1. Introduction
Two-photon imaging (2PI) is a fluorescence-based laser scanning microscopy technique commonly used in studies across various fields of research, including neurobiology, embryology, and tissue engineering [
1,
2]. In principle, it involves two infrared photons simultaneously exciting a single fluorophore in a sample, thereby causing it to emit light in a specific wavelength region, also called fluorescence emission spectrum. This fluorescence is normally detected in a wavelength region close to the maximum of this spectrum, allowing the sample to be identified based on its specific fluorescent characteristics. Compared to confocal single-photon microscopy, the main advantages of 2PI include reduced damage of tissues, allowing intravital studies, greater penetration depth of up to 2 mm in the brain, and ex vivo deep tissue imaging. Simultaneously, it maintains the typical characteristics of confocal microscopy, such as optical slicing capabilities with the option to perform 3-dimensional (3D) structural visualization and quantification, and its high subcellular resolution of around 400 nanometers [
3,
4,
5]. We also note that, while many clinical techniques, such as ultrasound, CT, PET, and MRI, can penetrate much deeper in tissues, their resolution is up to a factor 1000 worse and cannot image at a subcellular level. We conclude that, despite the multiple applications of 2PI in various scientific fields, there might be further potential for the application of 2PI in epilepsy research.
There is an enormous need to further understand the pathophysiology of epilepsy. Being one of the most common neurological disorders, epilepsy accounts for the highest disability-adjusted life years [
6], affecting around sixty million people worldwide [
7], of whom 30–40% are drug-resistant [
8]. The societal burden of chronic epilepsy is massive and encompasses around 80% of total epilepsy-related costs [
6].
In recent years, epilepsy research has made a shift toward a vascular-centered concept of epileptogenesis [
9,
10,
11,
12]. This has resulted in the identification of an important role of neurovascular alterations [
13] and increased blood–brain barrier permeability in the pathophysiology of epilepsy [
14,
15,
16]. Indeed, recent reports have emphasized similarities between alterations in epilepsy and microvascular dysfunction [
17,
18,
19]. Additionally, oxidative stress has been implicated in epilepsy—as this process has been shown to induce structural cell damage and to facilitate the formation of lipofuscin, which itself also induces cellular damage [
20,
21,
22]. Nevertheless, further research into the different aspects of the pathophysiology of epilepsy is needed to connect these areas.
In this regard, the application of 2PI in epilepsy research entails high potential to improve our understanding of seizure-induced anatomical and pathophysiological changes across different scales, ranging from neuronal structures to neuronal microcircuits and the neurovasculature [
23,
24]. Moreover, advances in two-photon laser scanning have enabled in vivo imaging of the brain while preserving the natural neuronal environment [
25]. Consequently, pathological activity in these in vivo models may represent epileptic conditions more accurately [
1]. Furthermore, two-photon uncaging is a powerful technique that is of significance in epilepsy research. It essentially involves the use of the two-photon excitation laser to photolyse or activate certain biological compounds such as neurotransmitters while also allowing the visualization of the immediate effects of this photochemical excitation without any other interference. In addition, fluorescence lifetime imaging, a technique based on differences in the decay rates (on the nanoseconds time-scale) of fluorophores in samples rather than intensity [
26] can be advantageous when studying specific types of environments and the effects thereof on fluorophore. Lifetime imaging can be used with two-photon excitation (2P-FLIM) as well as with confocal microscopy and multiphoton tomography. When probing molecular environments wherein intensity-based measurements alone are often insufficient, fluorescence lifetime-based measurements may be capable of yielding additional data [
26]. Finally, the development of a wide range of fluorescent markers has made it possible to study various aspects of the pathophysiology of epilepsy, such as neurotransmitter levels [
27,
28], metabolism [
28,
29], pathological neuronal activity [
27], and hypersynchronous network firing [
30].
Previous studies that employed 2PI to evaluate the mechanisms associated with epileptic seizures have indeed contributed significantly to our understanding of this complex disorder. Here, we aim to review previous applications of 2PI in epilepsy research and to delineate the potential applications of 2PI in future research on epilepsy, particularly in relation to the pathophysiological role of the cerebral microvasculature.
4. Discussion
Our review includes 38 studies on the application of 2PI in epilepsy research. We observed the application of 2PI in a range of pathophysiological mechanisms underlying epilepsy. 2PI has demonstrated its value by shedding more light on a large spectrum of functional and morphological abnormalities that are associated with epilepsy. It allowed for the direct visualization of neurons, astrocytes, microglia, and neurites, revealing morphological changes. Alterations in biological compounds such as [Ca2+], glutamate, and NADH indicate functional changes. Furthermore, visualization of blood vessels, vasomotor responses, blood flow alterations, and partial oxygen pressure revealed a variety of neurovascular changes associated with epileptic activity. Since current research on the pathophysiology of epilepsy focuses increasingly on neurovascular alterations and oxidative stress, the application of 2PI in this field is emphasized in our discussion. However, we start this discussion with a more detailed description of the specific features and potential of 2PI in general.
4.1. Two-Photon Imaging
Novel advances in unraveling the pathomechanisms associated with epilepsy described in this review have benefitted greatly from the advent and development of 2PI as an imaging technique. This subsection describes specific features of 2PI and how these features were utilized within studies included in this review.
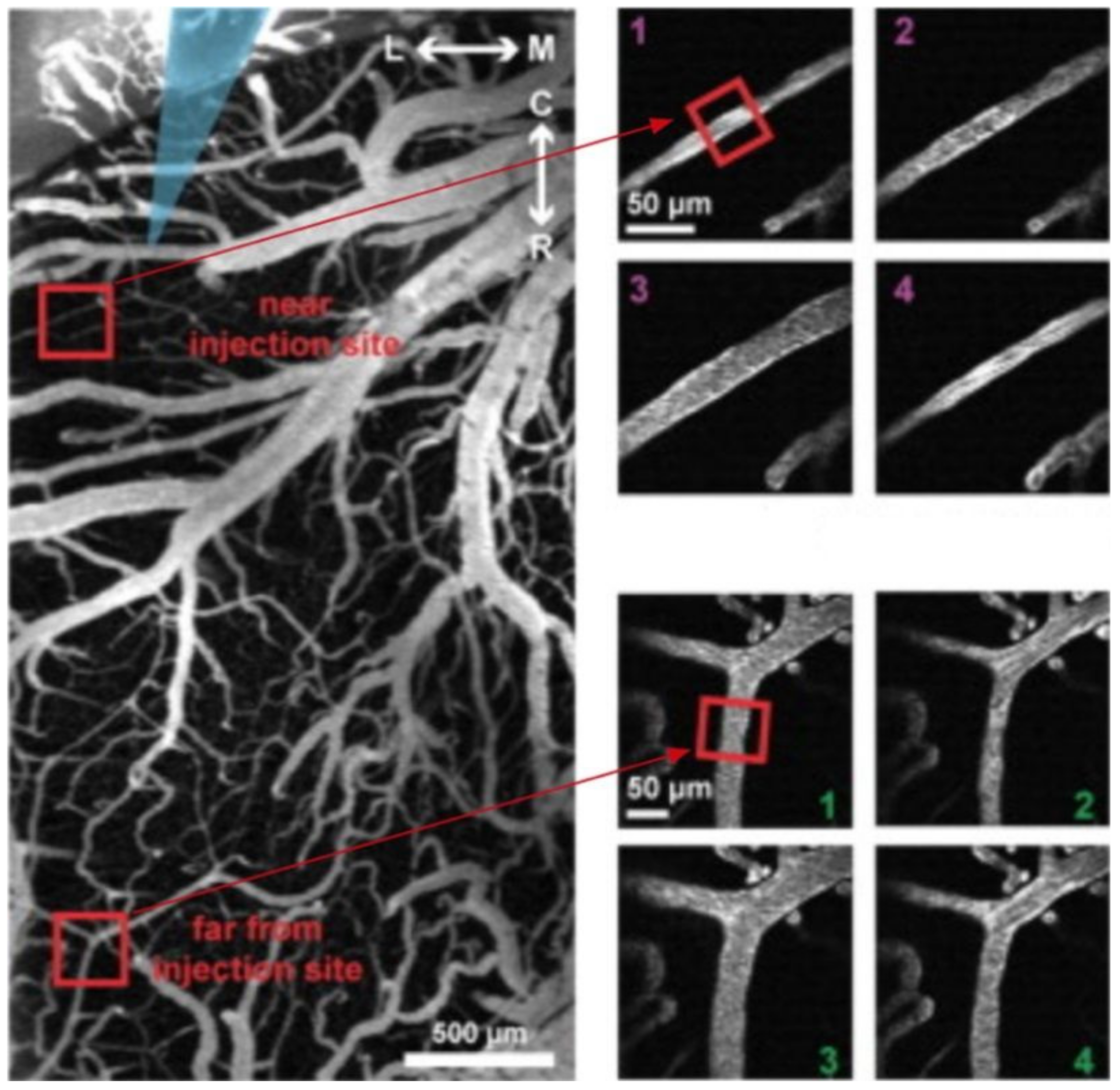
2PI enables high-resolution deep tissue imaging of vascular structures, neuronal cells, and fluorophores, with limited background signal [
1], and is suitable for molecular imaging of living tissues or in live animals. This feature was fully taken advantage of in some of the studies included in the present review. For example, three studies [
57,
59,
60] were able to visualize subtle changes in the microvasculature (namely vasodilation and vasoconstriction) that accompany epileptiform activity. In vivo 2PI can be used to quantify hemodynamics and vascular responses after epileptiform activity. Additionally, 2PI was even employed to measure capillary flow during epileptiform activity [
60], and visualization of neurons, microglia, astrocytes, and neurites revealed morphological changes accompanying seizures. As a result of these specific features, 2PI allowed for the identification of functional and structural alterations in epilepsy, which have not been described previously. In these in vivo instances, using confocal laser scanning microscopy would have likely been inadequate, as comparatively, 2PI significantly reduces photobleaching, increases cell viability, and allows for deeper tissue penetration [
69]. Furthermore, it has been reported that, when imaging neuronal cells specifically, single-photon microscopy suffers from increased interference by neuropil and decreased signal-to-noise ratio compared to 2PI [
70].
2PI is particularly well-suited for uncaging experiments, which involves the use of light to artificially activate biological compounds, such as glutamate or calcium, and therefore to increase their concentrations in small focal volumes [
71]. Due to the inherent nonlinearity of the two-photon system, 2PI is more suitable for uncaging than conventional single-photon excitation [
72]. Two-photon uncaging produces excited states exactly as UV excitation does while also overcoming major limitations when probing biological tissue, such as spatial resolution, tissue penetration, and toxicity [
73]. Two-photon uncaging is of special interest in epilepsy research, as imbalances between excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmission have been implicated in the pathogenesis of the disorder [
27,
28,
74]. Uncaging is used in four of the studies included in this review. Kelly and Beck [
62] performed glutamate uncaging in dendrites of granule cells, whereas Tian et al. [
53] and Ding et al. [
54] used uncaging to trigger astrocytic [Ca
2+] signaling and glutamatergic gliotransmission. The findings presented in the latter two publications were groundbreaking and paved the way for subsequent research into the role of astrocytes and astrocytic transformation in epilepsy. As such, the use of 2PI directly and unequivocally contributed to our understanding of a novel aspect of epilepsy [
72].
2P-FLIM was used in both papers on neurometabolism. Fluorescence decay profiles of intrinsic NADH were measured in vivo surrounding seizures and induced metabolic inhibition. This led to imaging and confirmation of the hypothesis that NADH binding and metabolic capacity are significantly impaired following focal seizure activity [
65,
66]. One paper successfully applied 2P-FLIM to image intrinsic NADH in living human brain tissue [
66]. Furthermore, 2P-FLIM was used to identify the lifetime of substances, such as [Ca
2+] and pO
2, providing interesting information on quantified fluctuations of concentration over time [
57,
58] while also allowing for identification and quantification of lipofuscin in the cerebral vasculature [
21]. Finally, in 20 of the 38 included studies, 2PI was used to image animal brains in vivo. With the surgical implantation of a cranial window in the skull of an animal following anesthesia administration; imaging experiments can subsequently be performed by focusing the excitation laser through this cranial window. In vivo animal models are more representative of the human epileptic state compared to ex vivo models, let alone histological imaging on thin sections. The findings from in vivo 2PI studies are significant because high-resolution information can be obtained, which is not possible with current clinical techniques, and is therefore better translatable. To improve translatability, further imaging of human cerebral tissue is warranted. To date, only three studies included human brain tissue [
21,
40,
66].
4.2. Two-Photon Imaging in Epilepsy Research
The papers included in this review described several interesting and sometimes novel findings in the field of epilepsy research. The relevance of pathological hyperexcitability, whereby individual neurons become increasingly capable of generating repeated action potentials, is a widely recognized phenomenon in epilepsy [
75,
76]. It is thought this pathological hyperexcitability arises due to inhibitory control failure [
77]. We found that several studies in this review applied 2PI to shed more light on this process. For example, the described “inhibitory model” of seizure propagation, wherein the collapse of local inhibition leads to neuronal recruitment during ictal discharges, was suggested by the findings of multiple included studies [
31,
32,
35,
39,
41]. In these studies, 2PI allowed monitoring of [Ca
2+] of neurons and interneurons, revealing their activity. In addition, the interactions between glial cells and neurons were revealed through 2PI calcium imaging, underlining the importance of the glial environment in epileptic states. Additionally, we found that morphological changes to dendrites and axons can lead to dysfunction, possibly contributing to pathological hyperactivity [
62]. Another characteristic feature of epilepsy is interictal spiking, which refers to abnormal neuronal discharges between seizures. Previous reports have addressed interictal spiking [
78]; however, 2PI was needed to demonstrate the widespread impact of interictal spiking on interconnected regions of the brain by visualizing [Ca
2+]. This finding suggested a prominent role of interictal spiking in epilepsy [
36,
37]. However, the exact mechanisms responsible for the emergence of spontaneous interictal activity remain unknown. Interestingly, 2PI calcium imaging of neuronal activity in the in vitro human neocortex seemed viable and comparable to patterns in experimental animal models.
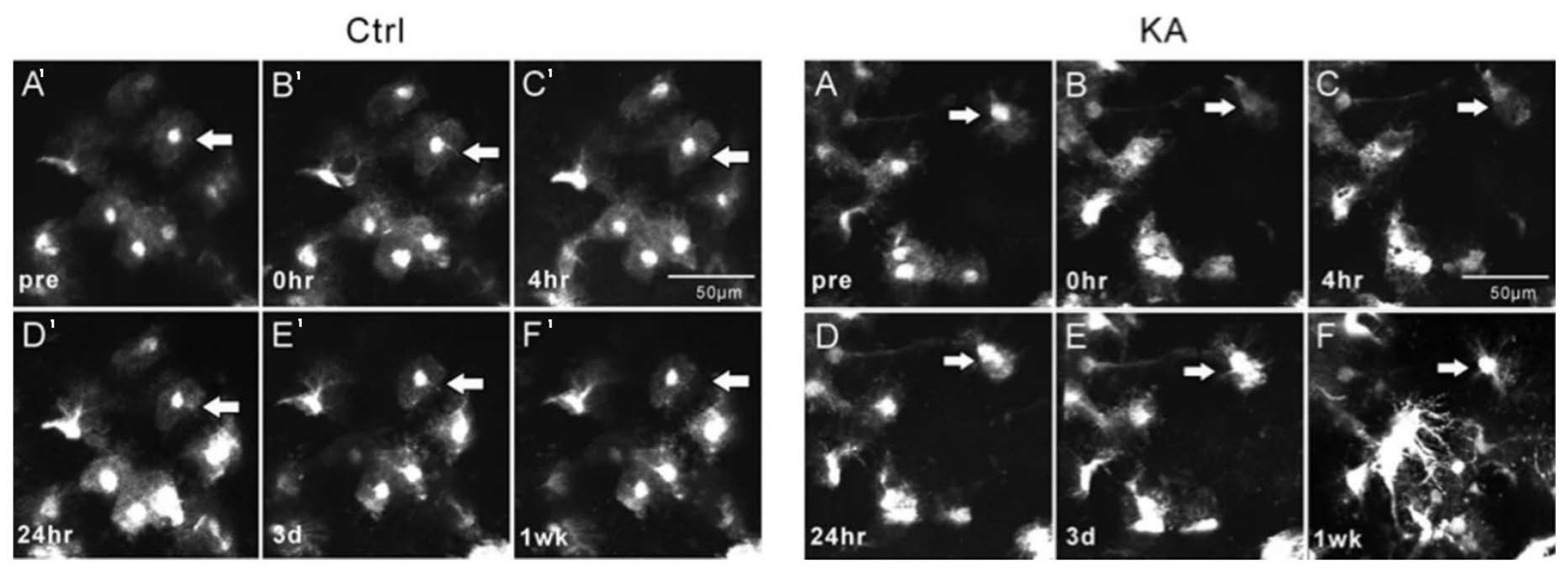
Important morphological changes in microglia, astrocytes, and neurites due to epileptiform activity were revealed in studies that applied 2PI. Using 2PI, changes in the mean sizes of cell bodies and the quantification of microglial processes before and after seizure-induction were recorded [
42,
45]. Such findings have not been reported previously. Alterations in the interactions of microglia and neurons in neuronal integration were described in epileptic states. Interestingly, microglia were identified to play a neurotoxic as well as a neuroprotective role in epileptic conditions, and the exact significance of this finding has yet to be explored. Furthermore, astrocytic [Ca
2+] signaling and subsequent glutamate release was identified as a key process contributing to the development and progression of ictal discharges. These findings are highly relevant, as astrocytic [Ca
2+] modulation provides a novel mechanism for seizure development and progression. This in turn indicates that astrocytes are capable of playing an active, dynamic role in modulating brain function [
55,
79]. This led to the understanding that the role of astrocytes goes far beyond its previously delineated supportive role in maintaining local structures and metabolism. This finding has revolutionized our understanding of epilepsy and has resulted in the concept that “astrocytic transformation” plays a major role in its pathophysiology [
80].
Given the recent change in epilepsy research from a neuronal-centered toward a vascular-centered concept of epileptogenesis [
9,
10,
11,
12], neurovascular alterations in epilepsy are of major interest. By using 2PI, changes in blood flow were visualized, with different states of vasoconstriction and vasodilation surrounding the epileptogenic focus [
59,
60,
61]. Preictal vasoconstriction was found to be correlated with increased excitatory and reduced inhibitory neuronal activity levels [
61]. These findings indicate that excitatory and inhibitory neurons affect surrounding neurons during epileptiform activity leading to dysfunctional local inhibition, which is supportive of the aforementioned “inhibitory model” of seizure propagation. Furthermore, the finding that astrocytic [Ca
2+] corresponds with vessel responses [
57] also suggests that astrocytes may play an active role in mediating changes in the neurovasculature in epilepsy.
Lastly, 2PI was employed to detect concentrations of NADH, an important cofactor central to metabolism, to identify neurometabolic alterations [
65,
66]. NADH 2PI fluorescence revealed prolonged seizure activity resulting in impairments of the electron transport chain as a consequence of inadequate oxygen supply, suggesting long-term altered metabolic capacity of neural tissue in epilepsy, possibly contributing further to pathological processes.
4.3. Oxidative Stress
Oxidative stress plays an important pathophysiological role in intracellular mechanisms in all of the aforementioned cells and processes [
68,
81,
82]. Most of the involved proteins and molecules in oxidative stress are difficult to assess due to their short lifespan and rapid reactivity with redox state regulating components [
83]. Hence, a robust analysis of the role of oxidative stress in epilepsy pathomechanisms using 2PI requires assessment of non-degradable fluorescent or autofluorescent composites. In this regard, lipofuscin forms an interesting end-product of oxidative stress as it is a nondegradable substance with strong and specific autofluorescent properties [
84,
85]. It is most commonly found in the cytoplasm of cells with a low rate of mitosis, such as neurons [
86]. Its aggregation is associated with the pathophysiology of epilepsy as well as with neurodegenerative disorders [
22,
87]. We recently associated lipofuscin with the pathophysiology of epilepsy using 2PI in vascular and tissue samples of epilepsy patients that were harvested following surgical resection of epileptic brain regions [
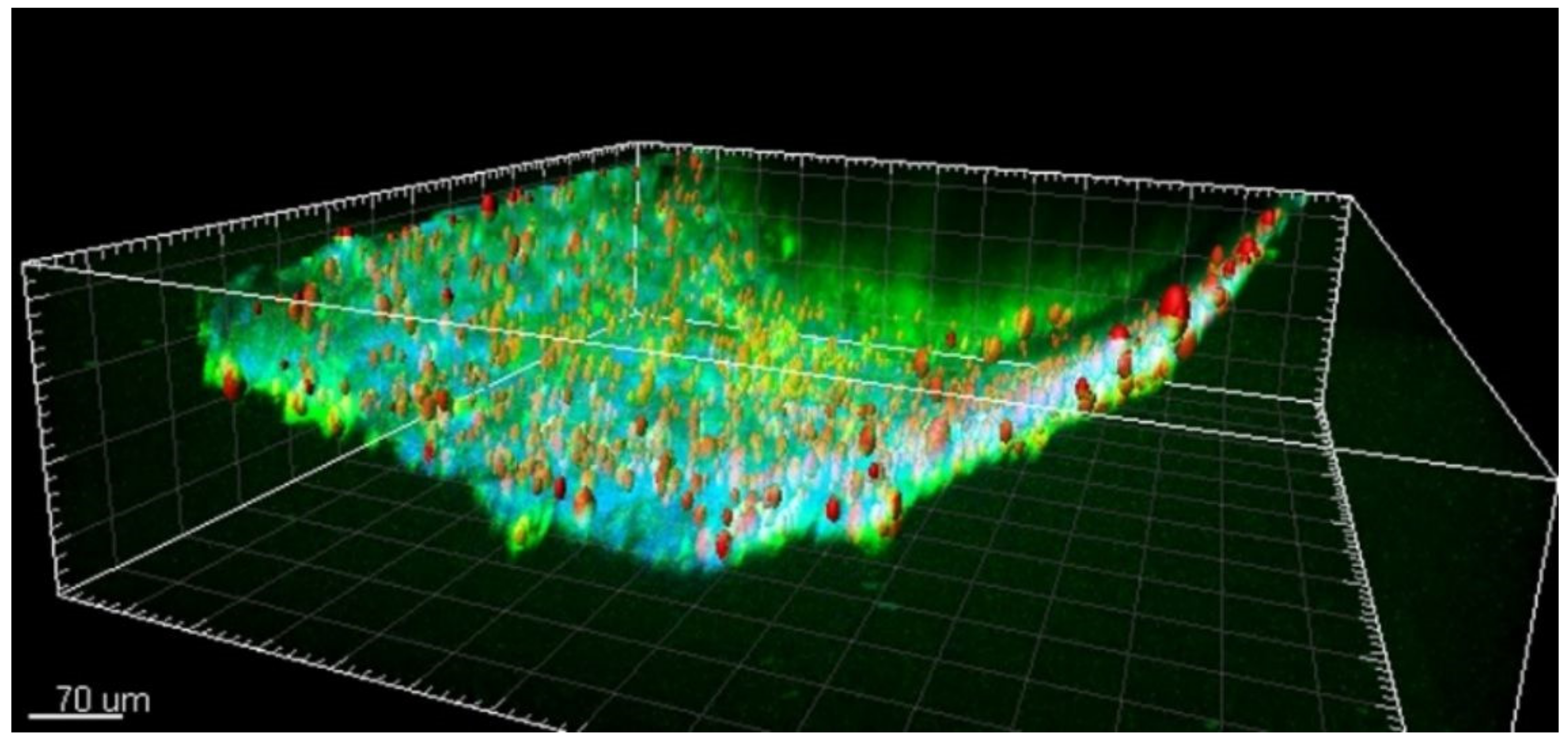
21]. We found that lipofuscin is present in the brain’s pial arterial wall and neocortical parenchyma in young epilepsy patients and age-matched controls. In the three-dimensional reconstruction of the 2PI image stack, the particles appeared to be mainly located within the adventitia and to a lesser extent in the tunica media (shown in
Figure 5) [
21]. Further quantitative analysis of lipofuscin particles suggested that progressive accumulation of lipofuscin in the vascular wall may be related to the pathophysiology of epilepsy.
Previous studies also reported on lipofuscin aggregation in association with human epilepsy. Liu et al. [
88] described excessive lipofuscin accumulation within dysmorphic neurons in young adult patients with focal frontal lobe epilepsy. Furthermore, lipofuscin has been found in mesial temporal lobe structures of epilepsy patients. Here, oxidative damage and neuronal degeneration induced by seizures seemed to contribute to the formation of lipofuscin in the hippocampus of kainate-treated mouse epilepsy models [
89]. In patients with temporal lobe epilepsy, the hippocampal antioxidative system was upregulated [
90]. This finding was most pronounced in patients with hippocampal sclerosis [
90]. Another article reported on the identification of lipofuscin granules within the entorhinal regions and amygdala of epileptic tissue [
91].
These findings provide a basis for understanding the potential involvement of oxidative alterations in the pathophysiology of epilepsy. Analysis of lipofuscin could be a new method to detect and quantify oxidative stress. Using 2PI, lipofuscin is recognized relatively easily based on its specific autofluorescent and lifetime characteristics [
21]. Furthermore, the finding of lipofuscin accumulation within the vascular wall in addition to lipofuscin-accumulation in post-mitotic cells such as neurons warrants further assessment.
4.4. Zebrafish Models of Epilepsy
While the majority of research in this area has so far been conducted using rodent models, zebrafish models of epilepsy are increasingly adopted. Two studies in this review used zebrafish models of epilepsy and 2PI [
39,
47]. Zebrafish are said to represent an excellent compromise between system complexity and experimental accessibility [
39].
A recent study demonstrated that brain trauma can induce epileptogenesis and seizures associated with inflammation and blood–brain barrier dysfunction in zebrafish, which resembles pathophysiological processes in human posttraumatic epilepsy [
92]. Additionally, the cerebral vasculature of zebrafish is morphologically and physiologically similar to humans and can readily be genetically manipulated [
93]. These properties make zebrafish models suitable for epilepsy research, especially considering the recent shift towards studying epilepsy as a vascular disorder.
The use of zebrafish models of epilepsy is rapidly increasing, and recent studies conducted using these models make a good case for their potential [
94]. Combining their use with 2PI is an exciting prospect that may aid our understanding of the different aspects of epilepsy.
4.5. Limitations
Despite a systematic and thoughtful search strategy, we may have missed some papers worthy of inclusion in this review. As all papers focused on their results, it was often challenging to extract which results were found specifically using 2PI. Discrepancies in methodologies such as the animal models utilized, seizure-induction methods, seizure durations and magnitudes, and other variables limit the comparability of the included studies. In fact, there are some instances of conflicting observations. Epilepsy is a syndrome referring to a group of distinct neurological disorders wherein epileptic seizures are a common symptom. Therefore, there can be significant variation and heterogeneity in terms clinical manifestation as well as the underlying biological and pathophysiological mechanisms and pathways responsible.
Additionally, animal models cannot fully replicate human epilepsy. While pharmacological agents such as 4-AP, kainic acid, and pilocarpine are capable of generating seizures, these seizure models are not representative of human epilepsy as a whole. Human epilepsy is characterized by several complex processes, including epileptogenesis and spontaneous interictal activity, which cannot be suitably replicated experimentally. It is also noteworthy that the use of anesthesia in in vivo 2PI experiments of live animals may represent a confounding variable by exerting unintended effects. These factors affect the translatability of findings presented in such studies. Hence, more research using human tissue is warranted.
With regard to the application of 2PI in epilepsy research, the surgical implantation of cranial windows for in vivo 2PI experiments can present some challenges. It has been reported that image quality may be diminished by craniotomy closure due to wound healing and inflammatory responses [
95]. However, this is not a major limitation as these effects can be nullified by employing sterile surgical techniques and by minimizing trauma [
95]. Although 2PI allows for deeper tissue penetration compared to single-photon microscopy, it is still limited to a depth of 2 mm into the brain. Beyond this depth, the scattering effects reduce image quality significantly. However, advances in the development of fiber-optic-based two-photon endomicroscopes potentially allow for in vivo probing of deep brain structures in experimental contexts [
96]. Another limitation of the application of 2PI in epilepsy research relates to the use of calcium-sensitive dyes to stain and subsequently visualize cells. Overexpression of such calcium indicators may cause unintended perturbations of baseline calcium levels. In fact, it has been reported that transgenic mice expressing the calcium indicator GCaMP6 can undergo modulations in electrical activity over large regions of cortex. These effects have been documented in mice of either sex across multiple laboratories [
97].
4.6. Future Perspectives
Currently, drug-resistant epilepsy is still awaiting new treatment options that may be aimed at as-of-yet unraveled mechanisms. The advantages of 2PI as demonstrated in this review have paved the way to visualize novel and specific processes associated with epilepsy. As such, the additional value of 2PI lies in the potential to find novel targets for antiepileptic therapies in the quest for seizure prevention. In this regard, studying the effects of epilepsy and anti-epileptic drugs on electrolytes such as Ca2+ and neurotransmitters such as glutamate and GABA have been shown to be highly relevant. Future studies that, for example, assess the application of K+-sensitive fluoroionophore FI3 for the visualization of epileptic seizures might be useful as an improved alternative for [Ca2+] imaging in studying specific features of epileptic activity.
Using 2PI, in vivo assessment of cerebral epileptic processes is warranted. In vivo imaging has so far mostly been limited to superficial cortical regions of rodent subjects. However, advances in endomicroscopy could provide the possibility to investigate deeper brain structures in vivo, such as the insular region [
96]. Endomicroscopy is a technique in which a small optical fiber can be inserted into a region of interest to visualize the target tissue. Following its employment, two-photon endomicroscopy could assess functional and morphological characteristics of the target tissue. In addition to applying this technique to animal models, in vivo 2PI and endomicroscopy could be applied for human research. Even though the possibility to capture a live seizure event in humans is unlikely, the profound effects of seizures on astrocytes, neurites, and microglia throughout the human brain could be visualized. In fact, in the field of neurosurgery, in vivo endomicroscopy is increasingly being adopted to visualize vascular structures and specific regions of interest [
98]. This is an exciting prospect that could potentially revolutionize the way we evaluate and understand epilepsy.
2PI has been proven to be feasible in visualizing neurovascular changes in epileptogenesis. Further research may be aimed at exploring microvascular alterations and blood–brain barrier dysfunction, perhaps finding novel therapeutic targets by restoring adequate vasomotor control, which can be visualized using 2PI. Furthermore, the aggregation of lipofuscin in the neurovasculature could play a role in the pathophysiology of epilepsy, contributing to our understanding of the disorder.
2PI is also of interest for use in studying the pathophysiology of other neurological disorders, such as stroke, neurodegenerative disease, and traumatic brain injury. Hence, the results of 2PI described in this review may also contribute to a better understanding of these neurological disorders. Furthermore, the potential application of 2PI using the current two-photon endoscopes under development opens avenues for 2PI as an in vivo tool to visualize metabolic processes and individual cell characteristics. As such, 2PI endoscopes could contribute to a new approach to brain imaging.