Gold-Modified Micellar Composites as Colorimetric Probes for the Determination of Low Molecular Weight Thiols in Biological Fluids Using Consumer Electronic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Equipment and Instrumentation
2.3. Experimental Procedure
2.4. Samples
3. Results and Discussion
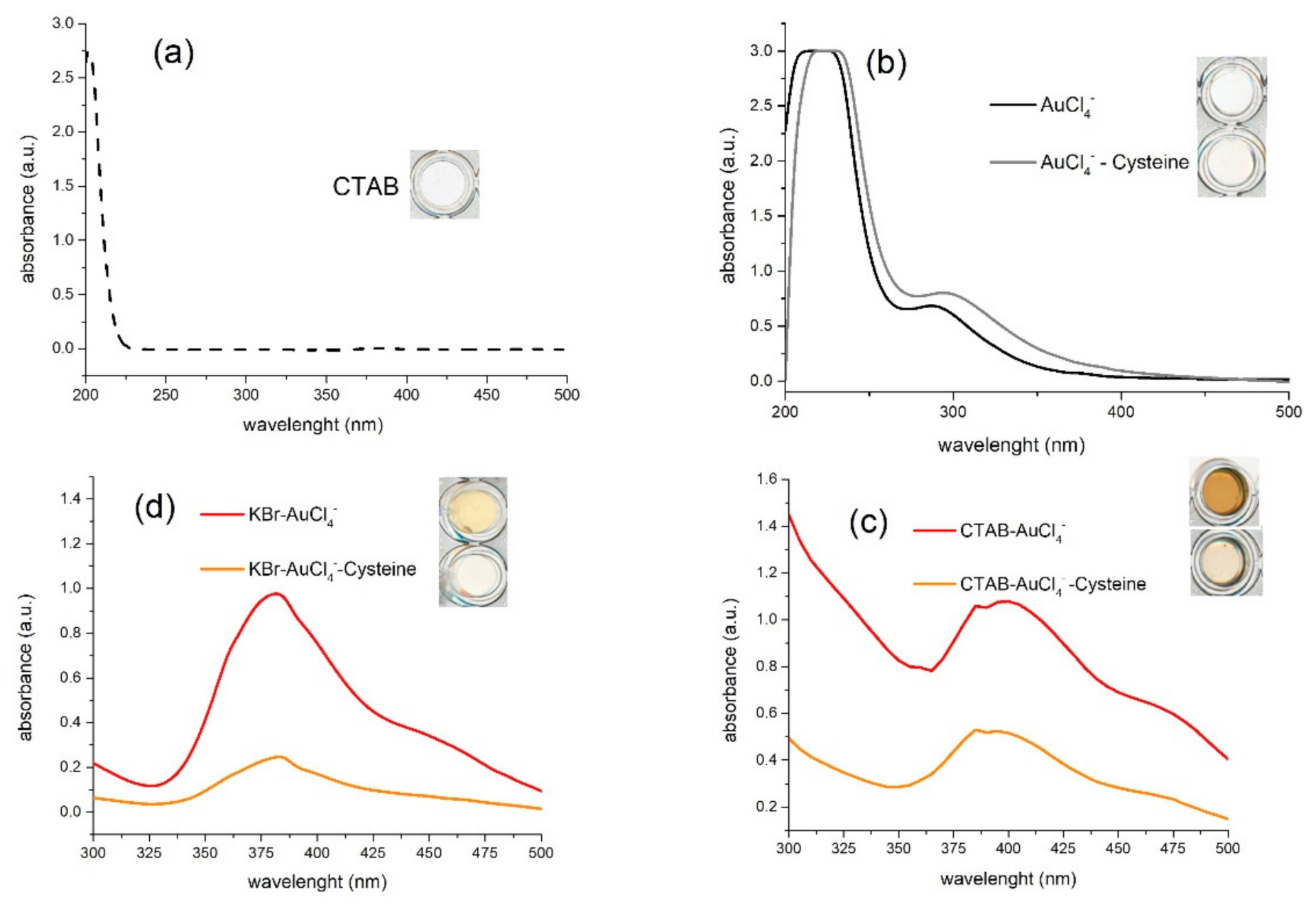
3.1. Mechanism of Biothiol Detection
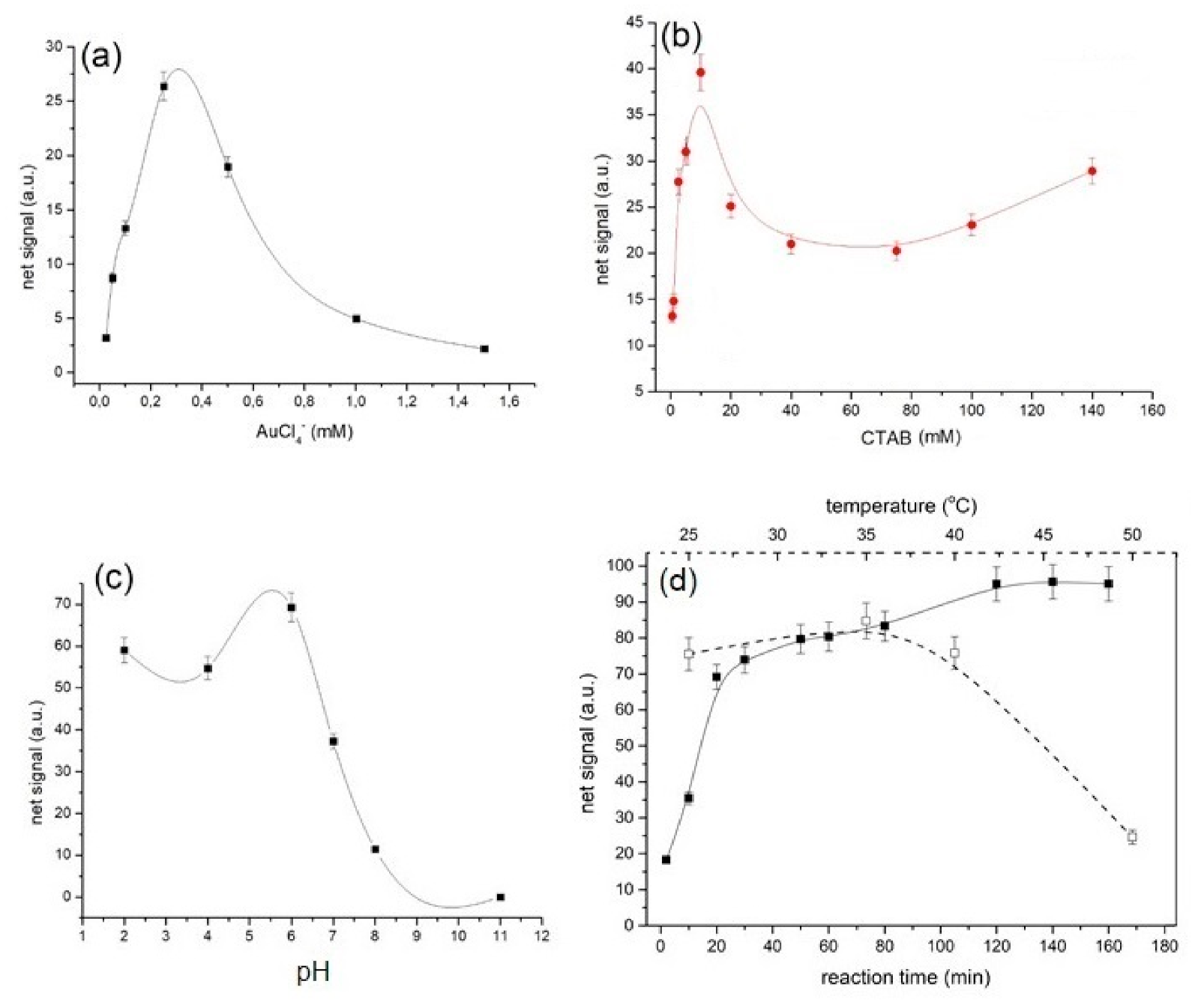
3.2. Effect of Gold Ion Concentration
3.3. Selection of Cationic Surfactant Concentration
3.4. Optimization of the Working pH
3.5. Optimization of the Reaction Kinetics
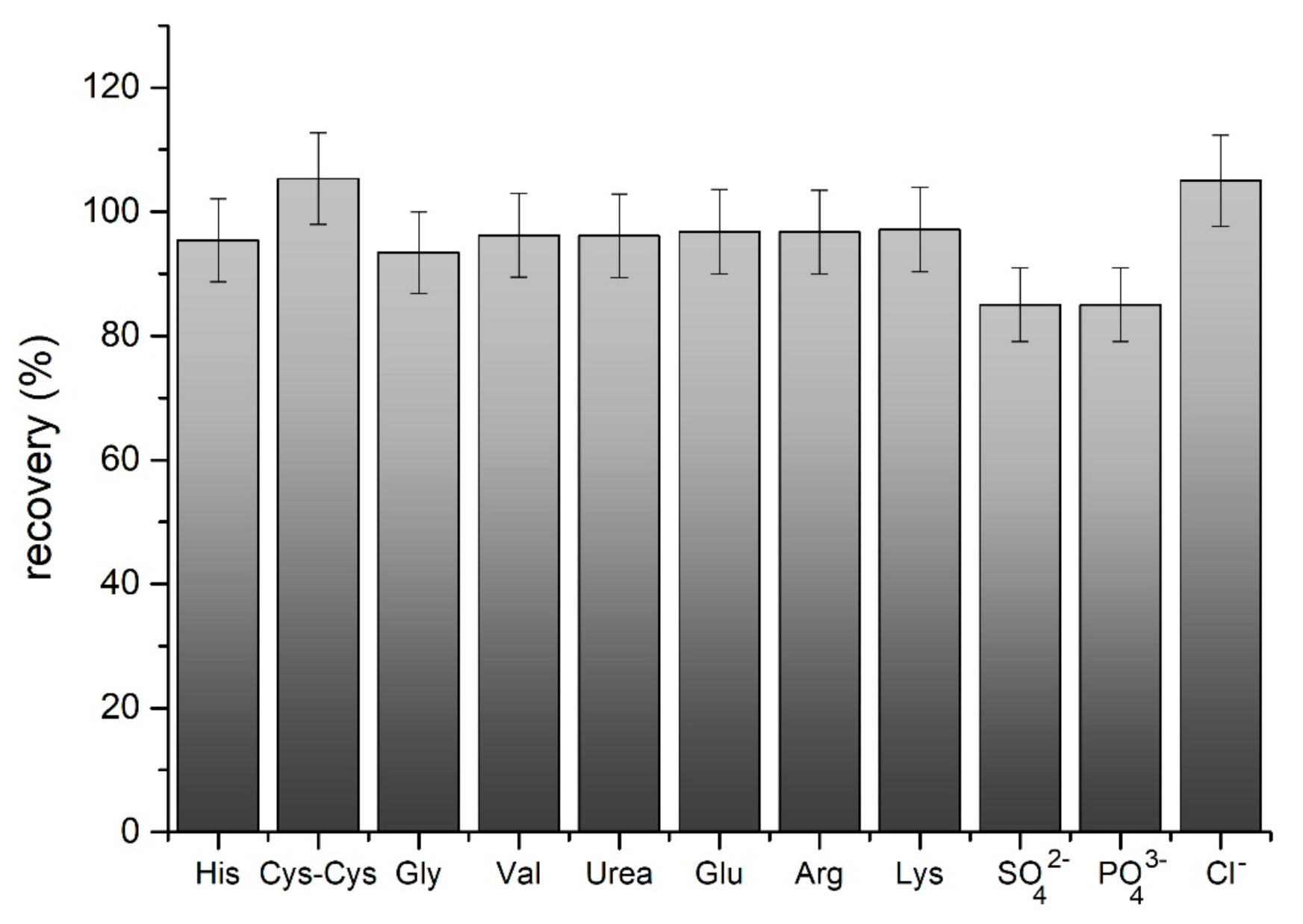
3.6. Interferences and Selectivity
3.7. Analytical Figures of Merit
3.8. Analysis of Biological Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free. Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Van Meurs, J.B.; Dhonukshe-Rutten, R.A.; De Groot, L.C.; Hofman, A.; Witteman, J.C.; Van Leeuwen, J.P.; Breteler, M.M.; Lips, P.; Pols, H.A.; Uitterlinden, A.G.; et al. Homocysteine Levels and the Risk of Osteoporotic Fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 87, pp. 141–159. ISBN 978-0-12-815203-4. [Google Scholar]
- Prendecki, M.; Florczak-Wyspianska, J.; Kowalska, M.; Ilkowski, J.; Grzelak, T.; Bialas, K.; Wiszniewska, M.; Kozubski, W.; Dorszewska, J. Biothiols and oxidative stress markers and polymorphisms of TOMM40 and APOC1 genes in Alzheimer’s disease patients. Oncotarget 2018, 9, 35207–35225. [Google Scholar] [CrossRef] [Green Version]
- Isokawa, M.; Kanamori, T.; Funatsu, T.; Tsunoda, M. Analytical methods involving separation techniques for determination of low-molecular-weight biothiols in human plasma and blood. J. Chromatogr. B 2014, 964, 103–115. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef] [PubMed]
- Tsogas, G.Z.; Kappi, F.A.; Vlessidis, A.G.; Giokas, D.L. Recent Advances in Nanomaterial Probes for Optical Biothiol Sensing: A Review. Anal. Lett. 2018, 51, 443–468. [Google Scholar] [CrossRef]
- Coronado-Puchau, M.; Saa, L.; Grzelczak, M.; Pavlov, V.; Liz-Marzán, L.M. Enzymatic modulation of gold nanorod growth and application to nerve gas detection. Nano Today 2013, 8, 461–468. [Google Scholar] [CrossRef]
- Shen, L.-M.; Chen, Q.; Sun, Z.-Y.; Chen, X.-W.; Wang, J.-H. Assay of Biothiols by Regulating the Growth of Silver Nanoparticles with C-Dots as Reducing Agent. Anal. Chem. 2014, 86, 5002–5008. [Google Scholar] [CrossRef]
- Jung, Y.L.; Park, J.H.; Kim, M.I.; Park, H.G. Label-free colorimetric detection of biological thiols based on target-triggered inhibition of photoinduced formation of AuNPs. Nanotechnology 2016, 27, 055501. [Google Scholar] [CrossRef]
- Kostara, A.; Tsogas, G.Z.; Vlessidis, A.G.; Giokas, D.L. Generic Assay of Sulfur-Containing Compounds Based on Kinetics Inhibition of Gold Nanoparticle Photochemical Growth. ACS Omega 2018, 3, 16831–16838. [Google Scholar] [CrossRef]
- Akrivi, E.; Kappi, F.; Gouma, V.; Vlessidis, A.G.; Giokas, D.L.; Kourkoumelis, N. Biothiol modulated growth and aggregation of gold nanoparticles and their determination in biological fluids using digital photometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119337. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, K.; Esumi, K. Preparation of colloidal gold by photoreduction of tetracyanoaurate(1-)-cationic surfactant complexes. Langmuir 1992, 8, 59–63. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Bai, T.; Tan, Y.; Zou, J.; Nie, M.; Guo, Z.; Lu, X.; Gu, N. AuBr2–-Engaged Galvanic Replacement for Citrate-Capped Au–Ag Alloy Nanostructures and Their Solution-Based Surface-Enhanced Raman Scattering Activity. J. Phys. Chem. C 2015, 119, 28597–28604. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Nemiroski, A.; Kumar, A.A.; Whitesides, G.M. Broadly Available Imaging Devices Enable High-Quality Low-Cost Photometry. Anal. Chem. 2015, 87, 9170–9178. [Google Scholar] [CrossRef] [PubMed]
- Eliceiri, K. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar]
- Perez-Juste, J.; Liz-Marzan, L.M.; Carnie, S.; Chan, D.Y.C.; Mulvaney, P. Electric-Field-Directed Growth of Gold Nanorods in Aqueous Surfactant Solutions. Adv. Funct. Mater. 2004, 14, 571–579. [Google Scholar] [CrossRef]
- Patel, V.; Dharaiya, N.; Ray, D.; Aswal, V.K.; Bahadur, P. pH controlled size/shape in CTAB micelles with solubilized polar additives: A viscometry, scattering and spectral evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 67–75. [Google Scholar] [CrossRef]
- Pacławski, K.; Fitzner, K. Kinetics of gold(III) chloride complex reduction using sulfur(IV). Met. Mater. Trans. A 2004, 35, 1071–1085. [Google Scholar] [CrossRef]
- O’Neil, M. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Ma, Q.; Fang, X.; Zhang, J.; Zhu, L.; Rao, X.; Lu, Q.; Sun, Z.; Yu, H.; Zhang, Q. Discrimination of cysteamine from mercapto amino acids through isoelectric point-mediated surface ligand exchange of β-cyclodextrin-modified gold nanoparticles. J. Mater. Chem. B 2020, 8, 4039–4045. [Google Scholar] [CrossRef]
- Khan, M.N.; Alam Khan, T.; Al-Thabaiti, S.A.; Khan, Z. Spectrophotometric evidence to the formation of AuCl4–CTA complex and synthesis of gold nano-flowers with tailored surface textures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 889–897. [Google Scholar] [CrossRef]
- Roy, J.C.; Islam, N.; Aktaruzzaman, G. The Effect of NaCl on the Krafft Temperature and Related Behavior of Cetyltrimethylammonium Bromide in Aqueous Solution. J. Surfactants Deterg. 2013, 17, 231–242. [Google Scholar] [CrossRef]
- Becker, R.; Liedberg, B.; Käll, P.-O. CTAB promoted synthesis of Au nanorods–Temperature effects and stability considerations. J. Colloid Interface Sci. 2010, 343, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R. Gram-Scale Synthesis of Soluble, Near-Monodisperse Gold Nanorods and Other Anisotropic Nanoparticles. Small 2005, 1, 875–882. [Google Scholar] [CrossRef]
- Zijlstra, P.; Bullen, C.; Chon, J.W.M.; Gu, M. High-Temperature Seedless Synthesis of Gold Nanorods. J. Phys. Chem. B 2006, 110, 19315–19318. [Google Scholar] [CrossRef]
- De, M.; Rana, S.; Akpinar, H.; Miranda, O.R.; Arvizo, R.R.; Bunz, U.H.F.; Rotello, V.M. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nat. Chem. 2009, 1, 461–465. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Kele, Z.; Baranyai, P.; Deák, A.; Juhász, Á.; Janovák, L.; Dékány, I. Influence of pH and aurate/amino acid ratios on the tuneable optical features of gold nanoparticles and nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 601–608. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Z.; Parkinson, J.A.; Chen, Y.; Sadler, P.J. Gold(III)-induced oxidation of glycine. Chem. Commun. 1999, 1359–1360. [Google Scholar] [CrossRef]
- Shaw, C.F.; Cancro, M.P.; Witkiewicz, P.L.; Eldridge, J.E. Gold(III) oxidation of disulfides in aqueous solution. Inorg. Chem. 1980, 19, 3198–3201. [Google Scholar] [CrossRef]
- Franskhaw, P.; Awitkiewic, C. Oxidative Cleavage of Peptide and Protein Disulphide Bonds by Gold(II1): A Mechanism for Gold Toxicity. J. Chem. Soc. Chem. Commun. 1981, 21, 1111–1114. [Google Scholar]
- Oelschlaeger, C.; Suwita, P.; Willenbacher, N. Effect of Counterion Binding Efficiency on Structure and Dynamics of Wormlike Micelles. Langmuir 2010, 26, 7045–7053. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Miao, Y.; Zhang, X.; Zhang, S.; Zhao, X. Colorimetric determination of cysteine by a paper-based assay system using aspartic acid modified gold nanoparticles. Microchim. Acta 2020, 187, 362. [Google Scholar] [CrossRef] [PubMed]
- Kappi, F.A.; Papadopoulos, G.A.; Tsogas, G.Z.; Giokas, D.L. Low-cost colorimetric assay of biothiols based on the photochemical reduction of silver halides and consumer electronic imaging devices. Talanta 2017, 172, 15–22. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Ortega-Muñoz, M.; Marín-Sánchez, A.; De Orbe-Payá, I.; Hernandez-Mateo, F.; Capitan-Vallvey, L.F.; Santoyo-Gonzalez, F.; Salinas-Castillo, A. A vinyl sulfone clicked carbon dot-engineered microfluidic paper-based analytical device for fluorometric determination of biothiols. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Yu, M.; He, K.; Liu, Y.; Cao, Y.; Shui, Y.; Li, J.; Farooq, M.; Wang, L. A novel colorimetric and fluorometric probe for biothiols based on MnO2 NFs-Rhodamine B system. Anal. Chim. Acta 2020, 1127, 39–48. [Google Scholar] [CrossRef]
- Tang, J.; Kong, B.; Wang, Y.; Xu, M.; Wang, Y.; Wu, H.; Zheng, G. Photoelectrochemical Detection of Glutathione by IrO2–Hemin–TiO2Nanowire Arrays. Nano Lett. 2013, 13, 5350–5354. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Thompson, M. Standard additions: Myth and reality. Analyst 2008, 133, 992–997. [Google Scholar] [CrossRef]

| Biothiol | Cysteine (Cys) | Homocysteine (Hcy) | Glutathione (GSH) |
|---|---|---|---|
| Linear range Regression function Quantification limit b | 5–100 μM y = 1.3x + 5.3, R2 = 0.97 5.0 μM | 5–100 μM y = 0.73x + 2.9, R2 = 0.99 3.5 μM | 5–100 μM Y = x + 8.6, R2 = 0.98 5.0 μM |
| Scanner image (transmittance mode) |  |  |  |
| Detection Technique | Material or Sensing Element | Real Samples | Linear Range (μM) a | LOD (μM) a | Recoveries (%) | Reference |
|---|---|---|---|---|---|---|
| Colorimetric (paper-based) | Asp-AuNPs | Blood plasma | 99.9–998.7 | 1.0 | 99.2–101.1 | [36] |
| Turbidimetry/Colorimetry | AgCl/AgNPs | Blood plasma | 10–100 | 8.1 | 92–97 | [37] |
| Fluorescence | VS-CDs | Spiked blood plasma b | 5–200 | 0.3 | 98.6–111.5 | [38] |
| Colorimetry/Fluorescence | RhB–MnO2 NFs | Spiked blood plasma b | 0–15 | 0.14 | 89.3–116.3% | [39] |
| Colorimetric | IrO2/rGO nanocomposites | 5 wt% BSA, Blood serum | 0.1–50 | 0.04 | 99.2–122 | [40] |
| Visual/Colorimetric | AuCl4−/AuNP seeds/AuNPs | Whole blood, blood plasma | 3–300 | 1.0 | 88.7–114 | [14] |
| Visual/Colorimetric | AuCl4− CTAB | Urine, Blood plasma | 5–100 | 5 | This method |
| Sample | Measured (μM) | Spiked (μM) | Found (μM) a | Recovery (%) b | RSD (%, n = 3) |
|---|---|---|---|---|---|
| Simulated urine c | 0 | 150.0 | 101.0 | 67.3 | 8.4 |
| 0 | 250.0 | 190.5 | 76.2 | 6.3 | |
| Simulated blood plasma c | 0 | 150.0 | 142.0 | 94.6 | 5.9 |
| 0 | 250.0 | 307.5 | 123.0 | 7.0 | |
| Urine d | 108 | 100.0 | 212.1 | 103.8 | 6.3 |
| Blood plasma d | 201 | 100.0 | 331.1 | 115.0 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrivi, E.A.; Vlessidis, A.G.; Giokas, D.L.; Kourkoumelis, N. Gold-Modified Micellar Composites as Colorimetric Probes for the Determination of Low Molecular Weight Thiols in Biological Fluids Using Consumer Electronic Devices. Appl. Sci. 2021, 11, 2705. https://doi.org/10.3390/app11062705
Akrivi EA, Vlessidis AG, Giokas DL, Kourkoumelis N. Gold-Modified Micellar Composites as Colorimetric Probes for the Determination of Low Molecular Weight Thiols in Biological Fluids Using Consumer Electronic Devices. Applied Sciences. 2021; 11(6):2705. https://doi.org/10.3390/app11062705
Chicago/Turabian StyleAkrivi, Elli A., Athanasios G. Vlessidis, Dimosthenis L. Giokas, and Nikolaos Kourkoumelis. 2021. "Gold-Modified Micellar Composites as Colorimetric Probes for the Determination of Low Molecular Weight Thiols in Biological Fluids Using Consumer Electronic Devices" Applied Sciences 11, no. 6: 2705. https://doi.org/10.3390/app11062705






