Featured Application
This study developed a robust optimization algorithm to minimize dose delivery uncertainties by potential applicator-positional errors for MRI or CT-based planning in the Cervix Brachytherapy. It is important to improve clinical outcomes to minimize the dose to the organ at risk (OAR) when covering the target.
Abstract
Brachytherapy is an important technique to increase the overall survival of cervical cancer patients. However, a possible shift of the applicators in relation to the target and organs at risk may occur between imaging and treatment. Without daily adaptive brachytherapy planning, these applicator displacements can lead to a significant change in dose distribution. In order to resolve it, a robust optimization method had been developed using a genetic algorithm combined with a median absolute deviation as a robustness evaluation function. The resulting robustness plans from our strategy might be worth considering according to the GEC-ESTRO guidelines. From the point of view of dose delivery uncertainty from applicator displacement, the robust optimization may be considered with caution in a single-plan approach for High Dose Rate brachytherapy treatment planning and should be confirmed by a more thorough investigation.
1. Introduction
Cervical cancer is the fourth most frequent cancer among women worldwide and ranked second for both incidence and mortality in the lower Human Development Index (HDI) [1,2]. According to previous reports, cervical cancer patients with initial stages (stages IB—IIA) who undergo appropriate treatment will develop a recurrence with a risk factor of 10–15% [3,4]. Therefore, early diagnosis and treatment are crucial for reducing the mortality rate.
One of the most common treatment strategies for cervical cancer, when high dose radiation is required to be curative, is a combination of external beam radiotherapy (EBRT), chemotherapy and brachytherapy, such as EBRT alone, EBRT plus brachytherapy, or combined EBRT plus brachytherapy with concurrent chemotherapy [5]. Several studies have verified that the combination of EBRT, chemotherapy, and brachytherapy improves treatment outcomes [6,7,8]. It is well known that the brachytherapy boost improves overall survival by 5 years due to its superiority of rapid dose fall-off with distance from the source and limited dose exposure to surrounding tissues in accordance with the inverse square law [9]. Moreover, a remote after-loading platform, including radioactive sources, allows for a more precise configuration of the dose to the target and the optimization of dwell times [10].
Excellent results were achieved with brachytherapy in combination with EBRT although this approach is not without limitations. The dose distribution in brachytherapy has a sharp dose gradient that is inherent to the radioactive source, and it is more sensitive to uncertainties of patient setup and applicator position [11]. Uncertainties in brachytherapy for cervical cancer are mainly related to source calibration, dose and dose–volume–histogram (DVH) calculation, reconstruction of applicators, contouring, intra- and inter-fraction uncertainties and dose delivery [12,13,14,15,16,17,18,19,20,21,22]. For the definition of the size and location of the cervical cancer, magnetic resonance imaging (MRI)/computed tomography (CT)-guided High Dose Rate (HDR) brachytherapy is currently defined by Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology (GEC–ESTRO) as MR imaging prior to the first fraction of irradiation with the applicator in place and subsequent treatment planning of each fraction [23]. However, an MRI is not routinely available in radiotherapy departments and this optimal approach requires a large amount of personnel, time and equipment infrastructure. An alternative to using CT or radiographs for subsequent fractions is the use of one implantation for several fractions of irradiation with a standardized constant bladder filling [24,25]. In the view from above, the dosimetric impacts of uncertainty should be considered to minimize therapy delivery variations and to improve patient outcomes: Grigsby et al. [26] investigated the interfractional tandem displacement between multiple insertions, and found a displacement of about 1.2 cm in the caudal–cranial direction when a point A based-plan was generated on orthogonal X-rays, and Hellebust et al. [27] applied one brachytherapy plan for several fractions and demonstrated that the average relative standard deviation for 13 series of 3 to 6 fractions were 15 and 17.5% for the rectum and bladder, respectively. Kiristis et al. [25] compared individual MRI-based 3D treatment planning for each of four fractions with the use of only one MRI treatment plan for 14 patients. They found significant mean differences the brachytherapy dose of 9–28% variations.
Applicator displacements especially occurred randomly during interfraction treatments so that the change-of-dose distribution could not be determined mathematically. For that reason, a robust optimization method may be considered to address uncertain conditions. There were many ways to deal with this uncertainty [28,29,30]; however, there is no direct literature minimizing dose delivery uncertainty by applicator displacements. The most relative and straightforward approach was to predict the worst-case objective value from all scenarios and minimize their dose delivery uncertainty similar to robust optimization strategies in intensity-modulated radiation therapy and proton therapy [31,32,33,34,35]. Consideration of the worst-case scenario for dose delivery uncertainties in cervical brachytherapy is useful for assessing resilience and robustness. In practice, the band of DVHs for a given structure represents the range of possible dose variations. The evaluation of the band width on the DVH can be used as a quantitative measure of robustness. The median absolute deviation (MAD) is used as one of the well-known robustness estimators. It is not only often used as an initial value for computing more efficient robust estimators but also as a good robust estimator in regression problems [36]. To cope with the measurements of bandwidth at the defined dose-volume points on the DVH, a MAD robust estimator was applied to design a robust optimization algorithm in this study.
In real-world optimization problems, objectives can conflict, so there cannot be a single solution [37]. Accordingly, the optimization of dwell times and dwell positions using an inverse optimization technique needed to be carefully considered to improve the target dose coverage and reduce the dose delivered to the organ at risk (OAR). The objective function for determining the dwell times at each of dwell position for the desired dose distributions is a multi-objective optimization problem requiring complex computation [38,39]. Classical optimization techniques are efficient at finding local minimum points; however, they may not yield the global minimum point. To solve this problem, we should search for the set of all optimal compromises. The so-called Pareto set, containing all Pareto-optimal points based on objective function, has more than one local minimum. As previously mentioned, the feasible solution to a multi-objective problem in real-world optimization is to find a set of solutions. Therefore, the genetic algorithm is well-suited to solving multi-objective optimization problems due to the population-based search approach. Given the above, a preferred efficient genetic algorithm that can search global minimum points as a multi-objective optimizer was selected to overcome the complexity of global searches [39,40,41,42]. The focus of this research study was to assess the feasibility of minimizing dose delivery uncertainty by potential applicator displacements of cervical brachytherapy treatment using a MAD-constrained robust optimization method with a multi-objective genetic algorithm.
2. Materials and Methods
2.1. Treatment Plans Data Set
Planning data from five patients who had previously been treated with EBRT followed by cervical HDR brachytherapy with a Tandem-and-Ring (TR) applicator set was used as a representative sample in this retrospective study. Each patient who underwent brachytherapy procedures was placed in a supine position with a CT/MRI applicator clamp attached to a base plate, immobilized and transported during imaging and treatment. All patients received the EBRT dose of 50.40 Gray (Gy) in 28 fractions with a consecutive brachytherapy boost dose of 25 to 30 Gy in 5–6 fractions. For the first fraction of a brachytherapy patient’s treatment, an MRI- or CT-based treatment plan was generated and delivered, and then for subsequent fractions (2–5 or 2–6). The mobile C-arm X-ray imaging unit (WHA-50N, Shimadzu, Japan) was used only for registration and applicator position verification prior to each fraction of treatment. It should be noted that the initial brachytherapy plan (made from the first fraction) was reused for subsequent fractions of treatment. This manually optimized “nominal plan” (also called “initial plan”) was generated from the first fraction image and compared with that of a robust optimized treatment plan taking into account applicator displacement assumptions. To incorporate dose delivery uncertainty by potential applicator displacements, the translation of the applicator displacement set up 1 mm steps in the upper and lower bound of 5 mm in the x, y and z directions based on assumptions in the pre-study reports: the MRI-based 3D treatment plan for each fraction using only one MRI treatment, interfractional tandem displacement between multiple insertions [26], interapplication variation of doses and spatial location of the OAR [27] and several reports by other groups [20,21,22]. Then, we applied our robust optimization method to anatomical structures and applicator models delineated from the first fraction image to generate robust treatment plans for all treatment fractions.
2.2. Optimization System Modelling
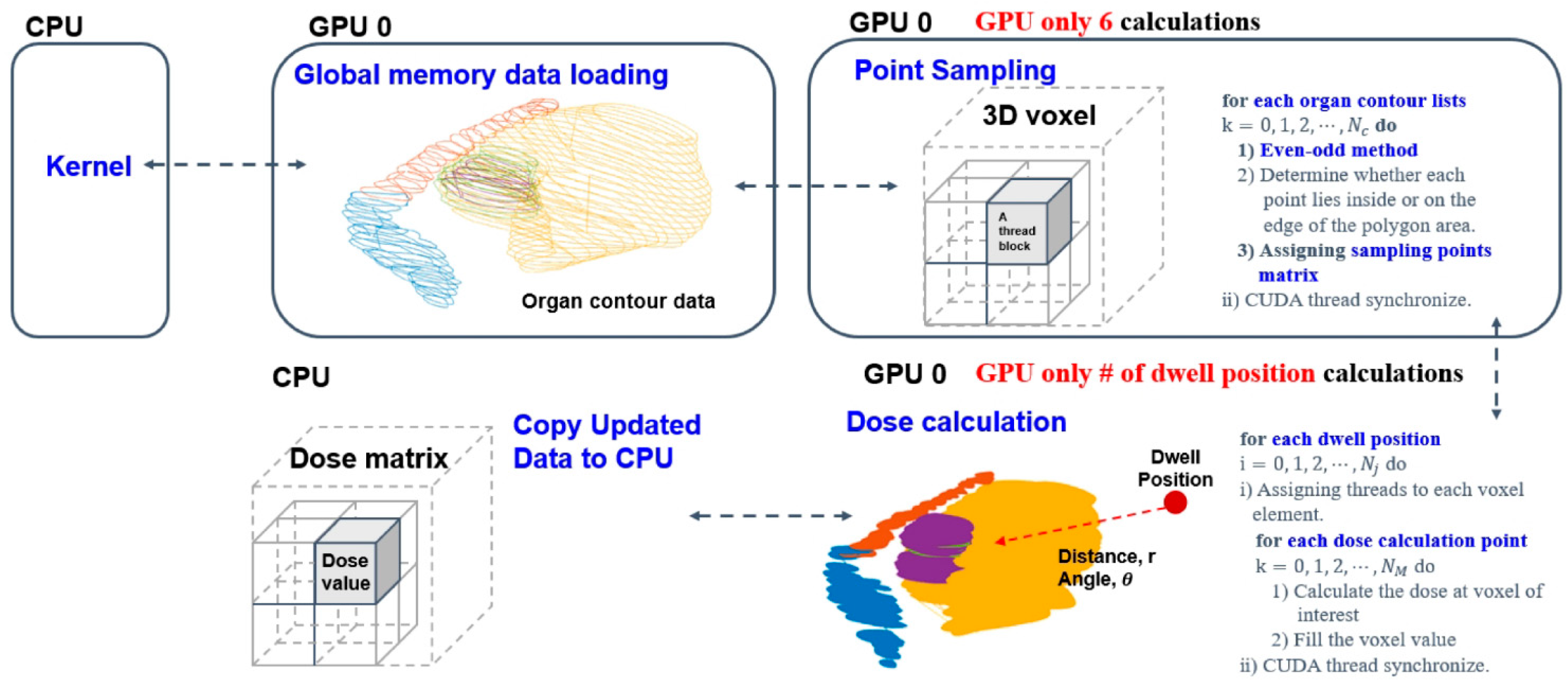
A In this study, Elekta microSelectronTM (Nucletron, Elekta AB, Stockholm, Sweden) HDR after-loader unit with 192 Ir source was used. OncentraTM (Oncentra, version 3.5, Elekta AB, Stockholm, Sweden) treatment planning system (TPS) was used for making brachytherapy plans. The voxel sizes of the T2 MRI data used for dose calculations as before were 0.5 × 0.5 × 4.0 mm3. Here, MR-compatible TR applicator sets (MR ring applicator, Elekta AB, Stockholm, Sweden) of two differently sized (small and large) rings were used, each with a 30-degree angle, 2.5 mm source position separation, and with a distance from the first source to the applicator tip defined as 7 mm. Traditionally, an optimization algorithm needs specific input data such as digitized positions, relevant anatomical structures and an applicator path to calculate a dose distribution matrix. Regions of interest (ROIs) including the high-risk clinical target volume (HRCTV), intermediate-risk clinical target volume (IRCTV), bladder, rectum, bowel, and sigmoid were manually contoured on each first-fraction image by oncologists and delineation was based on MRI findings. The contour-point lists for each structure were extracted in sequence from the Digital Imaging and Communications in Medicine–Radiation Therapy (DICOM–RT) structure files using C++ program language. Also, applicator paths were reconstructed and extracted as radiation source positions in the treatment-planning system. The source dwell positions provided in the applicator coordinate system were pre-defined according to the applicator ring structure. Overall, volume delineation and dose constraints were made in accordance with GEC–ESTRO recommendations and EMBRACE protocol guidelines for both the nominal and robust plan [18,43,44]. Given the above facts, we only used positional information of anatomical structures and source positions from the first fraction image to make the optimization model; then, after determining the positional characteristics of the source relative to the dose, calculations were completed with care in all robust optimization processes (Figure 1).
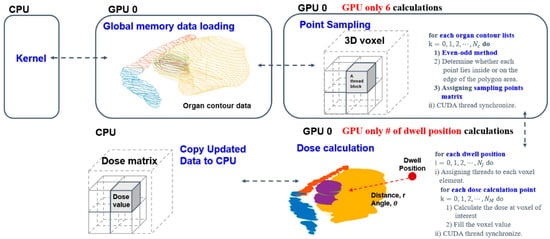
Figure 1.
Detailed dose matrix calculation process from the delineation dataset.
2.3. Robust Treatment Plan Optimization
The robust optimization quality evaluation of the treatment plan is defined by the relation of each objective function to an anatomical structure and source position. Thus, the robust optimization is based on minimizing the sum of objective functions, . During an optimization iteration, plan quality was optimized by adjusting dwell times at each dwell position, x, and (the certain feasible set). The dose rate to a given dose point from dwell position j () was calculated using TG-43 formalism [45,46,47]. The dose rate coefficient matrix under that dose delivery uncertainty scenario is denoted by . Let represent the set of all dose delivery uncertainties under consideration; then for all scenarios, let , and all possible dose distributions be [32]. In addition, the constraint functions are scalar, so this constrained optimization approach is bounded and feasible [35] and can be formulated as
The voxel dose was calculated as (dose rate to a given dose point i from a dwell position j in each structure) and , and are bounds on the values of the constraint functions and given in the unit Gy. In this formula, the trade-offs related to robustness against uncertainty across a variety of scenarios was important for maximizing plan quality and minimizing uncertainty during optimization. If the set of uncertainty were empty for all decision variables , then optimization could be a nominal problem. Therefore, we set up the manual plan, generated by the oncologist and medical physics staff, as a nominal scenario, and it was considered a treatment plan without the shifting position of the applicator (i.e., uncertainty is zero, s = 0). Additionally, we used the applicator displacement assumption mentioned in Section 2.1 to create a worst-case scenario.
2.4. Plan Robustness
In this study, the DVH of the target volumes and OARs for each dose distribution were calculated to encompass all applicator displacement scenarios and assess a large number of scenarios for statistical interpretation [48,49,50]. For the statistical approach, we considered the band width at critical dose-volume points on the DVH under uncertainties used as a quantitative measure of robustness. Thus, the MAD of the bandwidth at the critical dose-volume points on the DVH was calculated for how spread out a set of data is. The worst-case value, determined from a random dataset in a bounded range, may not reflect a normal distribution, so MAD was used as a robust estimator instead of the standard deviation. Using the MAD function as a selection criterion served to protect against potentially large noise in data uncertainties [36]. Therefore, we used the MAD function to minimize the overshot and undershot dose-delivery uncertainty to the target volume in this work. The median and MAD functions are
where is the median value of the DVH curve in a given region of interest (ROI) under uncertainties () and is a constant scale factor: was the unscaled default and was used for the scaled version to maintain consistency with Gaussian distribution.
2.5. Robust Optimization with a Genetic Algorithm Optimizer
In this study, given an n-dimensional decision variable vector of dwell times in the solution space , the decision variable vector x was called a chromosome. Normally, the first generation of a chromosome is created randomly and called the initial population [51,52,53]. Randomly generated initial values were used when solving the problem in this work. To find a vector that maximized or minimized a given set of k objectives functions we generated fitness functions to minimize the given objective functions and constraints for our purpose.
where and were objective functions for all contoured organs and was the set of all voxels inside each contoured structure. The and were the prescription dose of the target volume and the dose constraints of OARs, and was a robustness function of the bandwidth at the critical dose-volume points on the DVH of the target volume and OAR under uncertainty. The constant b was a scalar factor linked to the assumption of normality of the data and the disregard of abnormality induced by outliers. Then, we needed to set , as previously mentioned above. The following constraints of the problem were given.
where was the step function defined as = 1 if ; otherwise, = 0 if . was the set of all dwell positions. The DVH of the target volume and OAR were controlled by each organ’s prescription dose in accordance with the EMBRACE protocol guidelines as dose constraints, respectively.
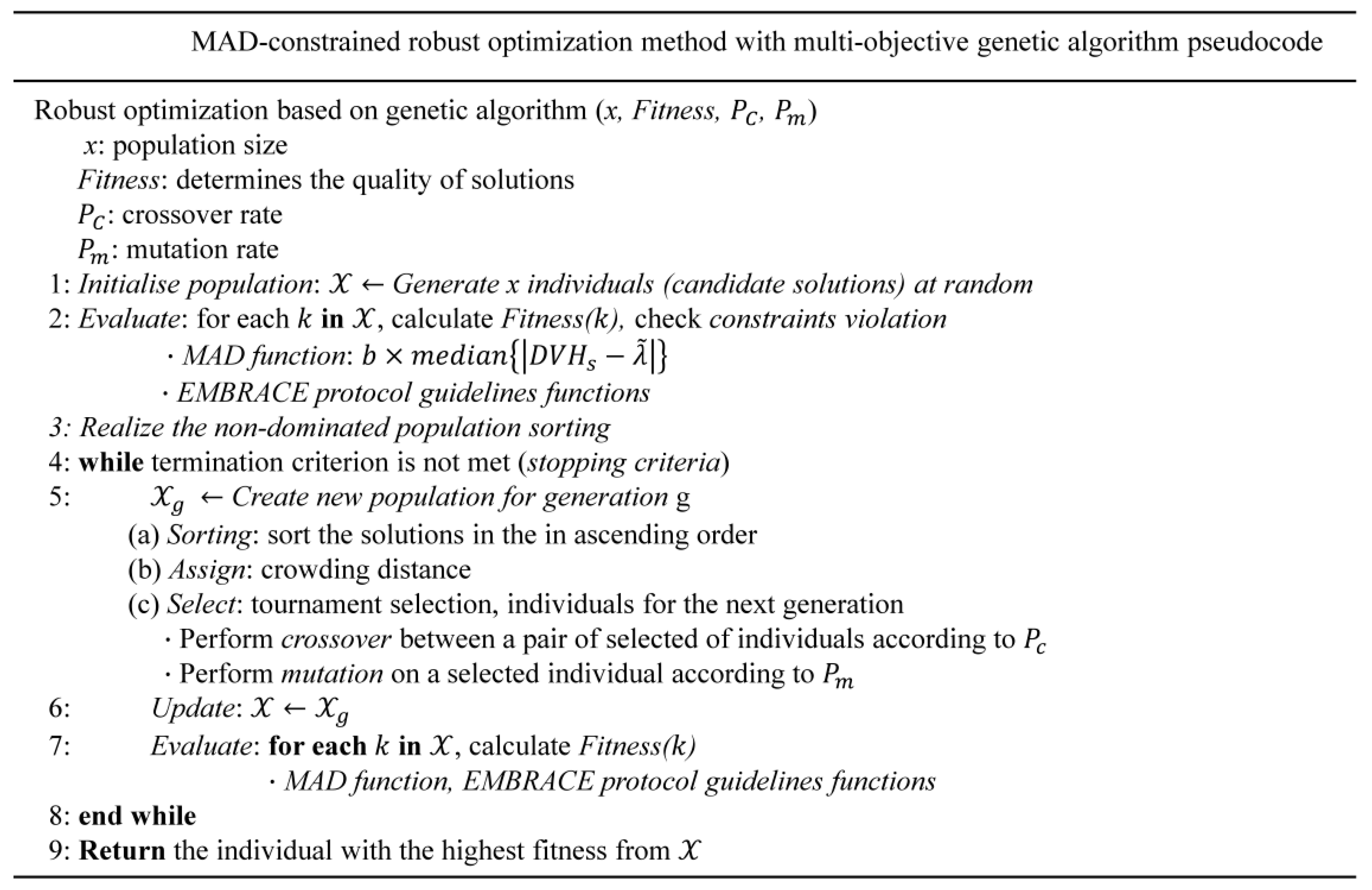
The overall objective functions were optimized by the genetic algorithm in a multicriteria optimization setting. This robust optimization was performed by calculating the delivered dose to the target and OAR in all worst-case scenarios and searching for the best alternative (a given fitness function) through chromosome evolution in each generation (Figure 2). We performed the multicriteria robust optimization problem with dose delivery uncertainty to maximize the fraction of the target volume receiving at least the prescribed dose and to minimize the maximum dose to the OAR [48].
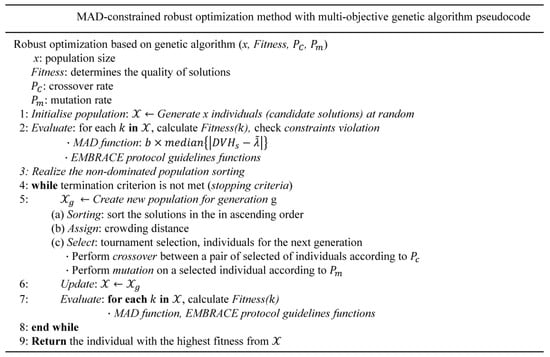
Figure 2.
Detailed algorithmic process of the robust optimization scheme.
3. Results
We demonstrated what occurs on the curve of the DVH when applicator displacements occurs and then validated the performance of the robust algorithm with random scenario sets of applicator displacements in any direction (x, y, and z). Lastly, we compared treatment plans between nominal and robust methods. Five patients’ MRI cases were analyzed with the cervical brachytherapy of 5 Gy × 5–6 fraction prescription after receiving the EBRT of 1.8 Gy × 28 fractions. All patients received the EBRT dose of 50.40 Gray (Gy) in 28 fractions and consecutive brachytherapy boost doses of 25–30 Gy in 5–6 fractions.
3.1. DVH Variation under Uncertainty
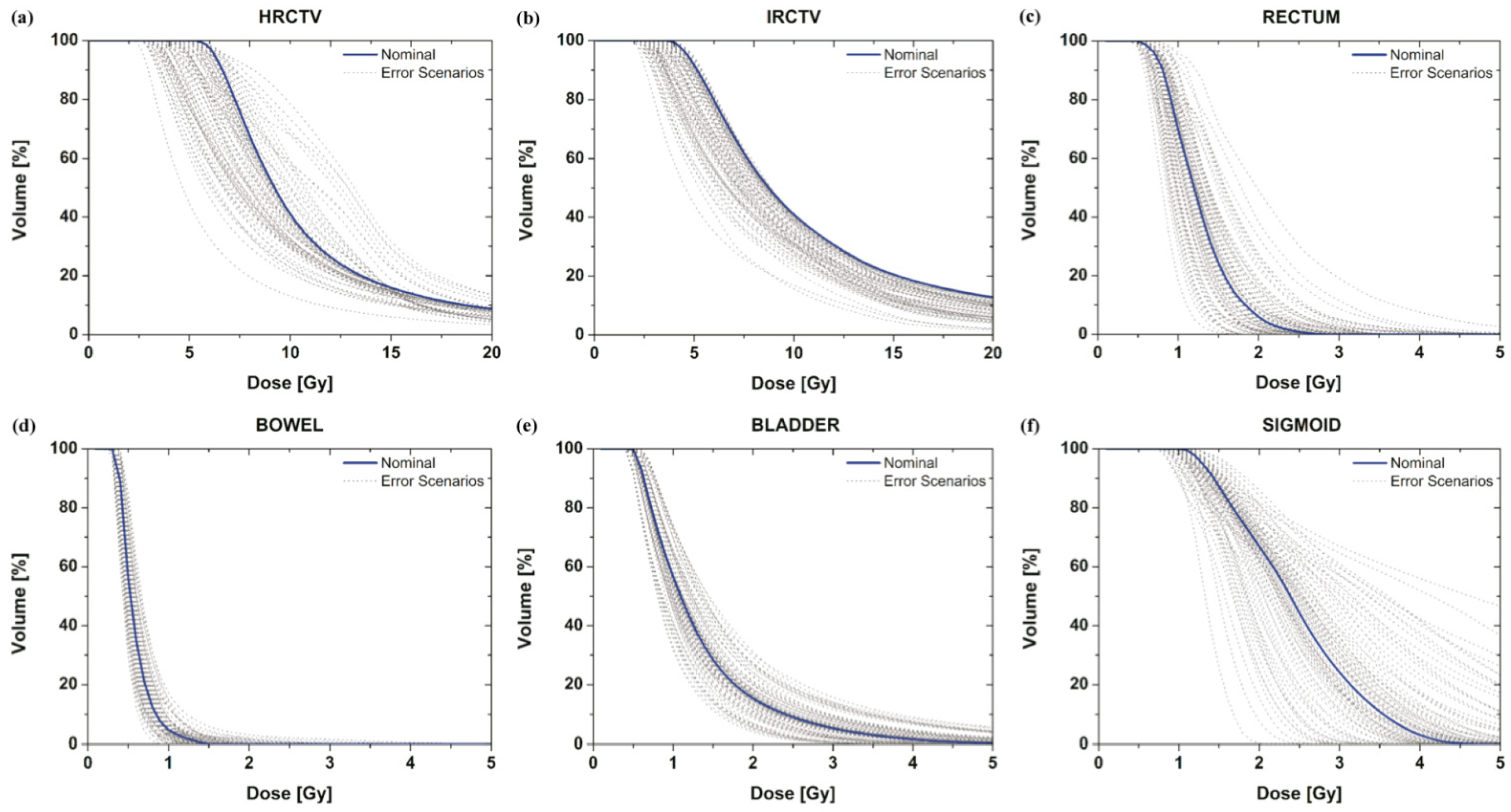
We investigated how DVH curves were changed to the targets and OARs by applicator displacements according to data uncertainty. With the assumption that the applicator moves randomly in any direction in accordance with pre-reported data [20,21,22,23,24,25,26,27], the dose-distribution variation from applicator positional errors for each target and organ was plotted (Figure 3, grey curve). We only considered a total of 79 error scenarios chosen randomly under certain assumptions and a nominal (manual) scenario generated by the oncologist and medical physics staff (Figure 3, blue curve).
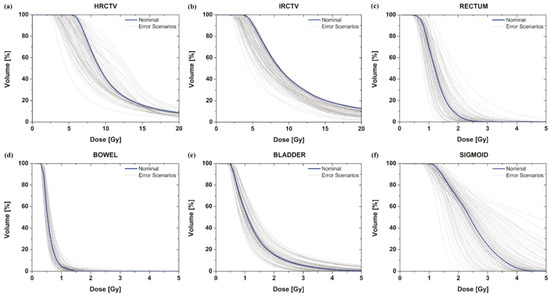
Figure 3.
DVH curve variation of the targets (a,b) and OAR (c–f) under applicator positional errors with the assumption of shift scenarios. The DVH for the nominal plan setting that does not contain uncertainties is the blue curve; the DVHs for 79 error scenarios containing dose uncertainties are the grey curves.
3.2. Performance of the Robust Optimization Algorithm
3.2.1. DVH Curves
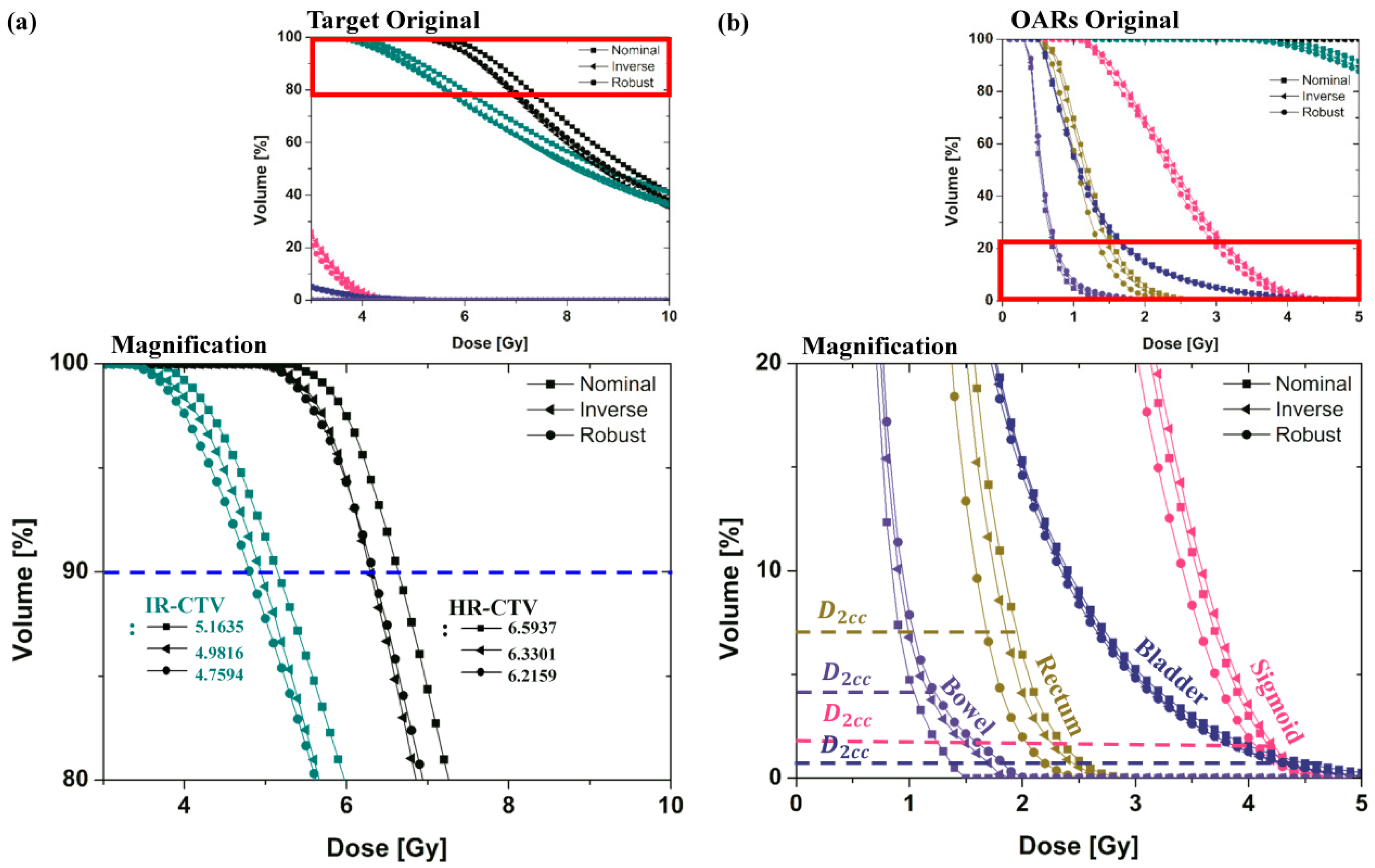
The effect of the proposed robust optimization is demonstrated in Figure 4 and Figure 5. Figure 4 illustrates the DVH curves from the nominal and robust planning for cervical brachytherapy, including inverse planning without considering the applicator displacement parameter. The target received a sufficient dose in all plans with improved rectal and sigmoid dose sparing. We found no severe violation of EMBRACE protocol guidance in any DVH curves of the target volume or OAR using the robust planning.
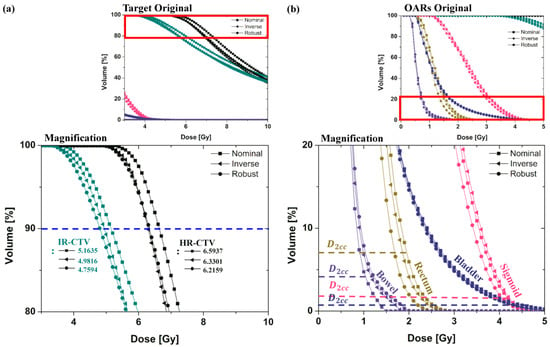
Figure 4.
DVH curves for the nominal (manual) plan, inverse plan, and robust plan in EBRT brachytherapy boost treatment of cervical cancer were plotted using colors indicating HRCTV (Black), IRCTV (Teal), Rectum (Dark Yellow), Sigmoid (Pink), Bladder (Royal) and Bowel (Violet). Graphs (a,b) are the magnification of the DVH curves of the target and OAR.
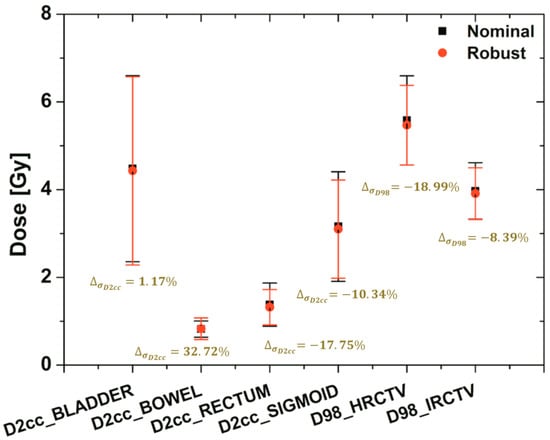
Figure 5.
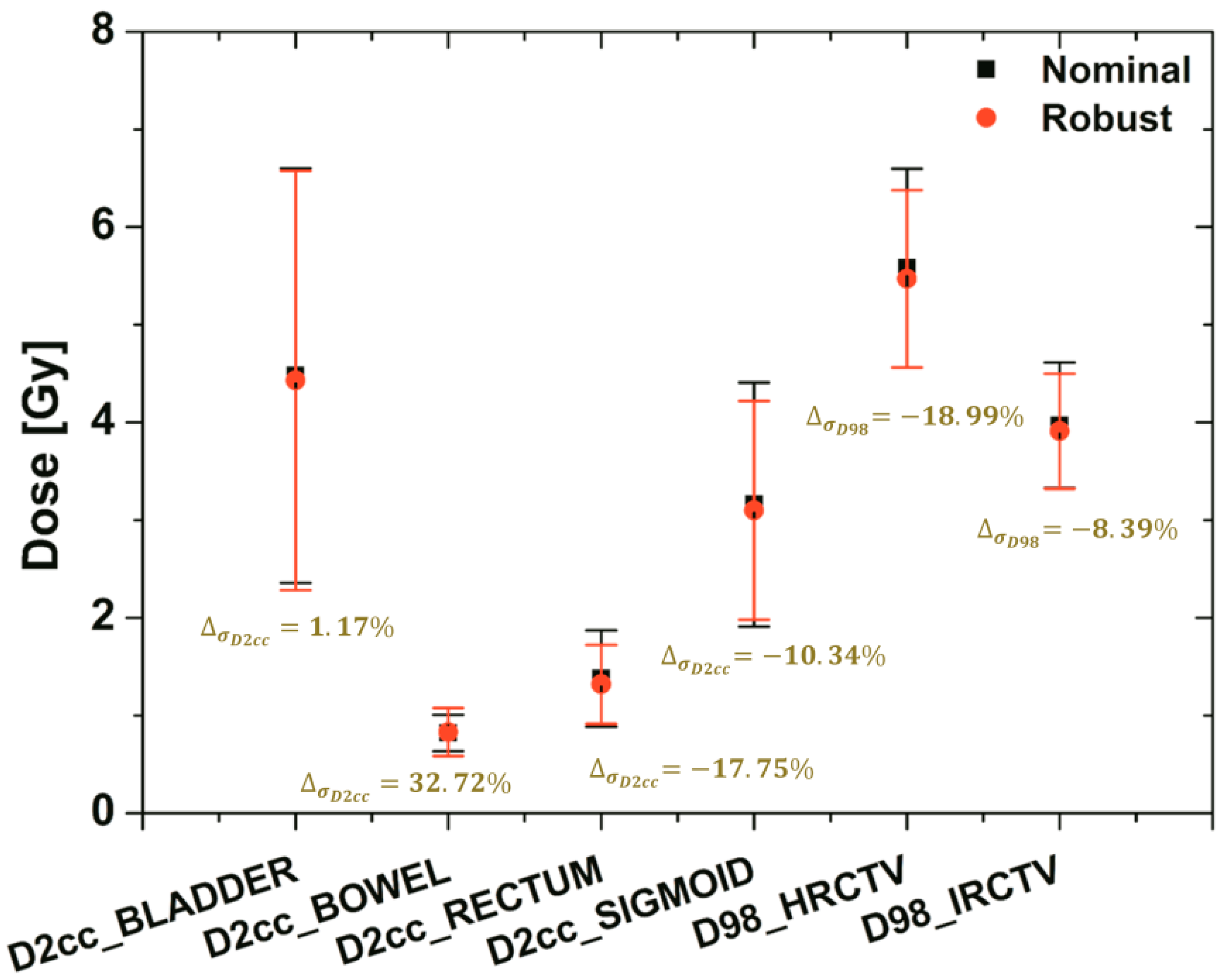
The bandwidth variation at the critical dose-volume points on the DVH of the targets (HRCTV and IRCTV) and OAR (BLADDER, BOWEL, RECTUM, and SIGMOID) under uncertainty with the applicator-shift scenario assumptions. The dose to 98% for target and dose to 2 cc for OAR were shown using a black square (nominal plan) and a red circle (robust plan) with a standard deviation of dose variation based on error scenarios.
In order to show the difference of dose variations between nominal and robust plans, in this case the dose to 98% for target and dose to 2 cc for OAR (with dose variations under applicator-displacement uncertainty) were plotted using colors. The standard deviations of robust planning were less than the others in most of the target and OARs. They ranged in standard deviation from 32.72 to −18.99%.
Moreover, although both plans satisfied the requirements for the dose coverage of the target and the OAR, the band width of the DVH curves under the uncertainty of the robust plan in all organs was much narrower than its nominal plan.
3.2.2. Isodose Lines
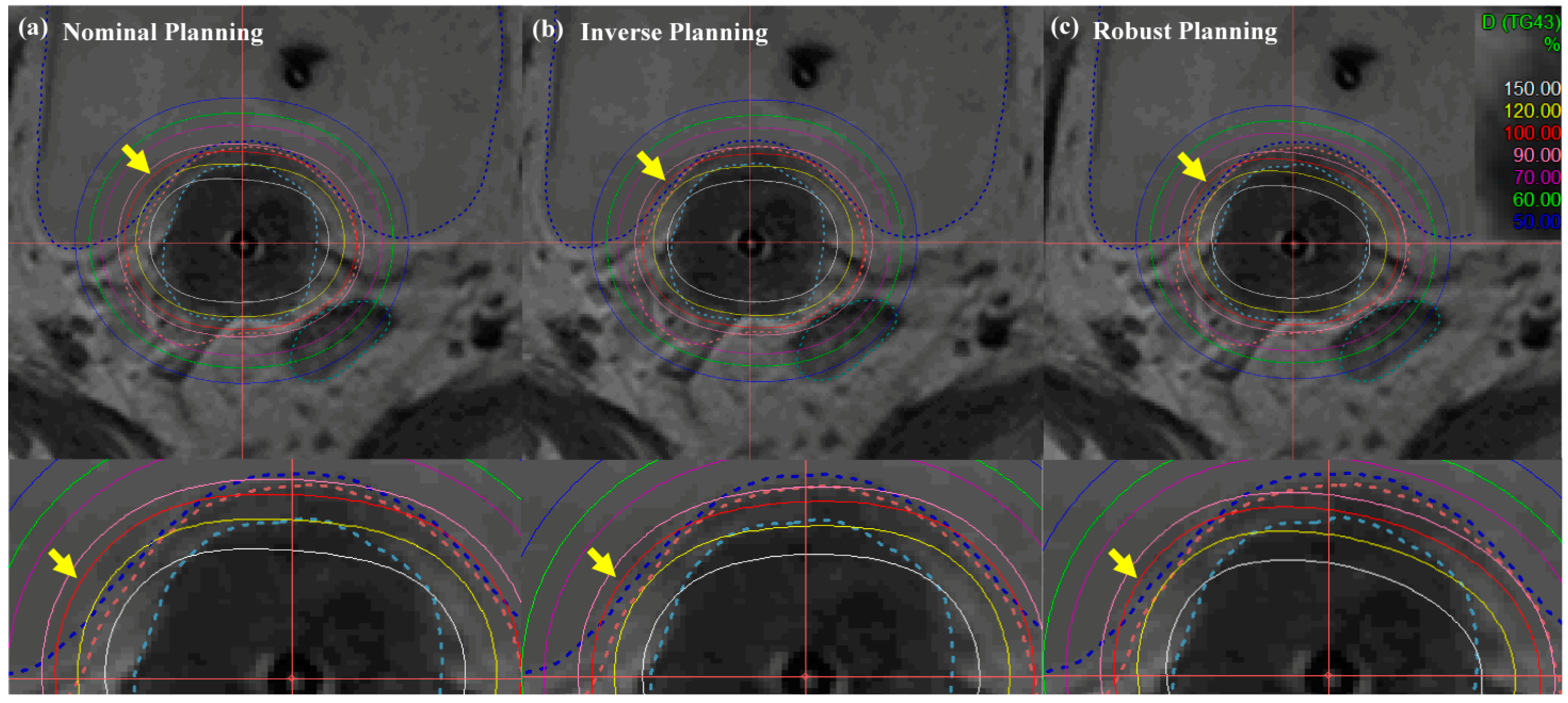
Figure 6 and Figure 7 illustrate the comparison of isodose lines calculated in the Oncentra Brachytherapy TPS of the nominal (manual) plan, inverse plan and robust plan for a cervical cancer patient. In all treatment plans, dwell times at each position for the desired target coverage were successfully encompassed based on EMBRACE protocol guidance. As shown in Figure 6, the bladder contour is encompassed by a minimum of percent isodose line in the robust planning while maintaining target-dose coverage and OAR sparing, rather than both coverage and sparing in the nominal and inverse planning.
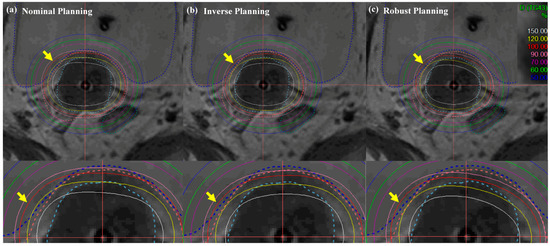
Figure 6.
Isodose-line comparison of (a) nominal (manual) planning, (b) inverse planning, and (c) robust planning standard planning in EBRT brachytherapy boost treatment of cervical cancer: axial view. An EBRT total dose of 50.40 Gy was delivered in 28 fractions, and an ICR total dose of 30 Gy was delivered in 6 fractions using a TR applicator.
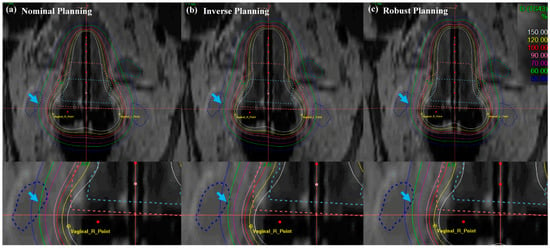
Figure 7.
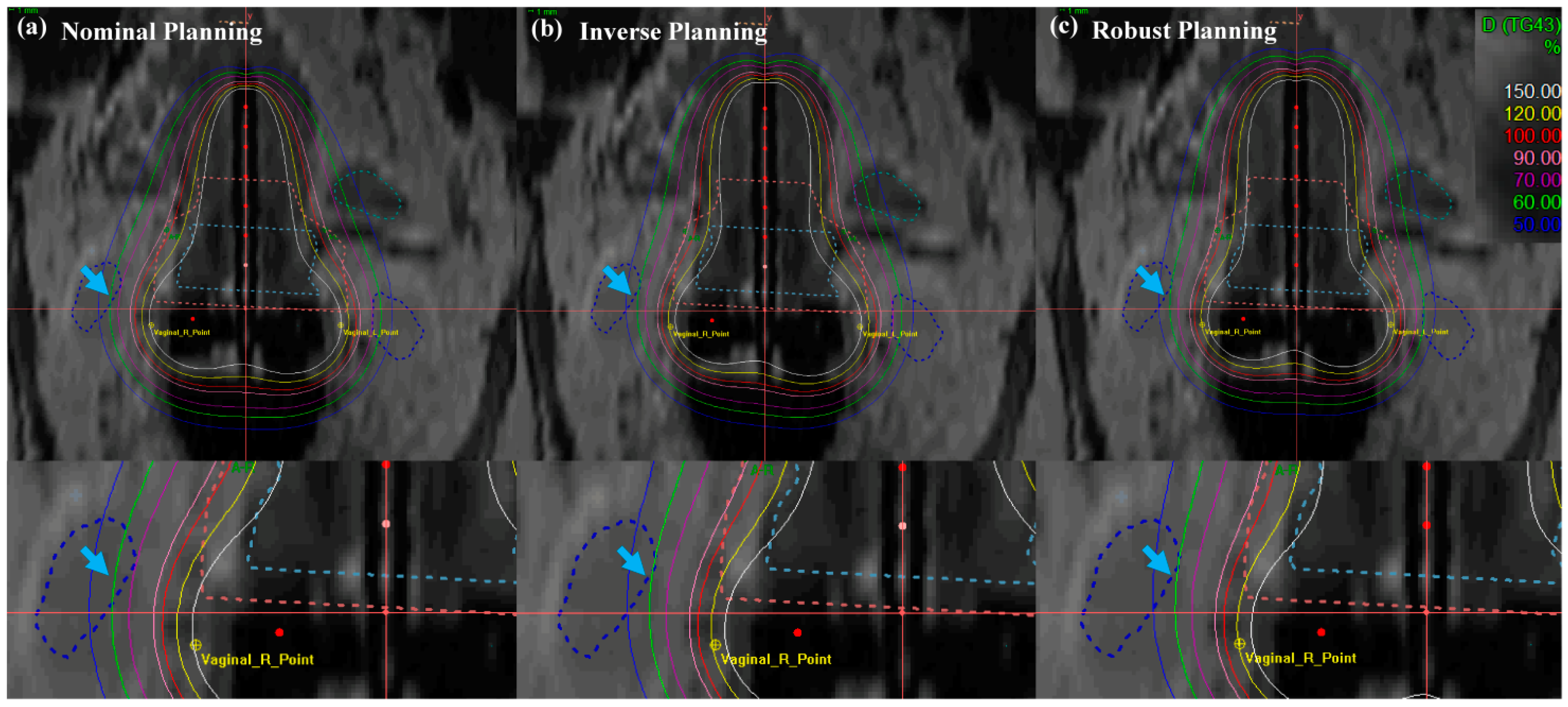
Isodose-line comparison of (a) nominal (manual) planning, (b) inverse planning and (c) robust planning. Standard planning in EBRT brachytherapy boost treatment of cervical cancer: axial view. An EBRT total dose of 50.40 Gy was delivered in 28 fractions, and an ICR total dose of 30 Gy was delivered in 6 fractions using a TR applicator.
In Figure 7, the cervical brachytherapy plan showed the isodose line from a coronal view. Figure 7 demonstrated a reduced dose to the international commission on radiation units (ICRU) vaginal right (R), left (L) point, and sigmoid than nominal and inverse plans (blue arrow with a single arrowhead). Furthermore, the robust plan not only minimized the dose delivery of the ICRU vaginal R and L point, but it also minimized doses to the OAR, while maintaining coverage of uneven HR- and IR-CTV contours. The most distinct difference between the methods in the results for the target and OAR is that the robust optimization method improved the dose distribution and decreased the dose delivery uncertainty. Table 1 displays the numerical results corresponding to Figure 6 and Figure 7.

Table 1.
The dose constraints in accordance with EMBRACE recommendation and the total EQD2 (EBRT + BT) of each target and OAR for nominal, inverse and robust plan strategy.
The quantitative results in Table 1 indicate the EQD2 and the standard deviation of each nominal, inverse and robust plan strategy. For cervical cancer treatment, a prescription dose of 50.40 Gy to the target was delivered by EBRT as previously mentioned. Then, the total EQD2 (EBRT + BT) of HRCTV D98 for all plans (nominal, inverse, and robust plans) was estimated at 88.59 , 86.71 , and 84.84 , respectively, and the standard deviation for each plan was ±1.0177, ±0.9393, and ±0.9085, respectively. Also, EQD2 and the standard deviation of the rectum for all plans were also estimated at 55.29 (±0.4927), 54.94 (±0.4620) and 54.09 (±0.4052), respectively.
3.3. Evaluation of Robust Optimization Algorithm with Various Cases
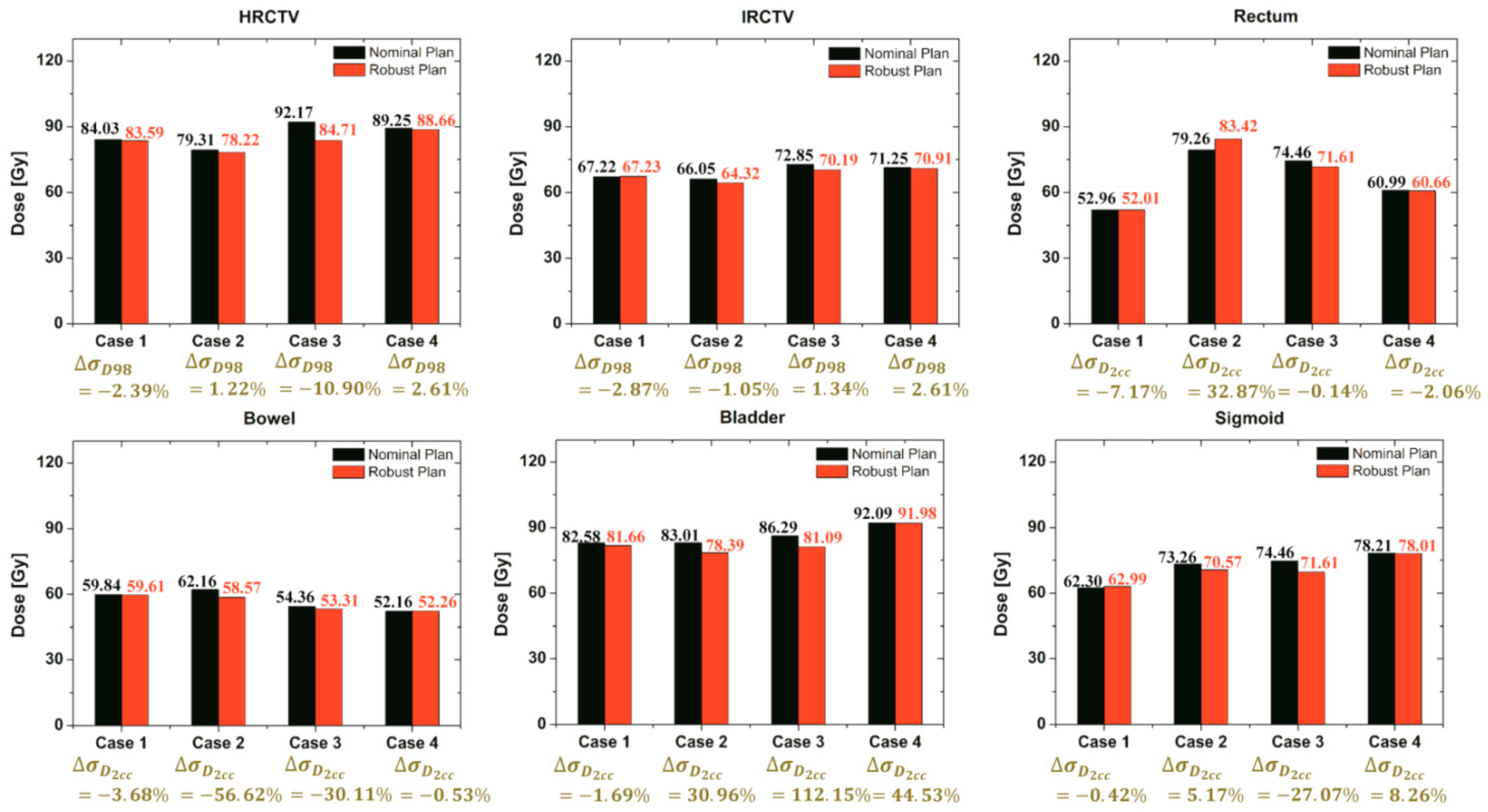
Further validation of our robust strategy compared plan robustness in the nominal and robust plans. Four case-sets of applicator displacements can be divided into two categories. Two cases were a challenge to fit with the EMBRACE protocol guidance, but the other two were easier because of the shape of targets. There was not an enormous difference in total time between the two nominal and robust plans, whereas the dose-delivered results of EQD2 of all cases were affected differently as shown in Figure 8.
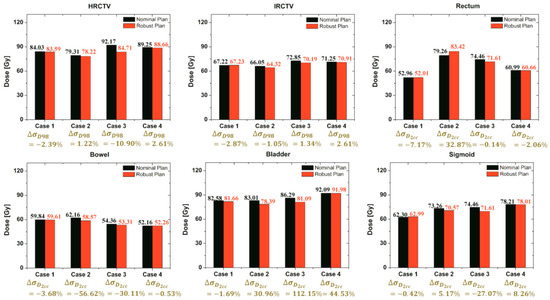
Figure 8.
Comparison of the total EQD2 (EBRT + BT) of each target and OAR for both the nominal and robust plan for all cases.
It shows the comparison of the EQD2 and the standard deviation values of each target and OAR using both strategies. Regarding quantitative results, this proposed robust optimization could reduce the standard deviation of the target and OAR compared to the nominal plan in most of the cases, whereas the standard deviation of a few organs was not promising. In addition, the proposed robust optimization also reduced EQD2 in most of the cases. Although the standard deviation in a few organs was not sufficiently minimized, the resulting robustness plans from our strategy might be worth considering according to EMBRACE protocol guidance.
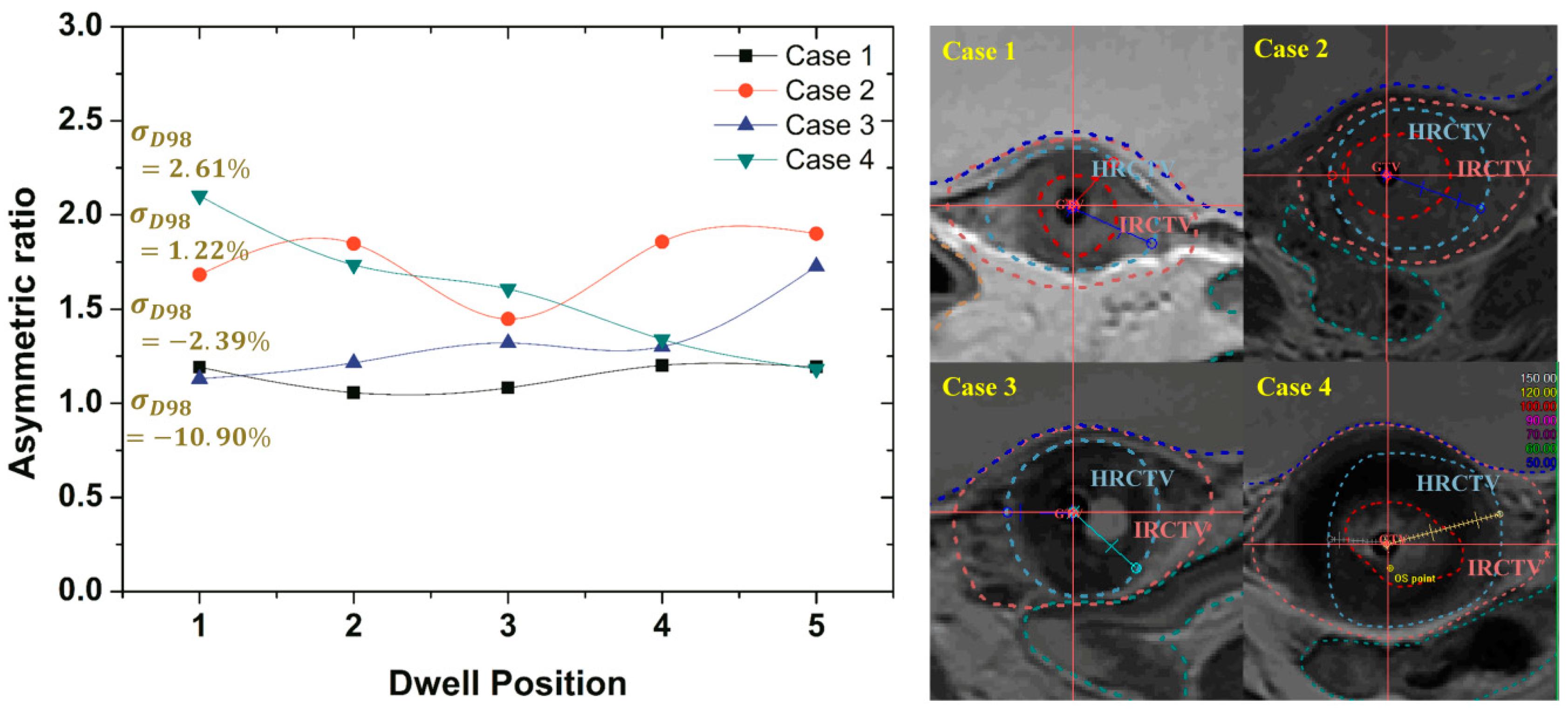
For the evaluation of the effects of irregularly shaped targets on robust optimization outcomes, the degree of asymmetry for HRCTV was measured. The measurements of asymmetric ratios were obtained from the distance difference between the maximum and minimum distances from the activated dwell positions inside the HRCTV to the surface of the HRCTV.
Figure 9 gives a comparison of the measured asymmetric ratios along the given dwell positions inside the HRCTV. When the asymmetric ratio is close to the value of 1, the target shape has symmetry. The results of cases that have lower asymmetric ratio variations along the given dwell positions had a greater standard deviation reduction than the results from the cases of the higher asymmetric ratio as compared with the optimized results as shown in Figure 8.
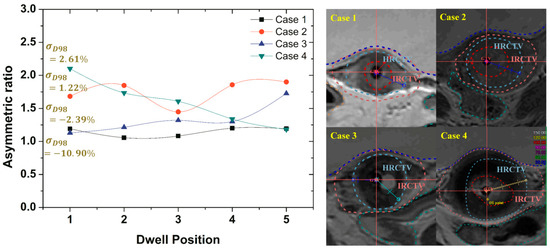
Figure 9.
The asymmetric ratio variations along the given dwell positions located inside the HRCTV in all cases.
4. Discussion
We would like to emphasize that the applicator displacements during brachytherapy treatment for cervical cancer led to radical changes in dose distribution [54]. With the advent of MRI-guided brachytherapy, the opportunity to improve outcomes by increasing the target dose while minimizing the dose to the OAR is essential for the management of locally advanced cervical cancer. Hence, the dose-delivery uncertainty by applicator displacements is of significant relevance within the dose distribution. Many studies have demonstrated that applicator displacement is a problem of critical importance.
Joshua et al. [22] quantified the dosimetric impact of applicator displacements and applicator reconstruction uncertainties through simulated planning studies of virtual applicator shifts. In addition, Tanderup et al. [21] reported 2 mm errors in applicator reconstruction. However, the reconstruction approach was rather limited due to the finite slice thickness, resulting in several sources of uncertainty. Junyi et al. [29] developed a real-time applicator position monitoring system with a 1 mm accuracy during long wait times between imaging and treatment. Nevertheless, the real-time monitoring system still depends on the accuracy of distance computability between the camera and marker. Furthermore, the applicator shift and intra- and inter-fractional organ movement are inevitable, and this leads to errors in fractional dose delivery. De Leeuw et al. [16] found geometrical shifts as large as 6 ± 7 mm in the posterior direction during PDR delivery. Several studies have previously reported such movements relative to target and organs [16,17,18,28]. While these efforts may reduce required dose-delivery errors, there will always remain residual uncertainties.
As mentioned in the above sections, the variations of the DVH parameter due to the applicator displacements could have occurred (Figure 3). The incorporation of applicator displacement worst-case scenarios into the robust optimization with the use of a MAD function may enable the robust optimization approach to minimize the dose delivery uncertainty in a single plan approach for HDR brachytherapy treatment planning. Meerschaert et al. [55] found the importance of adaptive planning method for cervical cancer HDR brachytherapy; however, a simulation device is not routinely available in some institution. Hence, a robust optimization method with a multi-objective genetic algorithm in a single plan approach for minimizing dose delivery uncertainty by potential applicator displacements was proposed. In our study, the robustness of the treatment plan was quantified using the MAD function and could have found the optimal solution of the area of DVH bands defined by worst-case scenarios. We demonstrated the feasibility of using our robust optimization strategy to minimize the dose-delivery uncertainty by potential applicator positional displacements. The dwell positions and times were determined in a way that optimally improved the plan quality according to GEC–ESTRO Gyn Working Group recommendations. Compared to manual plan approaches, our robust optimization method resulted in a reduced standard deviation on DVH parameters while maintaining the target-dose coverage and sparing the OAR, as well as reducing EQD2 in most of the cases. Similarly, the EQD2 and the standard deviation of DVH parameters of robust optimized plan in the Table 1 showed their values to be lower compared to those of the inverse plan without considering applicator displacement assumption. The resulting robustness plans from our strategy might be worth considering according to EMBRACE protocol guidance. Evaluation of the effects of irregularly shaped targets on robust optimization outcomes shows that it was difficult to find an optimized plan for the target with a higher asymmetric ratio because the dose distribution of brachytherapy sources was symmetrically isotropic. As a result of irregularly shaped targets, the OAR (bladder) received a slightly higher radiation dose in some robust optimization process. This difficulty is unavoidable: optimization may not improve on, or reduce, variation under specific circumstances. We should consider the asymmetric ratio of the target to deal with exceptional cases such as overshot and undershot of the target and OAR. Furthermore, applicator displacement relative to important anatomical structures can occur during treatment delivery due to organ mobility [56]. To minimize the intrafractional dose variations, EM tracking [57] and real-time applicator position monitoring system using an infrared camera and reflective markers may also be considered [29]. Lastly, the quality assurance (QA) could eventually decrease the source of uncertainties whether technical (source/equipment related) or clinical [58].
On another issue, optimized dwell times at certain dwell positions may have been very high compared to adjacent dwell positions, and there may have been almost no dwell times in adjacent source positions. This may carry a very high risk if a small volume of OARs is close to certain source positions. In the manual planning, the planner makes the safer plan by smoothing out dwell times manually over adjacent positions. To resolve this problem in the robust optimization, the algorithm can make the constraint of the difference between dwell times at adjacent source positions in the iteration step.
Nonetheless, one advantage of the proposed method is its applicability to an intensity-modulated brachytherapy (IMBT) system with modifications on the dose matrix and robust measure function. The IMBT, which is a promising method for cervix brachytherapy, is capable of increasing the dose to the target by 36.3% while decreasing the dose to the OAR by 4.7% to 22.4% compared with conventional HDR brachytherapy [59]. However, there are still a number of uncertainties related to the dose delivery in the IMBT system that include (a) uncertainty in the orientation of the shields, (b) uncertainty in source positioning and (c) uncertainty in patient/applicator relative movement [60]. These uncertainties occurring during treatment can decrease target coverage and increase the dose to organs at risk. There is a rising question about minimizing the dose-delivery uncertainty in the IMBT system. For that reason, the implementation of the proposed algorithm into the IMBT system will be investigated in a future study.
5. Conclusions
Here, our results with proposed algorithm show the feasibility of robust planning to reduce dose-delivery uncertainty by potential applicator displacements and EQD2 dose distribution compared to conventional brachytherapy plans. The fundamental assumption is that only random displacements of applicators were used for objective functions. Subsequently, a mathematical robust optimization algorithm was used to minimize the objective function value for meeting the best planning goals. Indeed, although this work concentrated only on a limited number of cases, the implication, at least from the point of view of dose-delivery uncertainty by applicator displacement, is that the robust optimization may be cautiously considered in a brachytherapy plan. Furthermore, our proposed algorithm in treatment planning systems could additionally be updated to incorporate both dwell position rotation and shield angle as degrees of freedom for minimizing the dose-delivery uncertainty in the promising IMBT system. Reducing the dose delivery uncertainty by applicator displacement should be confirmed by a more thorough investigation using different geometries and clinical cases. Additionally, the results mentioned earlier are only used for research reporting and not real treatment planning, including dose constraints.
Author Contributions
B.J. and H.K. conceived and coordinated the study, designed, performed, and analyzed the experiments, and wrote the manuscript. B.J., K.P., H.-J.K., D.S., Y.K.L., J.H.J., S.B.L. and H.K. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2017R1D1A1B03035002) and the Korean National Cancer Center Fund (1810272-3).
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Ni, L.Q.; Wang, S.S.; Lv, Q.L.; Chen, W.J.; Ying, S.P. Outcome and prognostic factors in cervical cancer patients treated with surgery and concurrent chemoradiotherapy: A retrospective study. World J. Surg. Oncol. 2018, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakagawa, K.; Tago, M.; Shiraishi, K.; Nakamura, N.; Ohtomo, K.; Taketani, Y. Comparison between conventional surgery and radiotherapy for FIGO stage I-II cervical carcinoma: A retrospective Japanese study. Gynecol. Oncol. 2005, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Kamrava, M. Brachytherapy in the treatment of cervical cancer: A review. Int. J. Women’s Health 2014, 6, 555–564. [Google Scholar]
- Al Feghali, K.A.; Elshaikh, M.A. Why brachytherapy boost is the treatment of choice for most women with locally advanced cervical carcinoma? Brachytherapy 2016, 15, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, O.; Kilic, S.; Khan, A.J.; Beriwal, S.; Small Jr, W. External beam techniques to boost cervical cancer when brachytherapy is not an option—theories and applications. Ann. Transl. Med. 2017, 5, 207. [Google Scholar] [CrossRef]
- Korenaga, T.R.K.; Pierson, W.; Swanson, M.; Chapman, J.S. Better late than never: Brachytherapy is more important than timeframe in cervical cancer outcomes [Abstracts]. Gynecol. Oncol. 2019, 154, 7. [Google Scholar] [CrossRef]
- Nikoofar, A.; Hoseinpour, Z.; Mahdavi, S.R.; Hasanzadeh, H.; Tavirani, M.R. High-Dose-Rate 192Ir Brachytherapy Dose Verification: A Phantom Study. Iran. J. Cancer Prev. 2015, 8, e2330. [Google Scholar] [CrossRef] [PubMed]
- Henschke, U.K. “Afterloading” applicator for radiation therapy of carcinoma of the uterus. Radiology 1960, 74, 834. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, B.; Ravikumar, M.; Palled, S.R.; Supe, S.S.; Sathiyan, S. Dosimetric comparison of high dose rate brachytherapy and intensity-modulated radiation therapy for cervical carcinoma. J. Med. Phys. 2011, 36, 111–116. [Google Scholar]
- DeWerd, L.A.; Ibbott, G.S.; Meigooni, A.S.; Mitch, M.G.; Rivard, M.J.; Stump, K.E.; Venselaar, J.L. A dosimetric uncertainty analysis for photon-emitting brachytherapy sources: Report of AAPM Task Group No. 138 and GEC-ESTRO. Med. Phys. 2011, 38, 782–801. [Google Scholar] [CrossRef]
- Berger, D.; Dimopoulos, J.; Georg, P.; Georg, D.; Pötter, R.; Kirisits, C. Uncertainties in assessment of the vaginal dose for intracavitary brachytherapy of cervical cancer using a tandem-ring applicator. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1451–1459. [Google Scholar] [CrossRef]
- Swamidas, J.; Mahantshetty, U.; Tanderup, K.; Malvankar, D.; Sharma, S.; Engineer, R.; Deshpande, D.D. Inter-application variation of dose and spatial location of D2cm3 volumes of OARs during MR image based cervix brachytherapy. Radiother. Oncol. 2013, 107, 58–62. [Google Scholar]
- Vargo, J.A.; Beriwal, S. Image-based brachytherapy for cervical cancer. World J. Clin. Oncol. 2014, 5, 921–930. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, A.A.; Moerland, M.A.; Nomden, C.; Tersteeg, R.H.; Roesink, J.M.; Jürgenliemk-Schulz, I.M. Applicator reconstruction and applicator shifts in 3D MR-based PDR brachytherapy of cervical cancer. Radiother. Oncol. 2009, 93, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T.P.; Tanderup, K.; Bergstrand, E.S.; Knutsen, B.H.; Røislien, J.; Olsen, D.R. Reconstruction of a ring applicator using CT imaging: Impact of the reconstruction method and applicator orientation. Phys. Med. Biol. 2007, 52, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T.P.; Kirisits, C.; Berger, D.; Perez-Caltayud, J.; De Brabandere, M.; De Leeuw, A.; Tanderup, K. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (III): Considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother. Oncol. 2010, 96, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.E.; Noe, K.Ø.; Sørensen, T.S.; Nielsen, S.K.; Fokdal, L.; Paludan, M.; Tanderup, K. Simple DVH parameter addition as compared to deformable registration for bladder dose accumulation in cervix cancer brachytherapy. Radiother. Oncol. 2013, 107, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Nesvacil, N.; Kirisits, C.; Georg, P.; Dimopoulos, J.C.; Fedrico, M.; Potter, R. Uncertainty analysis for 3D image-based cervix cancer brachytherapy by repetitive MR imaging: Assessment of DVH variations between two HDR fractions within one applicator insertion and their clinical relevance. Radiother. Oncol. 2013, 107, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Hellebust, T.P.; Lang, S.; Granfeldt, J.; Potter, R.; Lindegaard, J.C.; Kirisits, C. Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother. Oncol. 2008, 89, 156–163. [Google Scholar] [CrossRef]
- Schindel, J.; Zhang, W.; Bhatia, S.K.; Sun, W.; Kim, Y. Dosimetric impacts of applicator displacements and applicator reconstruction uncertainties on 3D image-guided brachytherapy for cervical cancer. J. Contemp. Brachyther. 2013, 5, 250–257. [Google Scholar] [CrossRef]
- Haie-Meder, C.; Potter, R.; Van Limbergen, E.; Briot, E.; De Brabandere, M.; Dimopoulos, J.; Wachter-Gerstner, N. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group* (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef]
- Lang, S.; Georg, P.; Kirists, C.; Dimopoulos, J.; Kuzucan, A.; Georg, D.; Potter, R. Uncertainty analysis for 3D image based cervix cancer brachytherapy by repeated MRI examinations: DVH-variations between two HDR fractions within one applicator insertion. Radiother. Oncol. 2006, 81 (Suppl. 1), S79. [Google Scholar]
- Kirisits, C.; Lang, S.; Dimopoulos, J.; Oechs, K.; Georg, D.; Potter, R. Uncertainties when using only one MRI-based treatment plan for subsequent high-dose-rate tandem and ring applications in brachytherapy of cervix cancer. Radiother. Oncol. 2006, 81, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.W.; Georgiou, A.; Jeffrey, F.; Ferez, C.A. Anatomic variation of gynecologic brachytherapy prescription points. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 725–729. [Google Scholar] [CrossRef]
- Hellebust, T.P.; Dale, E.; Skjonsberg, A.; Olsen, D.R. Inter fraction variations in rectum and bladder volumes and dose distributions during high dose rate brachytherapy treatment of the uterine cervix investigated by repetitive CT examinations. Radiother. Oncol. 2001, 60, 273–280. [Google Scholar] [CrossRef]
- Oku, Y.; Arimura, H.; Nguyen, T.T.T.; Hiraki, Y.; Toyota, M.; Saigo, Y.; Hirata, H. Investigation of whether in-room CT-based adaptive intracavitary brachytherapy for uterine cervical cancer is robust against interfractional location variations of organs and/or applicators. J. Radiat. Res. 2016, 57, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Waldron, T.; Kim, Y. A real-time applicator position monitoring system for gynecologic intracavitary brachytherapy. Med. Phys. 2014, 41, 011703. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.J.; Devic, S.; Vuong, T.; Han, D.Y.; Scanderbeg, D.; Choi, D.; Song, W.Y. HDR brachytherapy of rectal cancer using a novel grooved-shielding applicator design. Med. Phys. 2013, 40, 091704. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Bokrantz, R. A critical evaluation of worst case optimization methods for robust intensity-modulated proton therapy planning. Med. Phys. 2014, 41, 081701. [Google Scholar] [CrossRef]
- Fredriksson, A. A characterization of robust radiation therapy treatment planning methods—From expected value to worst case optimization. Med. Phys. 2012, 39, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Zinchenko, Y.; Henderson, S.G.; Sharpe, M.B. Robust optimization for intensity modulated radiation therapy treatment planning under uncertainty. Phys. Med. Biol. 2005, 50, 5463–5477. [Google Scholar] [CrossRef] [PubMed]
- Tilly, D.; Holm, A.; Grusell, E.; Ahnesjö, A. Probabilistic optimization of dose coverage in radiotherapy. Phys. Imag. Radiat. Oncol. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Chen, W.; Unkelbach, J.; Trofimov, A.; Madden, T.; Kooy, H.; Bortfeld, T.; Craft, D. Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Phys. Med. Biol. 2012, 57, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Rousseeuw, P.J.; Croux, C. Alternatives to the Median Absolute Deviation. J. Am. Stat. Assoc. 1993, 88, 1273–1283. [Google Scholar] [CrossRef]
- Hu, S.; Wu, X.; Liu, H.; Wang, Y.; Li, R.; Yin, M. Multi-Objective Neighborhood Search Algorithm Based on Decomposition for Multi-Objective Minimum Weighted Vertex Cover Problem. Sustainability 2019, 11, 3634. [Google Scholar] [CrossRef]
- Chiandussi, G.; Codegone, M.; Ferrero, S.; Varesio, F.E. Comparison of multi-objective optimization methodologies for engineering applications. Comput. Math. Appl. 2012, 63, 912–942. [Google Scholar] [CrossRef]
- Deist, T.M.; Gorissen, B.L. High-dose-rate prostate brachytherapy inverse planning on dose-volume criteria by simulated annealing. Phys. Med. Biol. 2016, 61, 1155–1170. [Google Scholar] [CrossRef][Green Version]
- Morén, B.; Larsson, T.; Tedgren, A.C. Mathematical optimization of high dose-rate brachytherapy derivation of a linear penalty model from a dose-volume model. Phys. Med. Biol. 2018, 63, 065011. [Google Scholar] [CrossRef]
- Ghaheri, A.; Shoar, S.; Naderan, M.; Hoseini, S.S. The Applications of Genetic Algorithms in Medicine. Oman Med. J. 2015, 30, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.U.; Bergen, S.W. A genetic algorithm approach to the inverse problem of treatment planning for intensity-modulated radiotherapy. Biomed. Signal Process. Control 2010, 5, 189–195. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; De Leeuw, A.; Krichheiner, K.; Nout, R.; Jurgenliemk-Schulz, I. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Lindegaard, J.C. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Nath, R.; Anderson, L.L.; Luxton, G.; Weaver, K.A.; Williamson, J.F.; Meigooni, A.S. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med. Phys. 1995, 22, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Saiful Huq, M.; Ibbott, G.S.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 2004, 31, 633–674. [Google Scholar] [CrossRef] [PubMed]
- Rivard, M.J.; Ballester, F.; Butler, W.M.; DeWerd, L.A.; Ibbott, G.S.; Meigooni, A.S.; Williamson, J.F. Supplement to the 2004 update of the AAPM Task Group No. 43 Report. Med. Phys. 2017, 34, 2187–2205. [Google Scholar] [CrossRef]
- Kirisits, C.; Rivard, M.J.; Baltas, D.; Ballester, F.; De Brabandere, M.; Van der Laarse, R.; Siebert, F.A. Review of clinical brachytherapy uncertainties: Analysis guidelines of GEC-ESTRO and the AAPM. Radiother. Oncol. 2014, 110, 199–212. [Google Scholar] [CrossRef]
- Park, P.C.; Cheung, J.P.; Zhu, X.R.; Lee, A.K.; Sahoo, N.; Tucker, S.L.; Dong, L. Statistical Assessment of Proton Treatment Plans Under Setup and Range Uncertainties. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.; Unkelbach, J.; DeLaney, T.F.; Bortfeld, T. Visualization of a variety of possible dosimetric outcomes in radiation therapy using dose volume histogram bands. Pract. Radiat. Oncol. 2012, 2, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Deb, K.; Pratap, A.; Agarwal, S.; Mayarivan, T.A.M.T. A Fast and Elitist Multiobjective Genetic Algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Konaka, A.; Coit, D.W.; Smith, A.E. Multi-objective optimization using genetic algorithms: A tutorial. Reliab. Eng. Syst. Saf. 2006, 91, 992–1007. [Google Scholar] [CrossRef]
- Hajela, P.; Lin, C.Y. Genetic search strategies in multicriterion optimal design. Struct. Optim. 1992, 4, 99–107. [Google Scholar] [CrossRef]
- Tanderup, K.; Eifel, P.J.; Yashar, C.M.; Pötter, R.; Grigsby, P.W. Curative radiation therapy for locally advanced cervical cancer: Brachytherapy is NOT optional. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Meerschaert, R.; Nalichowski, A.; Burmeister, J.; Paul, A.; Miller, S.; Hu, Z.; Zhuang, L. A comprehensive evaluation of adaptive daily planning for cervical cancer HDR brachytherapy. J. Appl. Clin. Med. Phys. 2016, 17, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Nesvail, N.; Tanderup, K.; Hellebust, T.P.; De Leeuw, A.; Lang, S.; Mohamed, S.; Kirisits, C. A multicenter comparison of the dosimetric impack of inter- and intra-fractional anatomical variations in fractionated cervix cancer brachytherapy. Radiother. Oncol. 2013, 107, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.L.; Biswanathan, A.N.; Don, S.M.; Hansen, J.L.; Cormack, R.A. A system to use electromagnetic tracking for the quality assureance of brachytherapy catheter digitization. Med. Phys. 2014, 41, 101702. [Google Scholar] [CrossRef]
- Soror, T.; Siebert, F.A.; Lanellotta, V.; Placidi, E.; Fionda, B.; Taliafei, L.; Kovacs, G. Quality Assurance in Modern Gynecological HDR-Brachytherapy (Interventional Radiotherapy): Clinical Considerations and Comments. Cancers 2021, 13, 912. [Google Scholar] [CrossRef]
- Callaghan, C.M.; Adams, Q.; Flynn, R.T.; Wu, X.; Xu, W.; Kim, Y. Systematic review of intensity-modulated brachytherapy (IMBT): Static and dynamic techniques. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 206–221. [Google Scholar] [CrossRef]
- Famulari, G.; Duclos, M.; Enger, S.A. A novel 169Yb-based dynamic-shield intensity modulated brachytherapy delivery system for prostate cancer. Med. Phys. 2020, 47, 859–868. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).