Clove Oil Protects ?-Carotene in Oil-in-Water Emulsion against Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion
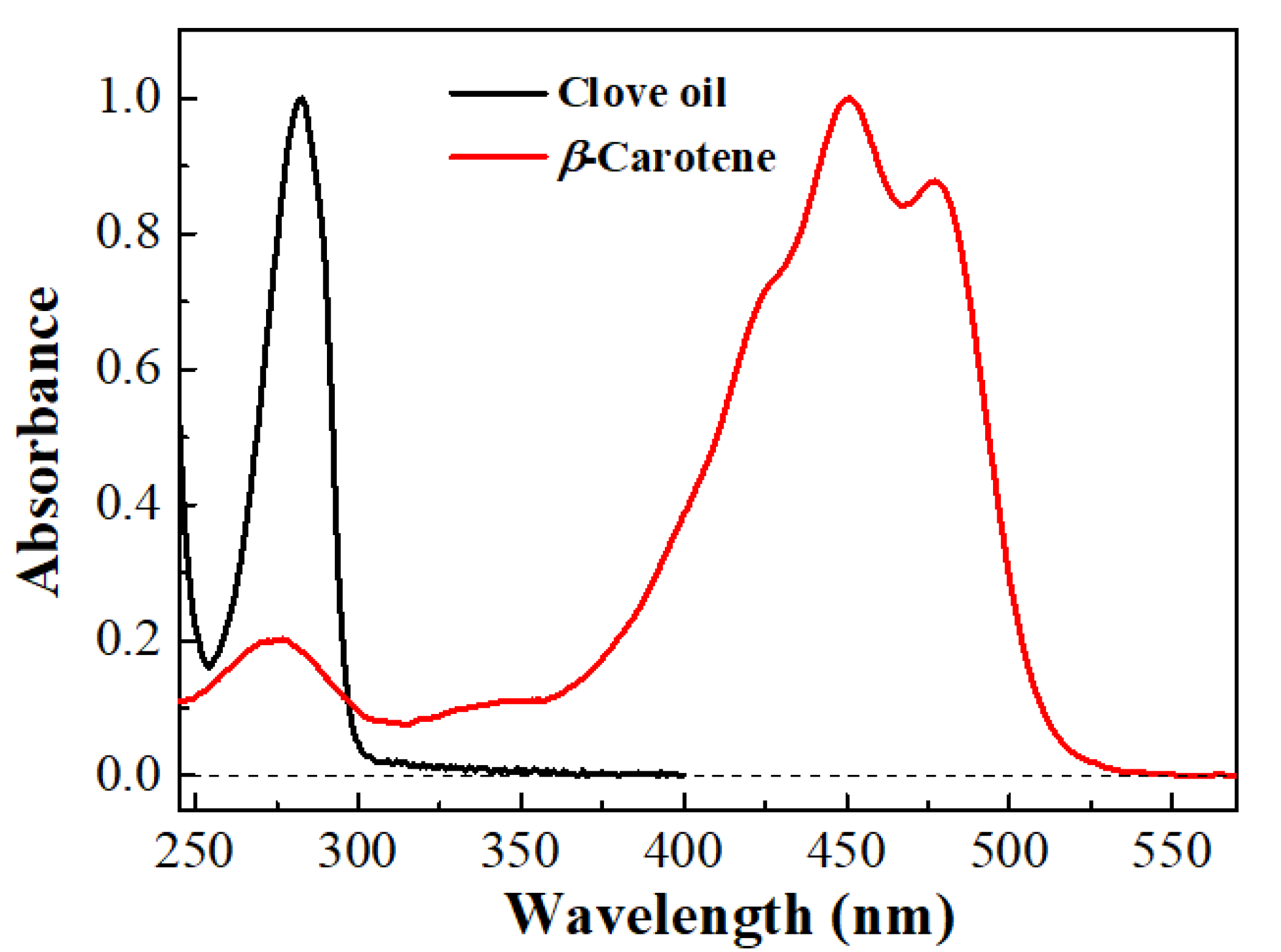
2.3. UV-Visible Absorption Spectroscopy
2.4. Color Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Vargas, R.; Galano, A. What is important to prevent oxidative stress? A theoretical study on electron-transfer reactions between carotenoids and free radicals. J. Phys. Chem. B 2009, 113, 12113–12120. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.T.; Becher, E.M.; Skibsted, L.H. Molecular mechanism of antioxidant synergism of tocotrienols and carotenoids in palm oil. J. Agric. Food Chem. 2006, 54, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, D.; Kim, M.; Gu, M.Y.; Kim, S.M.; Pan, C.H.; Kim, G.H.; Chung, D.J.F.C. Effects of temperature, light, and pH on the stability of fucoxanthin in an oil-in-water emulsion. Food Chem. 2019, 291, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ahmed, A.; Randhawa, M.A.; Atukorala, S.; Arlappa, N.; Ismail, T.; Ali, Z. Prevalence of vitamin a deficiency in south asia: Causes, outcomes, and possible remedies. J. Health Popul. Nutr. 2013, 4, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Amaya, D.B. Status of carotenoid analytical methods and in vitro assays for the assessment of food quality and health effects. Curr. Opin. Food Sci. 2015, 1, 56–63. [Google Scholar] [CrossRef]
- Cheng, H.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Electron transfer from plant phenolates to carotenoid radical cations. Antioxidant interaction entering the Marcus theory inverted region. J. Agric. Food Chem. 2014, 62, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Liang, R.; Li, D.D.; Xing, Y.D.; Han, R.M.; Zhang, J.P.; Skibsted, L.H. Beta-carotene radical cation addition to green tea polyphenols. Mechanism of antioxidant antagonism in peroxidizing liposomes. J. Agric. Food Chem. 2011, 59, 12643–12651. [Google Scholar] [CrossRef] [PubMed]
- Huvaere, K.; Skibsted, L.H. Flavonoids protecting food and beverages against light. J. Sci. Food Agric. 2015, 95, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Mihara, S.; Shibamoto, T. Photochemical reactions of eugenol and related compounds: Synthesis of new flavor chemicals. J. Agric. Food Chem. 1982, 30, 1982–1985. [Google Scholar] [CrossRef]
- Chang, H.T.; Cheng, H.; Han, R.M.; Wang, P.; Zhang, J.P.; Skibsted, L.H. Regeneration of beta-carotene from radical cation by eugenol, isoeugenol, and clove oil in the marcus theory inverted region for electron transfer. J. Agric. Food Chem. 2017, 65, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. Electron transfer reactions in chemistry. theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, 55–61. [Google Scholar] [CrossRef] [PubMed]
- De Cassia, S.S.R.; Pereira, M.M.; Freire, M.G.; Coutinho, J.A.P. Evaluation of the effect of ionic liquids as adjuvants in polymer-based aqueous biphasic systems using biomolecules as molecular probes. Sep. Purif. Technol. 2018, 196, 244–253. [Google Scholar]
- Taherpour, A.A.; Taherpour, A.; Taherpour, Z.; Taherpour, O. Relationship study of octanol–water partitioning coefficients and total biodegradation of linear simple conjugated polyene and carotene compounds by use of the Randićindex and maximum UV wavelength. Phys. Chem. Liq. 2009, 47, 349–359. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H. Kinetics and Mechanism of the Primary Steps of Degradation of Carotenoids by Acid in Homogeneous Solution. J. Agric. Food Chem. 2000, 48, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Boehm, F.; Edge, R.; Truscott, T.G.; Witt, C. A dramatic effect of oxygen on protection of human cells against γ-radiation by lycopene. FEBS Lett. 2016, 590, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skibsted, L.H. Anthocyanidins regenerating xanthophylls: A quantum mechanical approach to eye health. Curr. Opin. Food Sci. 2018, 20, 24–29. [Google Scholar] [CrossRef]
- Skibsted, L.H. Vitamin and non-vitamin antioxidants and their interaction in food. J. Food Drug. Anal. 2012, 20, 355–358. [Google Scholar] [CrossRef]

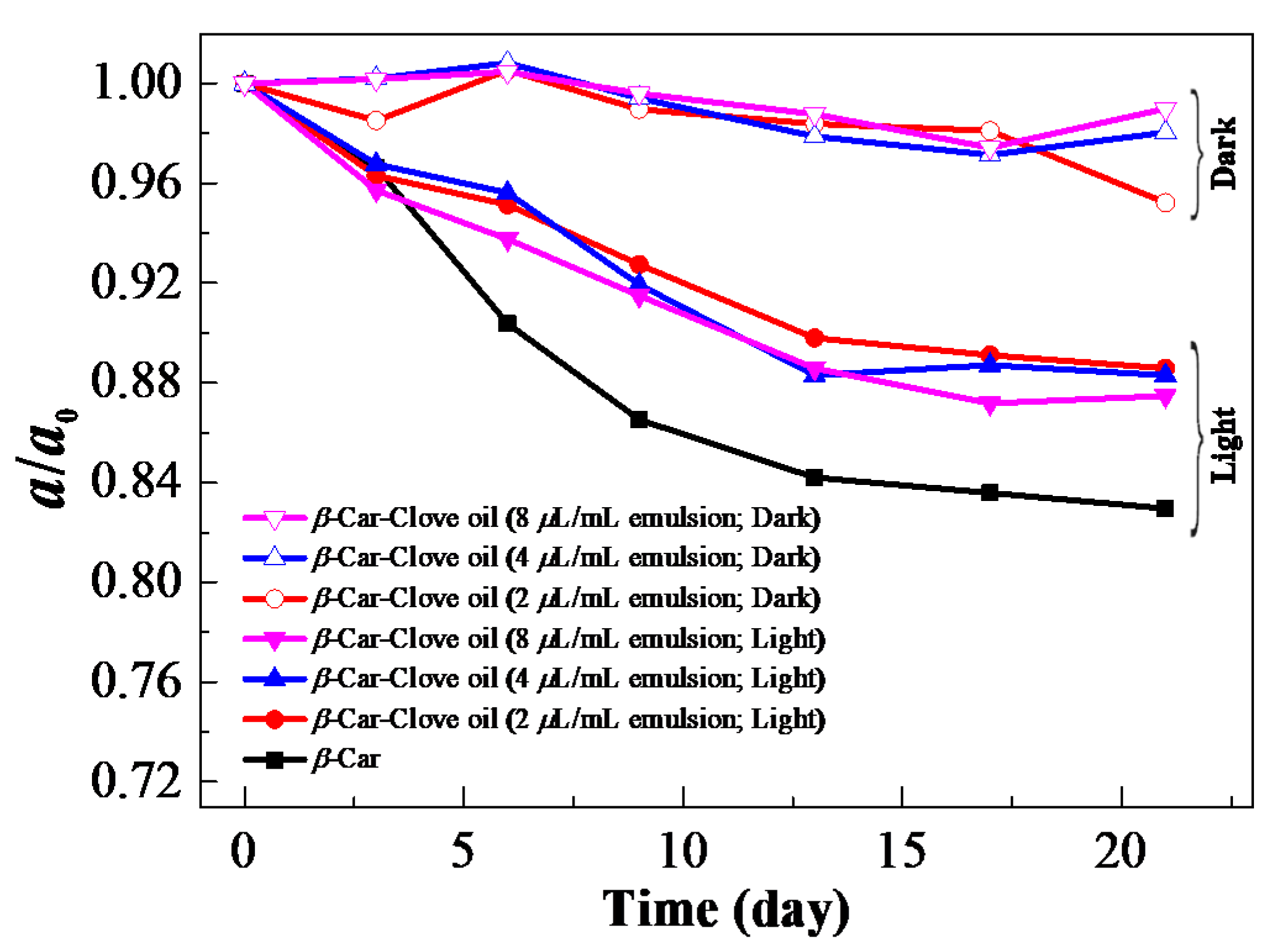
| Day | 0 | 3 | 6 | 9 | 13 | 17 | 21 | |
|---|---|---|---|---|---|---|---|---|
| Sample | ||||||||
| β-Car | 24.12 | 23.31 | 21.80 | 20.87 | 20.31 | 20.16 | 20.01 | |
| β-Car-clove oil (2 µL/mL emulsion; Light) | 24.69 | 23.78 | 23.49 | 22.90 | 22.17 | 22.00 | 21.87 | |
| β-Car-clove oil (4 µL/mL emulsion; Light) | 24.45 | 23.66 | 23.38 | 22.48 | 21.59 | 21.69 | 21.59 | |
| β-Car-clove oil (8 µL/mL emulsion; Light) | 23.39 | 22.39 | 21.93 | 22.48 | 20.72 | 20.39 | 20.46 | |
| β-Car-clove oil (2 µL/mL emulsion; Dark) | 24.27 | 23.91 | 24.40 | 24.02 | 23.88 | 23.81 | 23.11 | |
| β-Car-clove oil (4 µL/mL emulsion; Dark) | 24.07 | 24.12 | 24.27 | 23.93 | 23.56 | 23.38 | 23.60 | |
| β-Car-clove oil (8 µL/mL emulsion; Dark) | 22.93 | 22.97 | 23.04 | 22.84 | 22.65 | 22.34 | 22.70 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-M.; Chang, H.-T.; Zhang, J.-P.; Skibsted, L.H. Clove Oil Protects ?-Carotene in Oil-in-Water Emulsion against Photodegradation. Appl. Sci. 2021, 11, 2667. https://doi.org/10.3390/app11062667
Zhou Y-M, Chang H-T, Zhang J-P, Skibsted LH. Clove Oil Protects ?-Carotene in Oil-in-Water Emulsion against Photodegradation. Applied Sciences. 2021; 11(6):2667. https://doi.org/10.3390/app11062667
Chicago/Turabian StyleZhou, Yi-Ming, Hui-Ting Chang, Jian-Ping Zhang, and Leif H. Skibsted. 2021. "Clove Oil Protects ?-Carotene in Oil-in-Water Emulsion against Photodegradation" Applied Sciences 11, no. 6: 2667. https://doi.org/10.3390/app11062667
APA StyleZhou, Y.-M., Chang, H.-T., Zhang, J.-P., & Skibsted, L. H. (2021). Clove Oil Protects ?-Carotene in Oil-in-Water Emulsion against Photodegradation. Applied Sciences, 11(6), 2667. https://doi.org/10.3390/app11062667







