The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Topography and Nano-Indentation Analysis
2.3. Cell Culture and MTT Assay
2.4. Cell Live/Dead Assay
2.5. Contact Angle Analysis
2.6. Hemocompatibility Assay
2.7. Radiograph Evaluations In Vivo
2.8. Statistical Analysis
3. Results
3.1. Comparisons of Surface Characteristic and Young’s Modulus
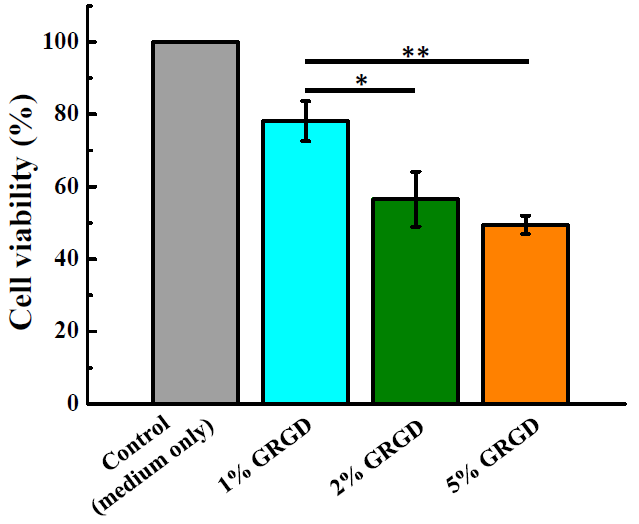
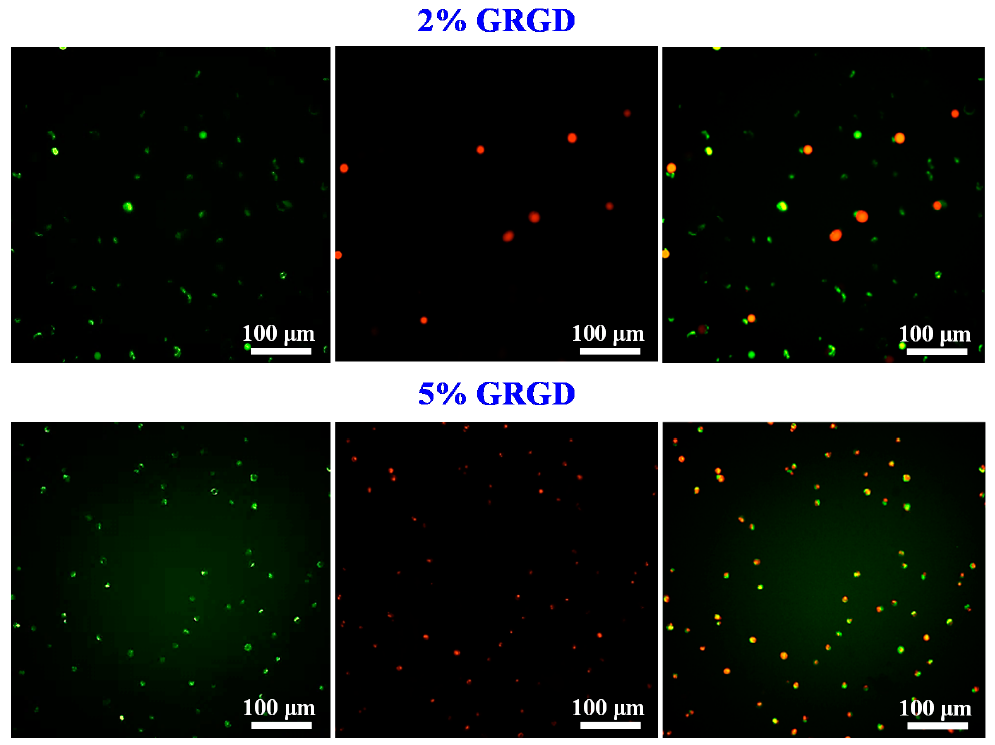
3.2. Biocompatibility in Different Concentrations of the GRGD Peptide
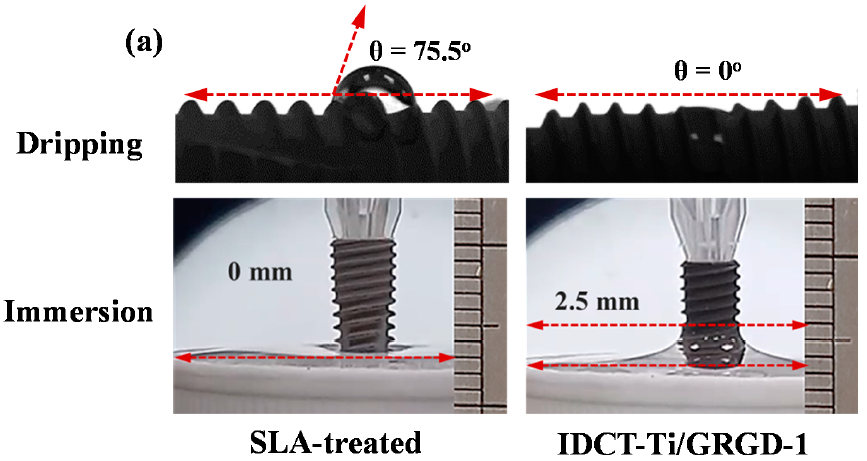
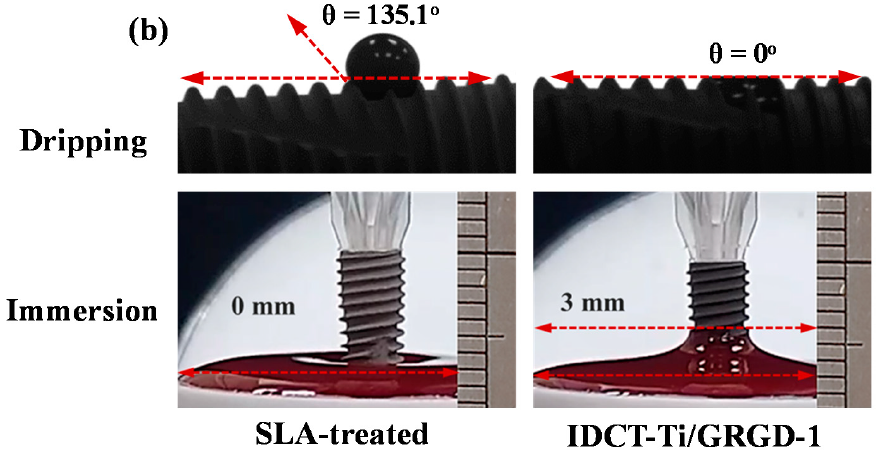
3.3. Wettability of the Investigated Implants
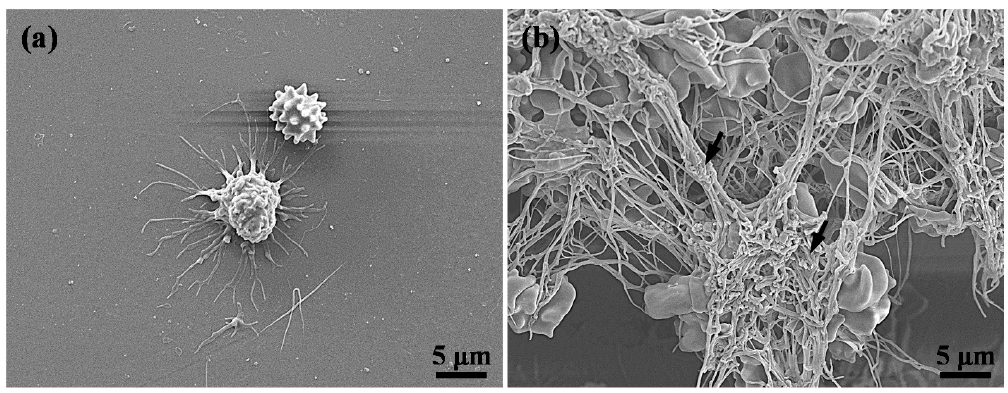
3.4. Hemocompatibility of the Investigated Implants
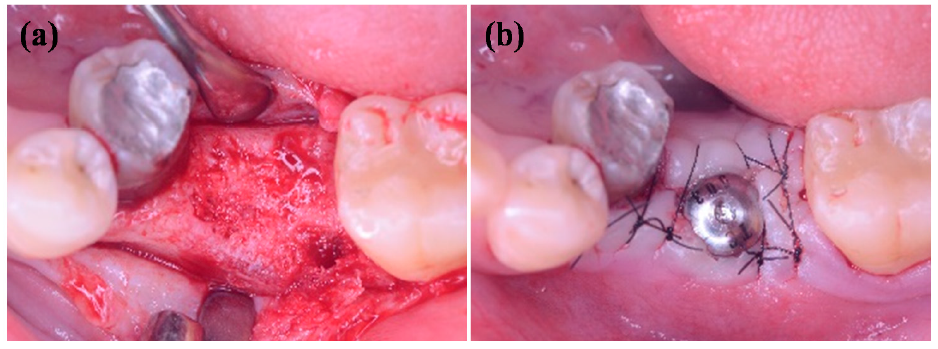
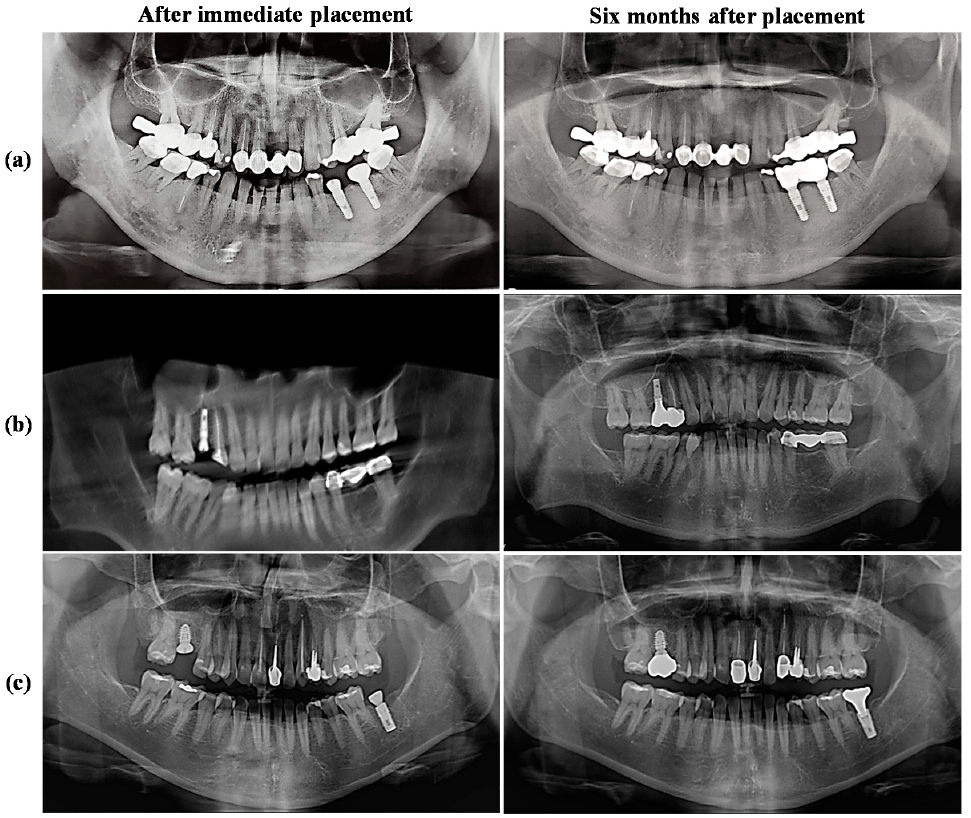
3.5. Radiograph Evaluations of the IDCT-Ti/GRGD-1 Implant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; You, Y.; Li, B.; Song, Y.; Ma, A.; Chen, B.; Han, W.; Li, C. Improved cell adhesion and osseointegration on anodic oxidation modified titanium implant surface. J. Hard Tissue Biol. 2019, 28, 8. [Google Scholar] [CrossRef]
- Albertini, M.; Fernandez-Yague, M.; Lazaro, P.; Herrero-Climent, M.; Rios-Santos, J.V.; Bullon, P.; Gil, F.J. Advances in surfaces and osseointegration in implantology. Biomimetic surfaces. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e316–e325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, S.F.; Wassall, T. In vitro evaluation of osteoblastic cell adhesion on machined osseointegrated implants. Braz. Oral Res. 2009, 23, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Park, K.; Choi, K.H.; Kim, S.H.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Cell adhesion and in vivo osseointegration of sandblasted/acid etched/anodized dental implants. Int. J. Mol. Sci. 2015, 16, 10324–10336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, N.; Gonzalez, A.; Monopoli, D.; Mentado, B.; Becerra, J.; Santos-Ruiz, L.; Vida, Y.; Perez-Inestrosa, E. Dendritic scaffold onto titanium implants. A versatile strategy increasing biocompatibility. Polymers 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Kim, H.-S.; Kim, J.-H.; Shim, J.-H.; Yun, M.-J.; Jeon, Y.-C.; Huh, J.-B.; Jeong, C.-M. Effects of anodized titanium implant coated with RGD peptides via chemical fixation on osseointegration and bone regeneration. Tissue Eng. Regen. Med. 2012, 9, 194–202. [Google Scholar] [CrossRef]
- Feller, L.; Chandran, R.; Khammissa, R.A.; Meyerov, R.; Jadwat, Y.; Bouckaert, M.; Schechter, I.; Lemmer, J. Osseointegration: Biological events in relation to characteristics of the implant surface. SADJ 2014, 69, 6. [Google Scholar]
- Likibi, F.; Jiang, B.; Li, B. Biomimetic nanocoating promotes osteoblast cell adhesion on biomedical implants. J. Mater. Res. 2011, 23, 3222–3228. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, X.C.; Liu, S.; Wu, R.F.; Aparicio, C.; Wu, J.Y. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Sameron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 16. [Google Scholar] [CrossRef]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.J.; Hsu, H.J.; Peng, P.W.; Wu, C.Z.; Ou, K.L.; Cheng, H.Y.; Walinski, C.J.; Sugiatno, E. Early bone response to machined, sandblasting acid etching (SLA) and novel surface-functionalization (SLAffinity) titanium implants: Characterization, biomechanical analysis and histological evaluation in pigs. J. Biomed. Mater. Res. A 2016, 104, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.-J.; Syam, S.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Chan, K.-C.; Tsai, C.-H.; Saito, T.; Liu, C.-M.; Chou, H.-H.; et al. Development of a surface-functionalized titanium implant for promoting osseointegration: Surface characteristics, hemocompatibility, and in vivo evaluation. Appl. Sci. 2020, 10, 8582. [Google Scholar] [CrossRef]
- Zanetti, E.M.; Pascoletti, G.; Cali, M.; Bignardi, C.; Franceschini, G. Clinical assessment of dental implant stability during follow-up: What is actually measured, and perspectives. Biosensors 2018, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic surfaces coated with covalently immobilized collagen type I: An X-ray photoelectron spectroscopy, atomic force microscopy, micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [Green Version]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Meda, L.; Fini, M.; Giavaresi, G.; Giardino, R. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: A rabbit model. Int. J. Oral Maxillofac. Implant. 2005, 20, 8. [Google Scholar]
- Dogan, A.; Yalvac, M.E.; Sahin, F.; Kabanov, A.V.; Palotas, A.; Rizvanov, A.A. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int. J. Nanomed. 2012, 7, 4849–4860. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-C.; Huang, H.-T.; Lan, W.-C.; Huang, M.-S.; Saito, T.; Huang, B.-H.; Tsai, C.-H.; Fan, F.-Y.; Ou, K.-L. The potential of a tailored biomimetic hydrogel for in vitro cell culture applications: Characterization and biocompatibility. Appl. Sci. 2020, 10, 9035. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Li, X.; Zhang, L. Covalent attachment of cell-adhesive peptide Gly-Arg-Gly-Asp (GRGD) to poly(etheretherketone) surface by tailored silanization layers technique. Appl. Surf. Sci. 2014, 320, 93–101. [Google Scholar] [CrossRef]
- Abiko, Y.; Pugdee, K.; Hirai, T.; Chu, C.-H.; Ando, T.; Shibata, Y. Attachment activity of RGD-motif peptides to osteoblast-like cell line MC3T3-E1. Int. J. Oral Med. Sci. 2007, 6, 3. [Google Scholar] [CrossRef]
- Piattelli, A.; Scarano, A.; Corigliano, M.; Piattelli, M. Effects of alkaline phosphatase on bone healing around plasma-sprayed titanium implants: A pilot study in rabbits. Biomaterials 1996, 17, 7. [Google Scholar] [CrossRef]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. Biomed. Res. Int. 2015, 2015, 171945. [Google Scholar] [CrossRef] [Green Version]
- Lebaron, R.G.; Athanasiou, K.A. Extracellular matrix cell adhesion peptides: Functional applications in orthopedic materials. Tissue Eng. 2000, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, K.M.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Shaikh, F.M.; Bellis, S.L. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials 2008, 29, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabbir, S.H.; Eisenberg, J.L.; Mrksich, M. An inhibitor of a cell adhesion receptor stimulates cell migration. Angew. Chem. Int. Ed. Engl. 2010, 49, 7706–7709. [Google Scholar] [CrossRef] [PubMed]
- Perlot, R.L., Jr.; Shapiro, I.M.; Mansfield, K.; Adams, C.S. Matrix regulation of skeletal cell apoptosis II: Role of Arg-Gly-Asp-containing peptides. J. Bone Miner. Res. 2002, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, P.; Bathala, L.R.; Sangur, R. Techniques for dental implant nanosurface modifications. J. Adv. Prosthodont. 2014, 6, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakral, G.; Thakral, R.; Sharma, N.; Seth, J.; Vashisht, P. Nanosurface—The future of implants. J. Clin. Diagn. Res. 2014, 8, ZE07–ZE10. [Google Scholar] [CrossRef] [PubMed]
- Ogle, O.E. Implant surface material, design, and osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Chu, K.T.; Shen, F.C.; Pan, Y.N.; Chou, H.H.; Ou, K.L. Stress effect on bone remodeling and osseointegration on dental implant with novel nano/microporous surface functionalization. J. Biomed. Mater. Res. A 2013, 101, 1158–1164. [Google Scholar] [CrossRef]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Perez, R.A.; Manero, J.M.; Gil Mur, J. Influence of the elastic modulus on the osseointegration of dental implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, P.J.; Ou, K.L.; Wang, C.C.; Huang, C.F.; Ruslin, M.; Sugiatno, E.; Yang, T.S.; Chou, H.H. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. J. Mech. Behav. Biomed. Mater. 2018, 79, 173–180. [Google Scholar] [CrossRef]
- Wennerberg, A.; Jimbo, R.; Stubinger, S.; Obrecht, M.; Dard, M.; Berner, S. Nanostructures and hydrophilicity influence osseointegration: A biomechanical study in the rabbit tibia. Clin. Oral Implant. Res. 2014, 25, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, I.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Shibli, J.A.; Ehrenfest, D.D. In dental implant surface, NanoWar has begun, but NanoQuest is still at stake. POSEIDO J. 2013, 1, 10. [Google Scholar]
- Sargeant, T.D.; Guler, M.O.; Oppenheimer, S.M.; Mata, A.; Satcher, R.L.; Dunand, D.C.; Stupp, S.I. Hybrid bone implants: Self-assembly of peptide amphiphile nanofibers within porous titanium. Biomaterials 2008, 29, 161–171. [Google Scholar] [CrossRef]
- Pereira, M.; Rybarczyk, B.J.; Odrljin, T.M.; Hocking, D.C.; Sottile, J.; Simpson-Haidaris, P.J. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J. Cell Sci. 2002, 115, 9. [Google Scholar]
- Villar, C.C.; Huynh-Ba, G.; Millis, M.P.; Cochran, D.L. Wound healing around dental implants. Endod. Top. 2012, 25, 19. [Google Scholar]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 19. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, L.; Scolaro, C. Blood wettability of haemocompatible carbon-based materials. J. Adv. Chem. Eng. 2017, 7, 1000179. [Google Scholar] [CrossRef]
- Koca, R.B.; Guven, O.; Celik, M.S.; Firatli, E. Wetting properties of blood lipid fractions on different titanium surfaces. Int. J. Implant Dent. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The surface anodization of titanium dental implants improves blood clot formation followed by osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, T.P.; Naves, M.M.; Menezes, H.H.M.; Pinto, P.H.C.; de Mello, J.D.B.; Costa, H.L. Topography and surface energy of dental implants: A methodological approach. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 1895–1907. [Google Scholar] [CrossRef]
- Nascimento, R.M.D.; Ramos, S.M.M.; Bechtold, I.H.; Hernandes, A.C. Wettability study on natural rubber surfaces for applications as biomembranes. ACS Biomater. Sci. Eng. 2018, 4, 2784–2793. [Google Scholar] [CrossRef]
- Homma, S.; Makabe, Y.; Sakai, T.; Morinaga, K.; Yokoue, S.; Kido, H.; Yajima, Y. Prospective multicenter non-randomized controlled study on intraosseous stability and healing period for dental implants in the posterior region. Int. J. Implant Dent. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Levin, L. Dealing with implant failures. J. Appl. Oral Sci. 2008, 16, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, R.Z.; de Vasconcelos, M.R.; Lopes-Guerra, I.M.; de Almeida, R.A.B.; de Campos-Felino, A.C. Implant stability in the posterior maxilla: A controlled clinical trial. Biomed. Res. Int. 2017, 2017, 6825213. [Google Scholar] [CrossRef]
- Tolstunov, L. Implant zones of the jaws: Implant location and related success rate. J. Implantol. 2007, 33, 10. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syam, S.; Wu, C.-J.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Lin, Y.-Y.; Saito, T.; Tsai, H.-Y.; Chuo, Y.-C.; Yen, M.-L.; et al. The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement. Appl. Sci. 2021, 11, 2616. https://doi.org/10.3390/app11062616
Syam S, Wu C-J, Lan W-C, Ou K-L, Huang B-H, Lin Y-Y, Saito T, Tsai H-Y, Chuo Y-C, Yen M-L, et al. The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement. Applied Sciences. 2021; 11(6):2616. https://doi.org/10.3390/app11062616
Chicago/Turabian StyleSyam, Syamsiah, Chia-Jen Wu, Wen-Chien Lan, Keng-Liang Ou, Bai-Hung Huang, Yu-Yeong Lin, Takashi Saito, Hsin-Yu Tsai, Yen-Chun Chuo, Ming-Liang Yen, and et al. 2021. "The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement" Applied Sciences 11, no. 6: 2616. https://doi.org/10.3390/app11062616





