Investigation of an Optical Imaging Platform Integrated with an Ultrasound Application System for In Vitro Verification of Ultrasound-Mediated Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Sample Preparations
2.2. Instrumentation of an Optical Imaging Platform and Imaging Procedures
2.3. Setup of an Ultrasound Application System for Ultrasound-Microbubble Cavitation
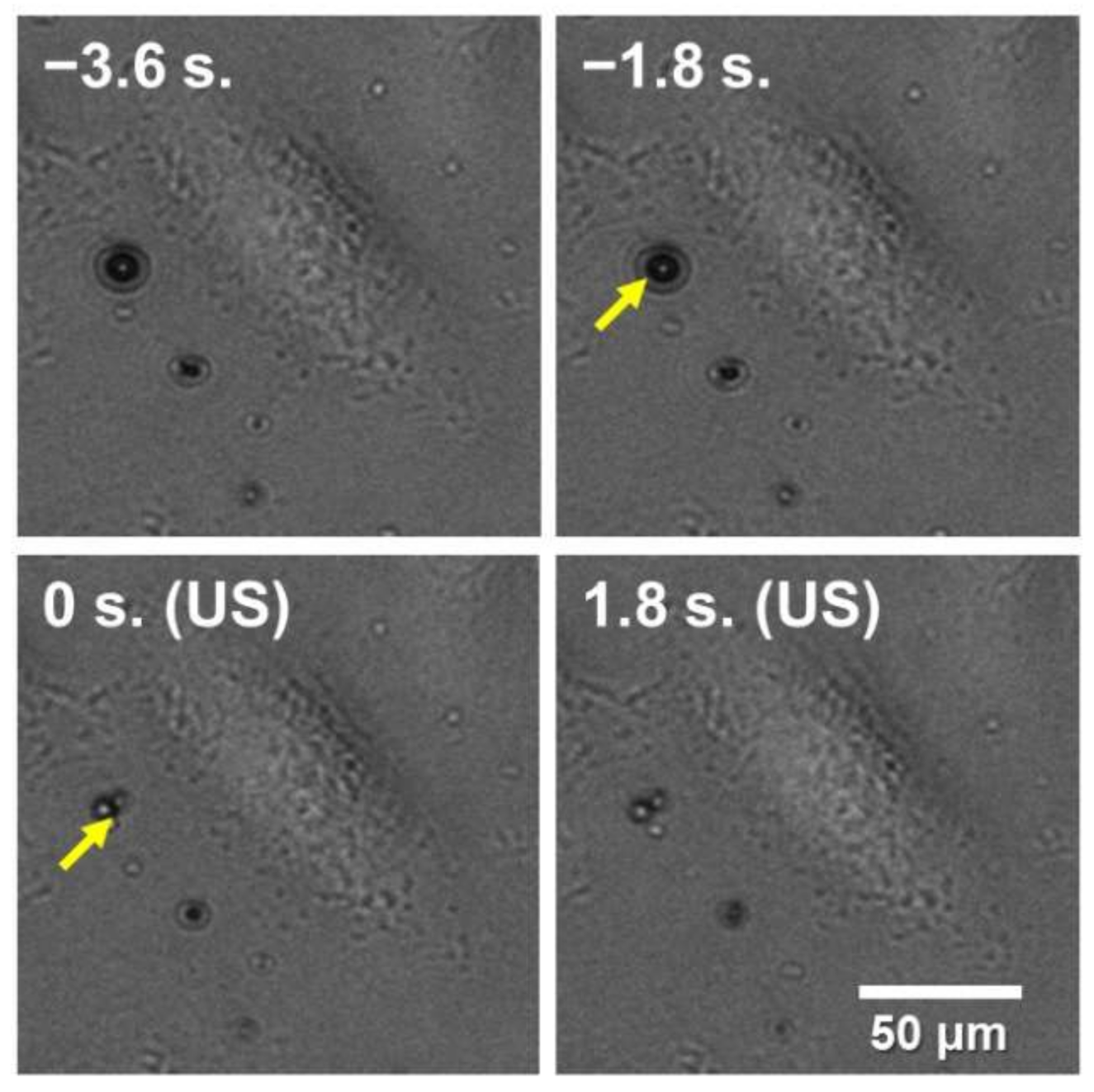
3. Results and Discussion
3.1. Imaging of Calcium Flux Changes of a Cervical Cell by Ultrasound-Microbubble Cavitation
3.2. Fluorescence Imaging of Propidium Iodide
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated re-view. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, X.; Akabar, M.D.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Ninave, K.M.; Karnam, S.; Venuganti, V.V.K. Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J. Liposome Res. 2018, 29, 153–162. [Google Scholar] [CrossRef]
- Belhadj, Z.; Ying, M.; Cao, X.; Fu, X.; Zhan, C.; Wei, X.; Gao, J.; Wang, X.; Yan, Z.; Lu, W. Design of Y-shaped targeting mate-rial for liposome-based multifunctional glioblastoma-targeted drug delivery. J. Control. Release 2017, 255, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef]
- Mehdipoor, E.; Adeli, M.; Bavadi, M.; Sasanpour, P.; Rashidian, B. A possible anticancer drug delivery system based on car-bon nanotube–dendrimer hybrid nanomaterials. J. Mater. Chem. 2011, 21, 15456–15463. [Google Scholar] [CrossRef]
- Bhutiani, N.; Agle, S.; Li, Y.; Li, S.; Martin, R.C. Irreversible electroporation enhances delivery of gemcitabine to pancreatic adenocarcinoma. J. Surg. Oncol. 2016, 114, 181–186. [Google Scholar] [CrossRef]
- Kulbacka, J.; Daczewska, M.; Dubińska-Magiera, M.; Choromańska, A.; Rembiałkowska, N.; Surowiak, P.; Kulbacki, M.; Kotulska, M.; Saczko, J. Doxorubicin delivery enhanced by electroporation to gastrointestinal adenocarcinoma cells with P-gp overexpression. Bioelectrochemistry 2014, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bu, W.; Pan, L.; Shi, J. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azo-benzene-modified mesoporous silica. Angew. Chem. 2013, 52, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, X.; Wang, S.; Qian, J.; He, S. Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 24368–24384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pepe, J.; Rincon, M. Sonoporation, anti-cancer drug and antibody delivery using ultrasound. Ultrasonics 2006, 44, e21–e25. [Google Scholar] [CrossRef]
- Maeda, H.; Tominaga, K.; Iwanaga, K.; Nagao, F.; Habu, M.; Tsujisawa, T.; Seta, Y.; Toyoshima, K.; Fukuda, J.-I.; Nishihara, T. Targeted drug delivery system for oral cancer therapy using sonoporation. J. Oral Pathol. Med. 2009, 38, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Kumon, R.E.; Park, J.; Deng, C.X. Intracellular delivery and calcium transients generated in sonoporation facilitated by microbubbles. J. Control. Release 2010, 142, 31–39. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.-Y.; Han, M.; Choi, J.-R.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood-brain barrier disruption in rat brain. Sci. Rep. 2016, 6, 31201. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Lee, T.; Willmann, J.K. Ultrasound-guided drug delivery in cancer. Ultrasonography 2017, 36, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Horsley, H.; Owen, J.; Browning, R.; Carugo, D.; Malone-Lee, J.; Stride, E.; Rohn, J. Ultrasound-activated microbubbles as a novel intracellular drug delivery system for urinary tract infection. J. Control. Release 2019, 301, 166–175. [Google Scholar] [CrossRef]
- Kato, S.; Shirai, Y.; Motozono, C.; Kanzaki, H.; Mori, S.; Kodama, T. In vivo delivery of an exogenous molecule into murine T lymphocytes using a lymphatic drug delivery system combined with sonoporation. Biochem. Biophys. Res. Commun. 2020, 525, 1025–1031. [Google Scholar] [CrossRef]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer per-meability. Colloids Surf. B 2018, 168, 83–93. [Google Scholar] [CrossRef]
- Ghaffarian, R.; Muro, S. Models and methods to evaluate transport of drug delivery systems across cellular barriers. J. Vis. Exp. 2013, 80, 50638. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro bar-rier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Trier, S.; Rahbek, U.L.; Dufva, M.; Kutter, J.P.; Andresen, T.L. A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS ONE 2018, 13, e0197101. [Google Scholar] [CrossRef]
- Asif, A.; Kim, K.H.; Jabbar, F.; Kim, S.; Choi, K.H. Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule. Microfluid. Nanofluidics 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Claudio, P.P.; Howard, C.M.; Nande, R. Ultrasound-mediated oncolytic virus delivery and uptake for increased therapeutic efficacy: State of art. Oncolytic Virotherapy 2015, 4, 193–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef]
- Krämer, C.E.M.; Wiechert, W.; Kohlheyer, D. Time-resolved, single-cell analysis of induced and programmed cell death via non-invasive propidium iodide and counterstain perfusion. Sci. Rep. 2016, 6, 32104. [Google Scholar] [CrossRef]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring cell death by propidi-um iodide uptake and flow cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087163. [Google Scholar]
- Oh, T.; Sung, J.H.; Tatosian, D.A.; Shuler, M.L.; Kim, D. Real-time fluorescence detection of multiple microscale cell culture analog devices in situ. Cytom. A 2007, 71A, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Muslimov, A.R.; Timin, A.S.; Bichaykina, V.R.; Peltek, O.O.; Karpov, T.E.; Dubavik, A.; Nominé, A.; Ghanbaja, J.; Sukhorukov, G.B.; Zyuzin, M.V. Biomimetic drug delivery platforms based on mesenchymal stem cells impregnated with light-responsive submicron sized carriers. Biomater. Sci. 2019, 8, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Patitsa, M.; Karathanou, K.; Kanaki, Z.; Tzioga, L.; Pippa, N.; Demetzos, C.; Verganelakis, D.A.; Cournia, Z.; Klinakis, A. Magnetic nanoparticles coated with polyarabic acid demonstrate enhanced drug delivery and imaging properties for cancer theranostic applications. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, M.; Sun, X.; Jia, H.; Liu, W. NIR-responsive cancer cytomembrane-cloaked carrier-free nanosystems for highly efficient and self-targeted tumor drug delivery. Biomaterials 2018, 159, 25–36. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-r.; Park, J. Investigation of an Optical Imaging Platform Integrated with an Ultrasound Application System for In Vitro Verification of Ultrasound-Mediated Drug Delivery. Appl. Sci. 2021, 11, 2846. https://doi.org/10.3390/app11062846
Choi J-r, Park J. Investigation of an Optical Imaging Platform Integrated with an Ultrasound Application System for In Vitro Verification of Ultrasound-Mediated Drug Delivery. Applied Sciences. 2021; 11(6):2846. https://doi.org/10.3390/app11062846
Chicago/Turabian StyleChoi, Jong-ryul, and Juyoung Park. 2021. "Investigation of an Optical Imaging Platform Integrated with an Ultrasound Application System for In Vitro Verification of Ultrasound-Mediated Drug Delivery" Applied Sciences 11, no. 6: 2846. https://doi.org/10.3390/app11062846
APA StyleChoi, J.-r., & Park, J. (2021). Investigation of an Optical Imaging Platform Integrated with an Ultrasound Application System for In Vitro Verification of Ultrasound-Mediated Drug Delivery. Applied Sciences, 11(6), 2846. https://doi.org/10.3390/app11062846





