) | |||
| 550 | [60] | (550, −7) | (550, 7) | (1000, 12) | (2000, −30) | |
| [66] | (500, 17) | (3000, −34) | (3000, −20) | |||
| 600 | [60] | (550, 14) | (1000, 4) | |||
| [66] | (500, −21) | (3000, −130) | ||||
| [54] | (100, 10) | (100, 20) | (100, 22) | (500, 5) | (500, 32) | |
| (500, 41) | (1500, −13) | (1500, −10) | (1500, 40) | (1500, 95) | ||
| This study | (1000, 45) | |||||
| Temperature (°C) | Reference | (Time (h), Corrosion Depth (μm)) | |
|---|---|---|---|
| 450 | [18] | (200, 4.2) | (500, 6.1) |
| 460 | [56] | (2000, 13) | (3000, 15) |
| 500 | [18] | (500, 12) | (1000, 17) |
| [67] | (800, 8) | (5000, 65) | |
| 550 | [67] | (5000, 50) | |
| 600 | This study | (1000, 25) | |
| Temperature (°C) | Reference | (Time (h), Corrosion Depth (μm)) | ||||
|---|---|---|---|---|---|---|
| 400 | [65] | (3000, 1) | ||||
| [62] | (5000, 1) | |||||
| 450 | [63] | (3000, 23) | ||||
| [60] | (550, 3) | (1000, 3) | ||||
| [59] | (3000, 4) | |||||
| [57] | (3000, 2.58) | |||||
| [54] | (2400, −25) | |||||
| 460 | [56] | (2000, 3) | (3000, 4) | |||
| 476 | [62] | (1200, 3) | ||||
| 500 | [67] | (800, 6) | (2000, 6) | (5000, 6) | (10000, −40) | |
| 535 | [66] | (500, 11) | (3000, −60) | |||
| 550 | [61] | (800, 10) | (2000, 20) | |||
| [60] | (550, −50) | (550, 7) | (1000, 8) | |||
| [57] | (3000, −22.41) | (500, 9) | (3000, −70) | (3000, −46) | ||
| [66] | (100, −9) | |||||
| [67] | (800, 15) | (2000, 15) | (5000, 15) | (10000, −200) | ||
| 600 | [60] | (550, 20) | (1000, 20) | |||
| [66] | (500, −55) | (3000, −156) | ||||
| [67] | (800, −15) | (2000, −20) | (5000, −20) | (10000, −180) | ||
| [54] | (100, 0.3) | (100, 8) | (100, 20) | (500, −40) | (500, 24) | |
| (500, 27) | (1500, −83) | (1500, −63) | (1500, 15) | (1500, 45) | ||
| This study | (1000, 20) | |||||
Appendix B
References
- Gromov, B.F. Use of lead-bismuth coolant in nuclear reactors and accerlerator-driven systmes. Nucl. Eng. Des. 1997, 173, 207–217. [Google Scholar] [CrossRef]
- Toshinsky, G.; Grigoriev, O.; Efimov, E.; Leonchuk, M.; Novikova, N.; Pankratov, D.; Skorikov, D.; Klimov, N.; Stepanov, V. Safety aspects of SVBR-75/100 reactor. In Proceedings of the Advanced Nuclear Reactor Safety Issues and Research Needs, Paris, France, 18–20 February 2002; pp. 71–86. [Google Scholar]
- Abderrahim, H.A.; Kupschus, P.; Malambu, E.; Benoit, P.; Van Tichelen, K.; Arien, B.; Vermeersch, F.; D’hondt, P.; Jongen, Y.; Ternier, S.; et al. MYRRHA: A multipurpose accelerator driven system for research & development. Nucl. Instrum. Methods Phys. Res. Sect. A 2001, 463, 487–494. [Google Scholar]
- Hwang, I.S.; Jeong, S.H.; Park, B.G.; Yang, W.S.; Suh, K.Y.; Kim, C.H. The concept of proliferation-resistant, environment-friendly, accident-tolerant, continual and economical reactor (PEACER). Prog. Nucl. Energy 2000, 37, 217–222. [Google Scholar] [CrossRef]
- Sekimoto, H.; Ryu, K.; Yoshimura, Y. CANDLE: The new burnup strategy. Nucl. Sci. Eng. 2001, 139, 306–317. [Google Scholar] [CrossRef]
- Stanisz, P.; Oettingen, M.; Cetnar, J. Monte Carlo modeling of Lead-Cooled Fast Reactor in adiabatic equilibrium state. Nucl. Eng. Des. 2016, 301, 341–352. [Google Scholar] [CrossRef]
- Choi, S.; Cho, J.-H.; Bae, M.-H.; Lim, J.; Puspitarini, D.; Jeun, J.H.; Joo, H.-G.; Hwang, I.S. PASCAR: Long burning small modular reactor based on natural circulation. Nucl. Eng. Des. 2011, 241, 1486–1499. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Choi, S.; Cho, J.; Kim, J.H.; Hwang, I.S. Advanced passive design of small modular reactor cooled by heavy liquid metal natural circulation. Prog. Nucl. Energy 2015, 83, 433–442. [Google Scholar] [CrossRef]
- Sienicki, J.J.; Spencer, B.W. Power optimization in the STAR-LM modular natural convection reactor system. In Proceedings of the 10th International Conference on Nuclear Engineering, Arlington, VA, USA, 14–18 April 2002. [Google Scholar]
- Wade, D.; Feldman, E.; Sienicki, J.; Sofu, T.; Barak, A.; Greenspan, E.; Saphier, D.; Brown, N.; Hossain, Q.; Carelli, M. ENHS: The encapsulated nuclear heat source—A nuclear energy concept for emerging worldwide energy markets. In Proceedings of the 10th International Conference on Nuclear Engineering, Arlington, VA, USA, 14–18 April 2002. [Google Scholar]
- Generation IV International Forum. Technology Roadmap Update for Generation IV Nuclear Energy Systems; OECD Nuclear Energy Agency: Paris, France, 2014. [Google Scholar]
- Li, N. Lead-alloy coolant technology and materials. Prog. Nucl. Energy 2007, 50, 140–151. [Google Scholar] [CrossRef]
- Allen, T.R.; Crawford, D.C. Lead-Cooled Fast Reactor Systems and the Fuels and Materials Challenges. Sci. Technol. Nucl. Install. 2007, 2007, 097486. [Google Scholar]
- OECD/NEA. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies, 2015 edition; Organisation for Economic Co-Operation and Development: Paris, France, 2015. [Google Scholar]
- Barbier, F.; Rusanov, A. Corrosion behavior of steels in flowing lead–bismuth. J. Nucl. Mater. 2001, 296, 231–236. [Google Scholar] [CrossRef]
- Müller, G.; Schumacher, G.; Zimmermann, F. Investigation on oxygen controlled liquid lead corrosion of surface treated steels. J. Nucl. Mater. 2000, 278, 85–95. [Google Scholar] [CrossRef]
- Weisenburger, A.; Heinzel, A.; Müller, G.; Muscher, H.; Rousanov, A. T91 cladding tubes with and without modified FeCrAlY coatings exposed in LBE at different flow, stress and temperature conditions. J. Nucl. Mater. 2008, 376, 274–281. [Google Scholar] [CrossRef]
- Lim, J.; Nam, H.O.; Hwang, I.S.; Kim, J.H. A study of early corrosion behaviors of FeCrAl alloys in liquid lead–bismuth eutectic environments. J. Nucl. Mater. 2010, 407, 205–210. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, I.S.; Kim, J.H. Design of alumina forming FeCrAl steels for lead or lead–bismuth cooled fast reactors. J. Nucl. Mater. 2013, 441, 650–660. [Google Scholar] [CrossRef]
- Dvoriashin, A.M.; Porollo, S.I.; Konobeev, Y.V.; Budylkin, N.I.; Mironova, E.G.; Ioltukhovskiy, A.G.; Leontyeva-Smirnova, M.V.; Garner, F.A. Mechanical properties and microstructure of three Russian ferritic/martensitic steels irradiated in BN-350 reactor to 50 dpa at 490 °C. J. Nucl. Mater. 2007, 367–370, 92–96. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Rong, L.; Li, D.; Li, Y. Effect of silicon on the oxidation resistance of 9 wt. % Cr heat resistance steels in 550 °C lead-bismuth eutectic. Corros. Sci. 2016, 111, 13–25. [Google Scholar]
- García Ferré, F.; Ormellese, M.; Di Fonzo, F.; Beghi, M.G. Advanced Al2O3 coatings for high temperature operation of steels in heavy liquid metals: a preliminary study. Corros. Sci. 2013, 77, 375–378. [Google Scholar] [CrossRef]
- Ballinger, R.G.; Lim, J. An overview of corrosion issues for the design and operation of high-temperature lead-and lead-bismuth-cooled reactor systems. Nucl. Technol. 2004, 147, 418–435. [Google Scholar] [CrossRef]
- Shankar Rao, V.; Lim, J.; Soon Hwang, I.L. Analysis of 316L stainless steel pipe of lead–bismuth eutectic cooled thermo-hydraulic loop. Ann. Nucl. Energy 2012, 48, 40–44. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Skrypnik, A.; Novotny, J.; Konys, J. Corrosion kinetics of Steel T91 in flowing oxygen-containing lead–bismuth eutectic at 450 °C. J. Nucl. Mater. 2012, 431, 105–112. [Google Scholar] [CrossRef]
- Kurata, Y. Corrosion behavior of cold-worked austenitic stainless steels in liquid lead–bismuth eutectic. J. Nucl. Mater. 2014, 448, 239–249. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Chen, Y.; Rusanov, A.E. Corrosion behaviors of US steels in flowing lead–bismuth eutectic (LBE). J. Nucl. Mater. 2005, 336, 1–10. [Google Scholar] [CrossRef]
- Heinzel, A.; Weisenburger, A.; Müller, G. Corrosion behavior of austenitic steels in liquid lead bismuth containing 10−6 wt% and 10−8 wt% oxygen at 400–500 °C. J. Nucl. Mater. 2014, 448, 163–171. [Google Scholar] [CrossRef]
- Martín-Muñoz, F.J.; Soler-Crespo, L.; Gómez-Briceño, D. Corrosion behaviour of martensitic and austenitic steels in flowing lead–bismuth eutectic. J. Nucl. Mater. s 2011, 416, 87–93. [Google Scholar] [CrossRef]
- Doubková, A.; Di Gabriele, F.; Brabec, P.; Keilová, E. Corrosion behavior of steels in flowing lead–bismuth under abnormal conditions. J. Nucl. Mater. 2008, 376, 260–264. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Novotny, J.; Skrypnik, A.; Konys, J. Selective leaching of nickel and chromium from Type 316 austenitic steel in oxygen-containing lead–bismuth eutectic (LBE). Corros. Sci. 2014, 84, 113–124. [Google Scholar] [CrossRef]
- Schroer, C.; Skrypnik, A.; Wedemeyer, O.; Konys, J. Oxidation and dissolution of iron in flowing lead–bismuth eutectic at 450 °C. Corros. Sci. 2012, 61, 63–71. [Google Scholar] [CrossRef]
- Hadjem-Hamouche, Z.; Auger, T.; Guillot, I. Temperature effect in the maximum propagation rate of a liquid metal filled crack: The T91 martensitic steel/Lead–Bismuth Eutectic system. Corros. Sci. 2009, 51, 2580–2587. [Google Scholar] [CrossRef]
- Yamaki, E.; Ginestar, K.; Martinelli, L. Dissolution mechanism of 316L in lead–bismuth eutectic at 500 °C. Corros. Sci. 2011, 53, 3075–3085. [Google Scholar] [CrossRef]
- Klueh, R.L.; Nelson, A.T. Ferritic/martensitic steels for next-generation reactors. J. Nucl. Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Garner, F.A.; Toloczko, M.B.; Sencer, B.H. Comparison of swelling and irradiation creep behavior of fcc-austenitic and bcc-ferritic/martensitic alloys at high neutron exposure. J. Nucl. Mater. 2000, 276, 123–142. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Novotny, J.; Skrypnik, A.; Konys, J. Performance of 9% Cr steels in flowing lead-bismuth eutectic at 450 and 550 °C, and 10−6 mass% dissolved oxygen. Nucl. Eng. Des. 2014, 280, 661–672. [Google Scholar] [CrossRef]
- Schroer, C.; Koch, V.; Wedemeyer, O.; Skrypnik, A.; Konys, J. Silicon-containing ferritic/martensitic steel after exposure to oxygen-containing flowing lead–bismuth eutectic at 450 and 550 °C. J. Nucl. Mater. 2016, 469, 162–176. [Google Scholar] [CrossRef]
- Kurata, Y.; Futakawa, M.; Saito, S. Corrosion behavior of steels in liquid lead–bismuth with low oxygen concentrations. J. Nucl. Mater. 2008, 373, 164–178. [Google Scholar] [CrossRef]
- Kurata, Y. Corrosion behavior of Si-enriched steels for nuclear applications in liquid lead–bismuth. J. Nucl. Mater. 2013, 437, 401–408. [Google Scholar] [CrossRef]
- Nam, H.O.; Lim, J.; Han, D.Y.; Hwang, I.S. Dissolved oxygen control and monitoring implementation in the liquid lead-bismuth eutectic loop: HELIOS. J. Nucl. Mater. 2008, 376, 381. [Google Scholar] [CrossRef]
- Weisenburger, A. Corrosion in HLM systems. In Proceedings of the International Workshop on Innovative Nuclear Reactors Cooled by Heavy Liquid Metals: Status and Prospectives, Pisa, Italy, 17–20 April 2012. [Google Scholar]
- Martinelli, L.; Balbaud-Célérier, F. Modelling of the oxide scale formation on Fe-Cr steel during exposure in liquid lead-bismuth eutectic in the 450–600° C temperature range. Mater. Corros. 2011, 62, 531–542. [Google Scholar] [CrossRef]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. Journal of the Institute of Metals 1923, 29, 529–582. [Google Scholar]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Appleby, W.; Tylecote, R. Stresses during the gaseous oxidation of metals. Corros. Sci. 1970, 10, 325–341. [Google Scholar] [CrossRef]
- Wood, G.C.; Stott, F.H. Oxidation of alloys. Mater. Sci. Technol. 1987, 3, 519–530. [Google Scholar] [CrossRef]
- Howes, V.; Richardson, C. The initial stresses developed during high temperature oxidation of Fe-Cr alloys. Corros. Sci. 1969, 9, 385–394. [Google Scholar] [CrossRef]
- Betz, W.; Brunetaud, R.; Coutsourais, D.; Fischmeister, H.; Gibbons, T.B.; Kvernes, I.; Lindblom, Y.; Marriott, J.B.; Meadowcroft, D.B. High Temperature Alloys for Gas Turbines and Other Applications; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Martynov, P.N.; Ivanov, K.D. Properties of Lead-Bismuth Coolant and Perspectives of Non-Electric Applications of Lead-Bismuth Reactor; Technical Report; IAEA: Vienna, Austria, 1997. [Google Scholar]
- Li, N.; Hang, W.; Darling, T. Oxygen sensor calibration for LBE coolant chemistry control. In Proceedings of the 11th International Conference on Nuclear Engineering (ICONE-11), Tokyo, Japan, 20–23 April 2003. [Google Scholar]
- Müller, G.; Heinzel, A.; Schumacher, G.; Weisenburger, A. Control of oxygen concentration in liquid lead and lead–bismuth. J. Nucl. Mater. 2003, 321, 256–262. [Google Scholar] [CrossRef]
- Wagner, C. Theoretical analysis of the diffusion processes determining the oxidation rate of alloys. J. Electrochem. Soc. 1952, 99, 369. [Google Scholar] [CrossRef]
- Soler, L.; Martín, F.J.; Hernandez, F.; Gomez-Briceno, D. Corrosion of Stainless Steels in Lead-bismuth Eutectic up to 600°C. J. Nucl. Mater. 2004, 335, 174. [Google Scholar] [CrossRef]
- Martinelli, L.; Balbaud-Celerier, F.; Bosonnet, S.; Terlain, A.; Santarini, G.; Delpech, S.; Picard, G. High Temperature Oxidation of Fe-9Cr Steel in Stagnant Liquid Lead-bismuth. Trans. Am. Nucl. Soc. 2008, 98, 1136–1137. [Google Scholar]
- Li, N.; He, X.; Rusanov, A.; Demishonkov, A.P. Corrosion Tests of US Steels in Lead-Bismuth Eutectic (LBE) and Kinetic Modelling of Corrosion in LBE Systems; LA-UR-01-5241; Los Alamos National Laboratory: Los Alamos, NM, USA, 2001. [Google Scholar]
- Kurata, Y.; Futakawa, M.; Saito, S. Comparison of the Corrosion Behavior of Austenitic and Ferritic/Martensitic Steels Exposed to Static Liquid Pb-Bi at 450 and 550C. J. Nucl. Mater. 2005, 343, 335. [Google Scholar] [CrossRef]
- Kondo, M.; Takahashi, M.; Suzuki, T.; Ishikawa, K.; Hata, K.; Qiu, S.; Sekimoto, H. Metallurgical Study on Erosion and Corrosion Behaviors of Steels Exposed to Liquid Lead-bismuth Flow. J. Nucl. Mater. 2005, 343, 349. [Google Scholar] [CrossRef]
- Gomez-Briceno, D.; Crespo, L.S.; Munoz, F.J.M.; Hernández, F. Oxide Formation Efficiency in Stagnant Lead-Bismuth; CIEMAT: Madrid, Spain, 2004. [Google Scholar]
- Gnecco, F.; Ricci, E.; Bottino, C.; Passerone, A. Corrosion Behaviour of Steels in Lead-bismuth at 823 K. J. Nucl. Mater. 2004, 335, 185. [Google Scholar] [CrossRef]
- Furukawa, T.; Müller, G.; Schumacher, G.; Weisenburger, A.; Heinzel, A.; Aoto, K. Effect of Oxygen Concentration and Temperature on Compatibility of ODS Steel with Liquid, Stagnant Pb45Bi55. J. Nucl. Mater. 2004, 335, 189. [Google Scholar] [CrossRef]
- Fazio, C.; Benamati, G.; Martini, C.; Palombarini, G. Compatibility Tests on Steels in Molten Lead and Lead-bismut. J. Nucl. Mater. 2001, 296, 243. [Google Scholar] [CrossRef]
- Deloffre, P.; Terlain, A.; Barbier, F. Corrosion and Deposition of Ferrous Alloys in Molten Lead-bismuth. J. Nucl. Mater. 2002, 301, 35. [Google Scholar] [CrossRef]
- Benamati, G.; Fazio, C.; Piankova, H.; Rusanov, A. Temperature Effect on the Corrosion Mechanism of Austenitic and Martensitic Steels in Lead-bismuth. J. Nucl. Mater. 2002, 301, 23. [Google Scholar] [CrossRef]
- Aiello, A.; Azzati, M.; Benamati, G.; Gessi, A.; Long, B.; Scaddozzo, G. Corrosion Behaviour of Stainless Steels in Flowing LBE at Low and High Oxygen Concentration. J. Nucl. Mater. 2004, 335, 169. [Google Scholar] [CrossRef]
- Martın, F.; Soler, L.; Hernandez, F.; Gomez-Briceno, D. Oxide layer stability in lead–bismuth at high temperature. J. Nucl. Mater. 2004, 335, 194–198. [Google Scholar] [CrossRef]
- Müller, G.; Heinzel, A.; Konys, J.; Schumacher, G.; Weisenburger, A.; Zimmermann, F.; Engelko, V.; Rusanov, A.; Markov, V. Behavior of steels in flowing liquid PbBi eutectic alloy at 420–600 C after 4000–7200 h. J. Nucl. Mater. 2004, 335, 163–168. [Google Scholar] [CrossRef]


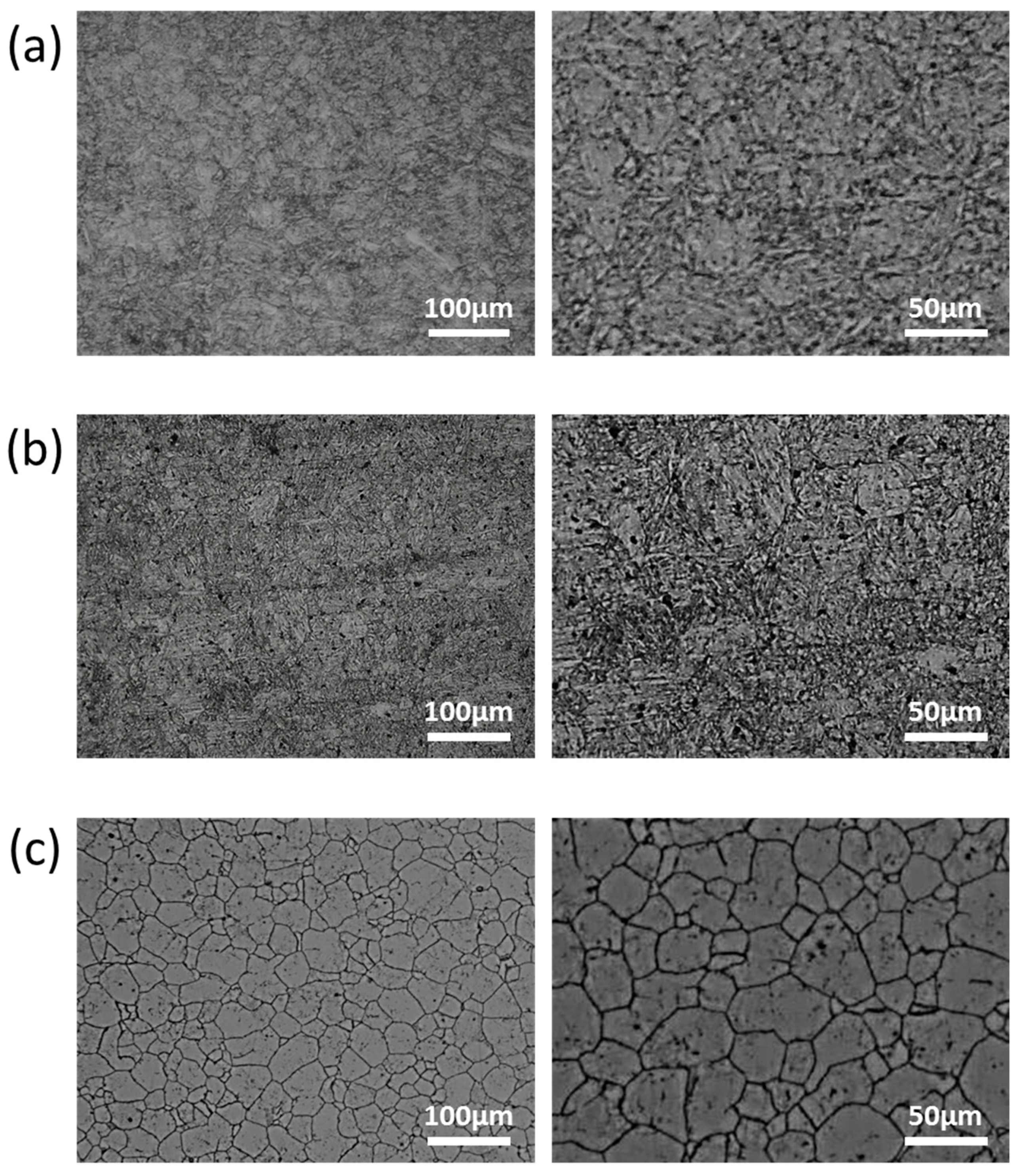

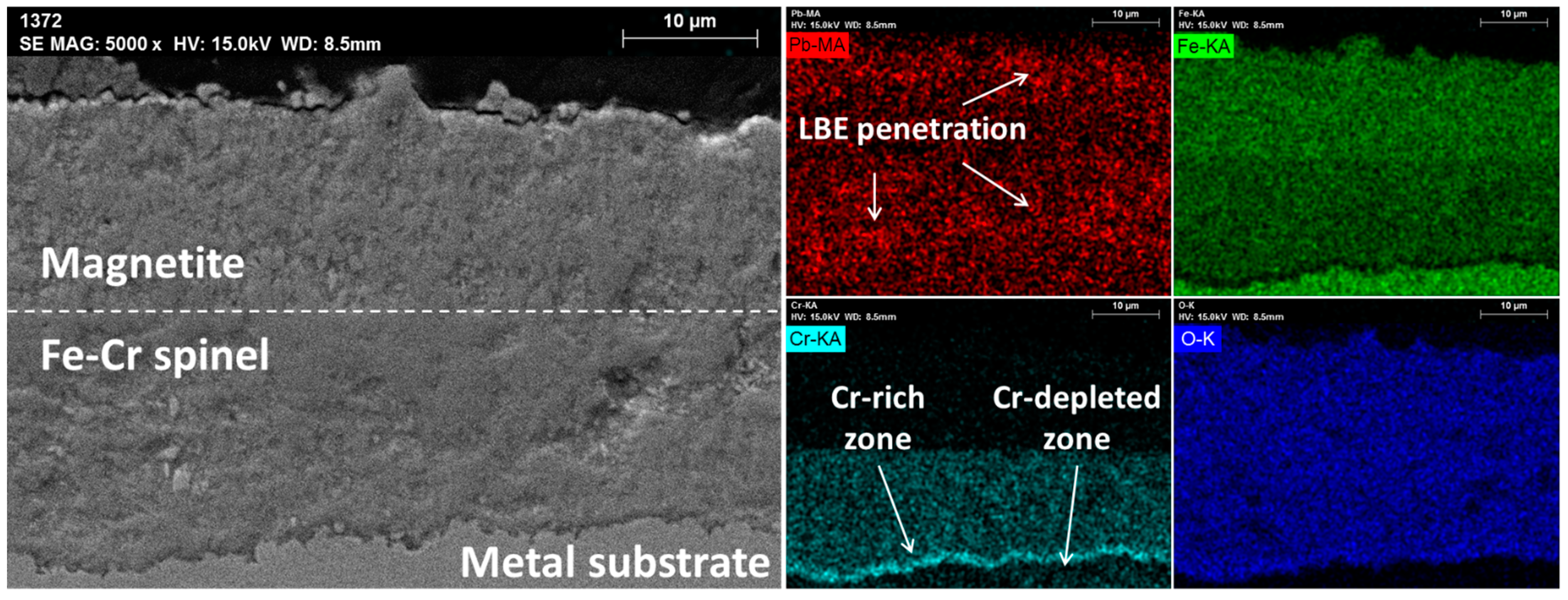

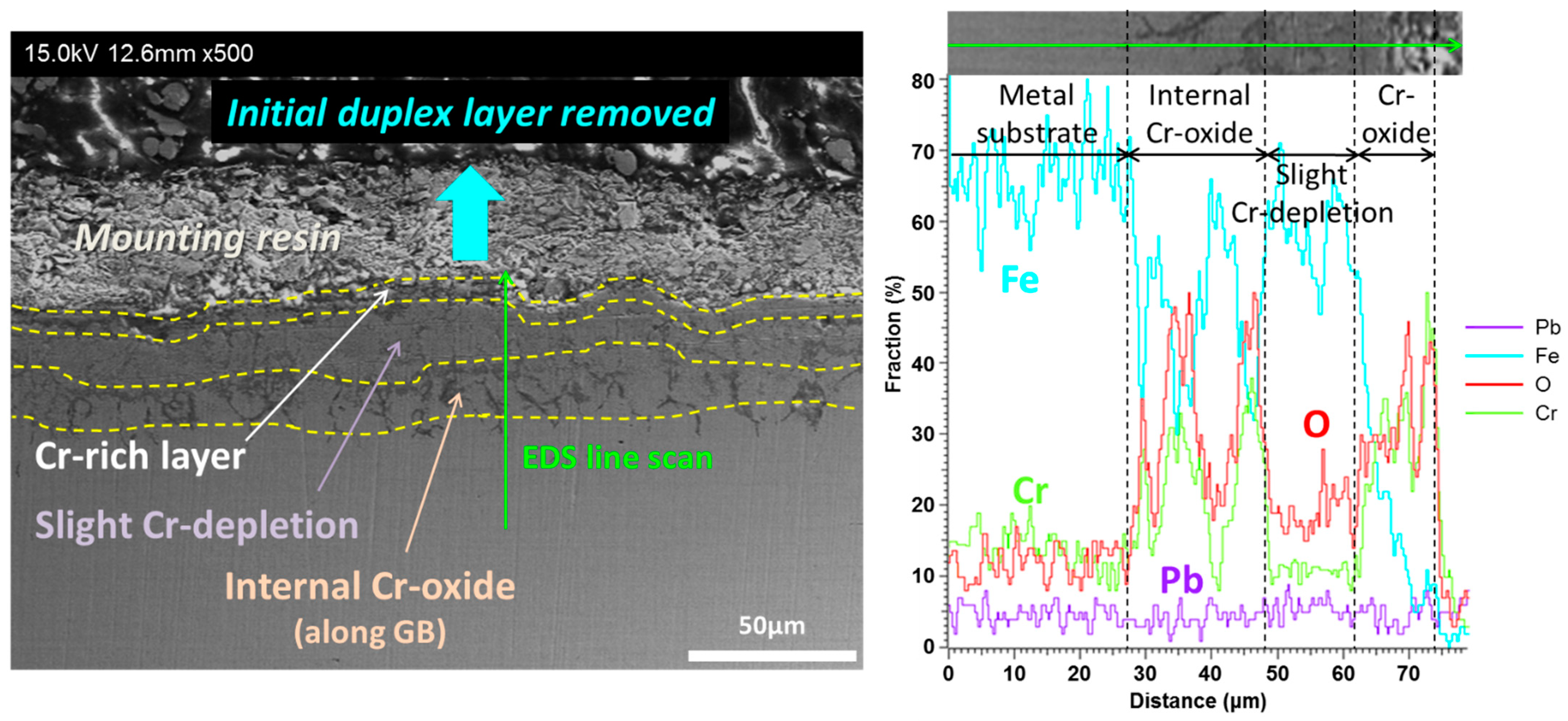

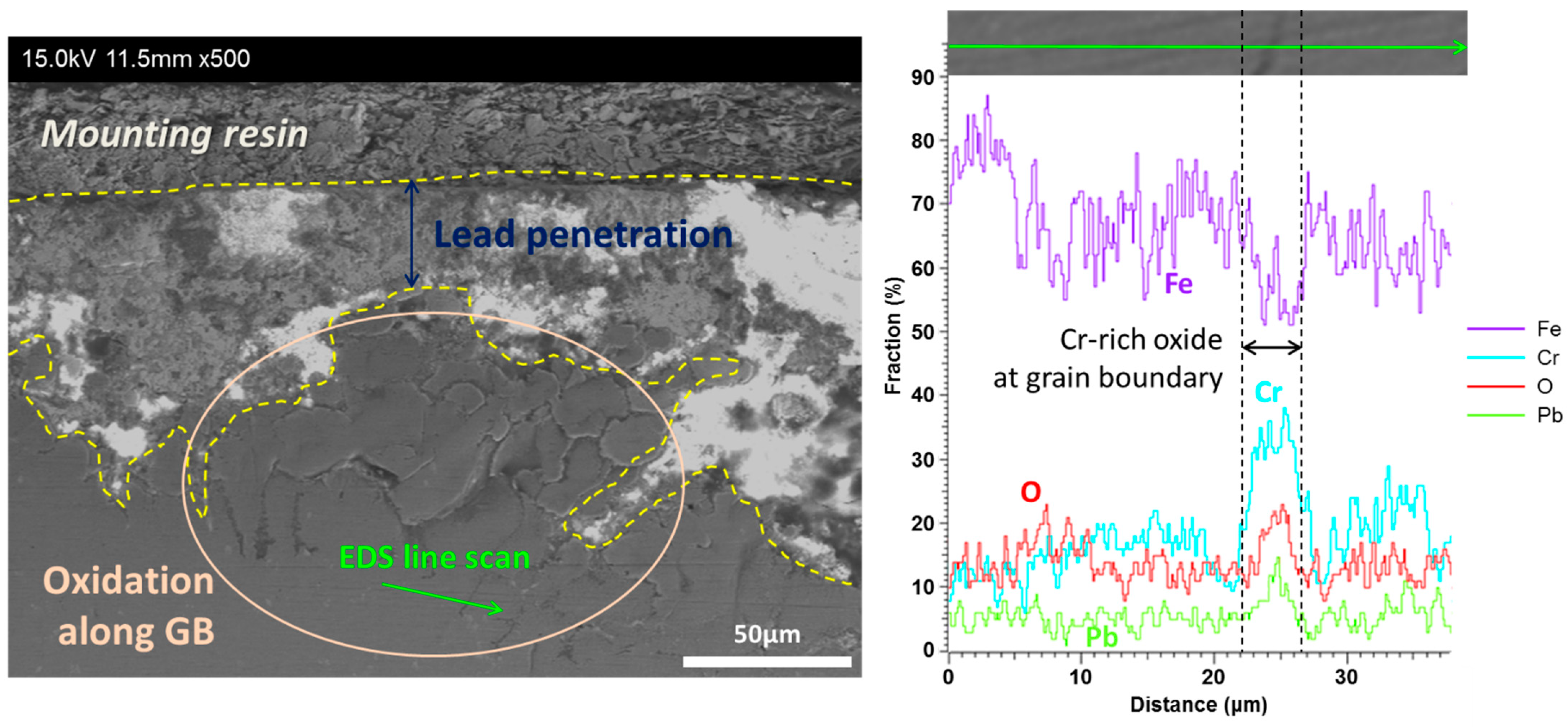



| Main Elements | Fe | Cr | Ni | Mo | Mn | Si | W | Nb | V | C | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T91 | Nominal | Bal. | 8.5 | 0.2 | 1 | 0.5 | 0.3 | - | 0.06 | 0.2 | 0.1 |
| Measured | Bal. | 8.61 | 0.18 | 0.96 | 0.45 | 0.229 | - | 0.07 | 0.22 | 0.09 | |
| HT9 | Nominal | Bal. | 11.5 | 0.5 | 1 | 0.6 | 0.4 | 0.5 | - | 0.3 | 0.1 |
| Measured | Bal. | 11.63 | 0.50 | 1.03 | 0.55 | 0.36 | 0.52 | - | 0.28 | 0.11 | |
| SS316L | Nominal | Bal. | 17.0 | 10.0 | 2.2 | 0.9 | 0.4 | - | - | - | 0.02 |
| Measured | Bal. | 16.87 | 10.31 | 2.07 | 0.92 | 0.44 | - | - | - | 0.021 | |
| Materials | Heat Treatment Procedure | Etchant |
|---|---|---|
| T91, HT9 | 1. Normalization (1050 °C, 1 h) and air-cooling 2. Tempering (750 °C, 2 h) and air-cooling | Vilella’s reagent (5 mL HCl + 2 g picric acid + 100 mL ethanol) |
| SS316L | Solid solution heat treatment (1050 °C, 1 h) and water-quenching | 5% Nital (5 mL HNO3 + 95 mL ethanol, 3 V electrolytic) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.G.; Shin, Y.-H.; Park, J.; Hwang, I.S. High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application. Appl. Sci. 2021, 11, 2349. https://doi.org/10.3390/app11052349
Lee SG, Shin Y-H, Park J, Hwang IS. High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application. Applied Sciences. 2021; 11(5):2349. https://doi.org/10.3390/app11052349
Chicago/Turabian StyleLee, Seung Gi, Yong-Hoon Shin, Jaeyeong Park, and Il Soon Hwang. 2021. "High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application" Applied Sciences 11, no. 5: 2349. https://doi.org/10.3390/app11052349
APA StyleLee, S. G., Shin, Y.-H., Park, J., & Hwang, I. S. (2021). High-Temperature Corrosion Behaviors of Structural Materials for Lead-Alloy-Cooled Fast Reactor Application. Applied Sciences, 11(5), 2349. https://doi.org/10.3390/app11052349










