Evaluation of Algae-Based Biodiesel Production Topologies via Inherent Safety Index (ISI)
Abstract
:1. Introduction
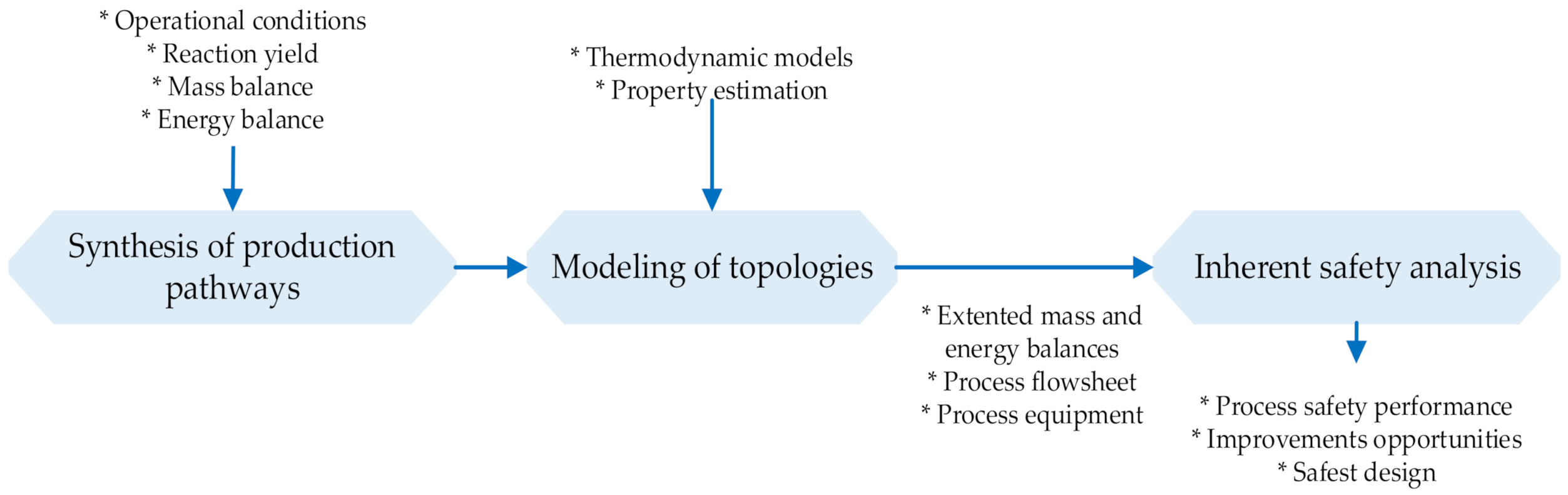
2. Materials and Methods
2.1. Process Description
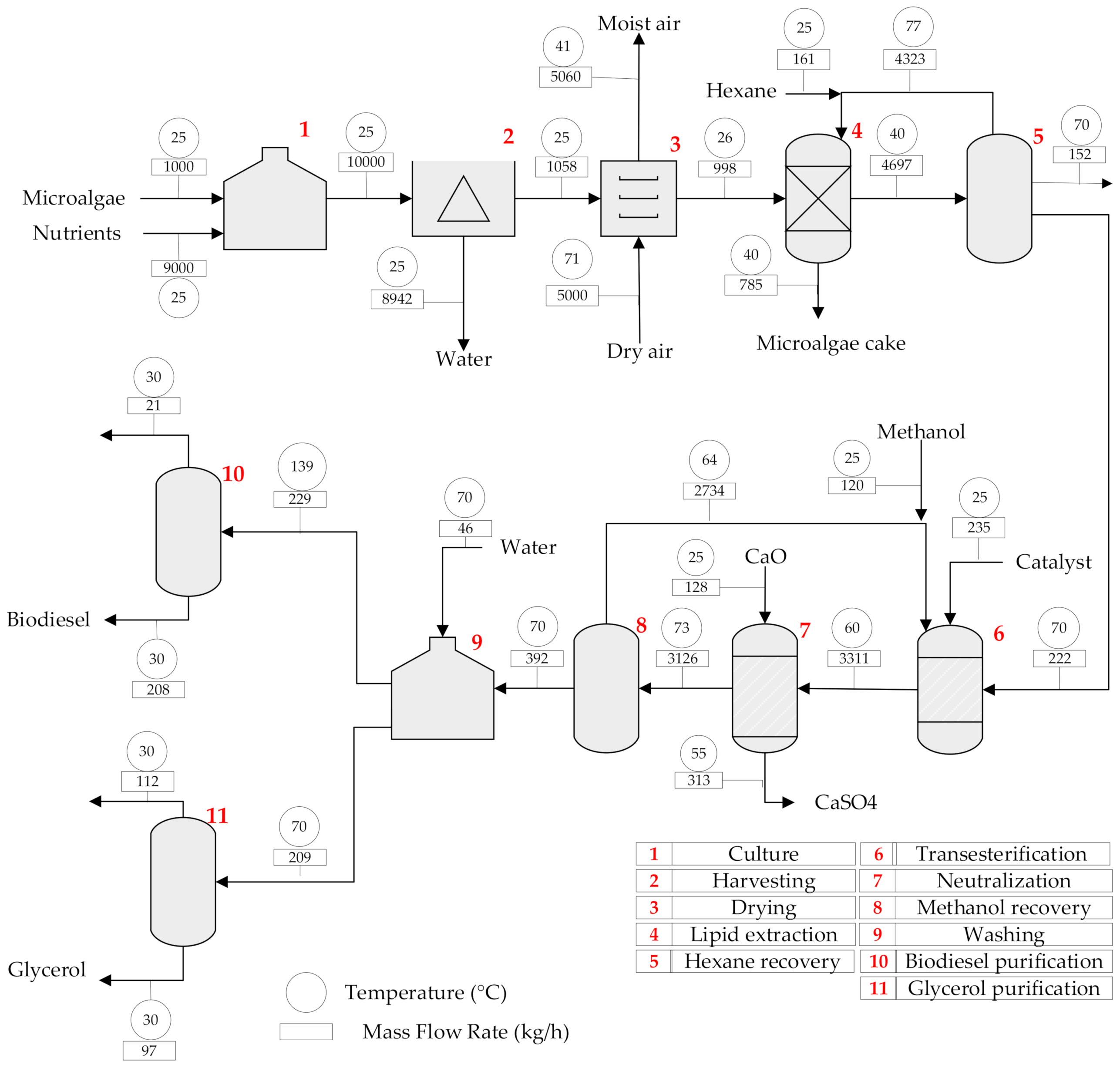
2.1.1. Topology 1. Conventional Method (Lipid Extraction and Transesterification)
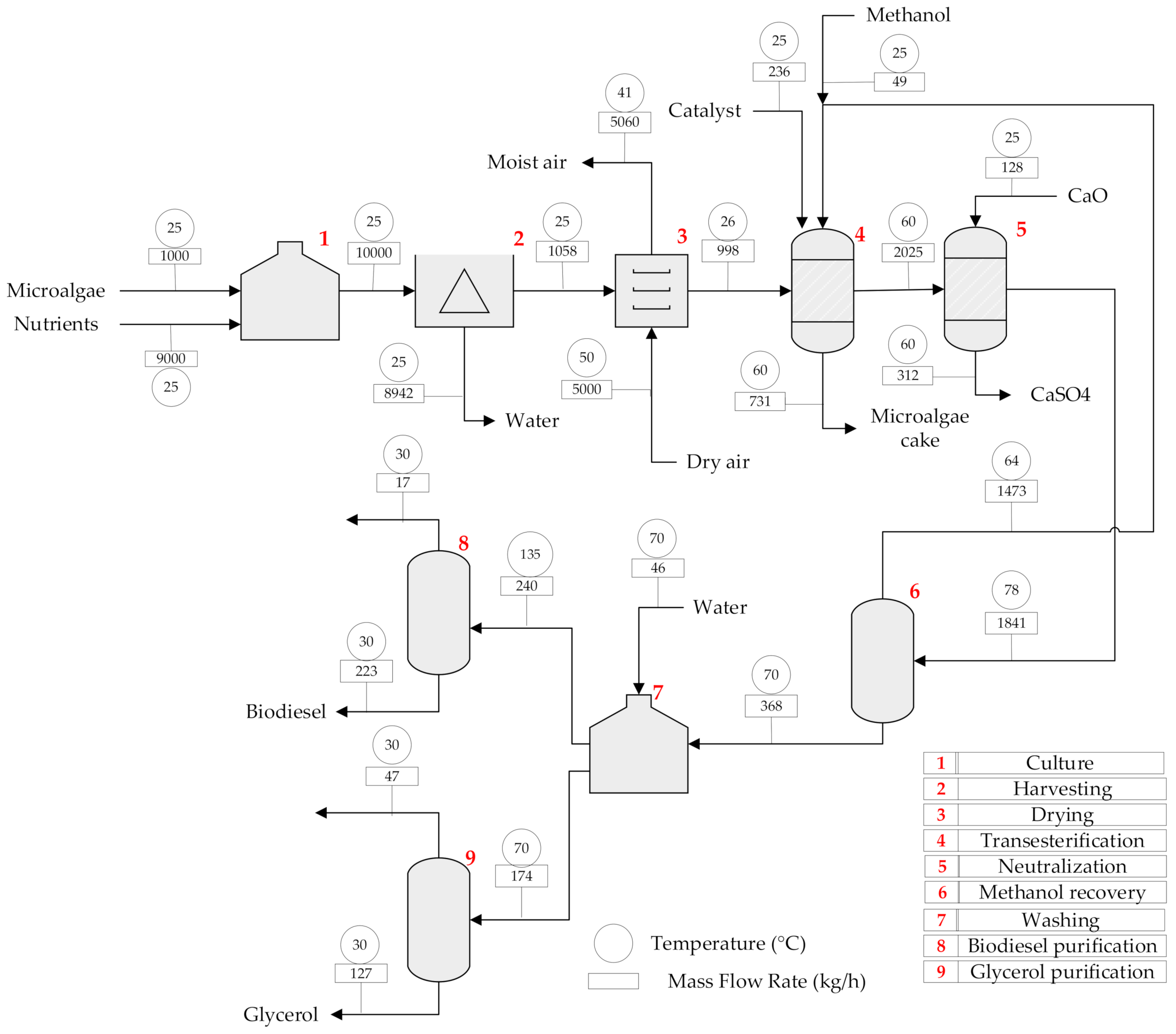
2.1.2. Topology 2. In-Situ Transesterification
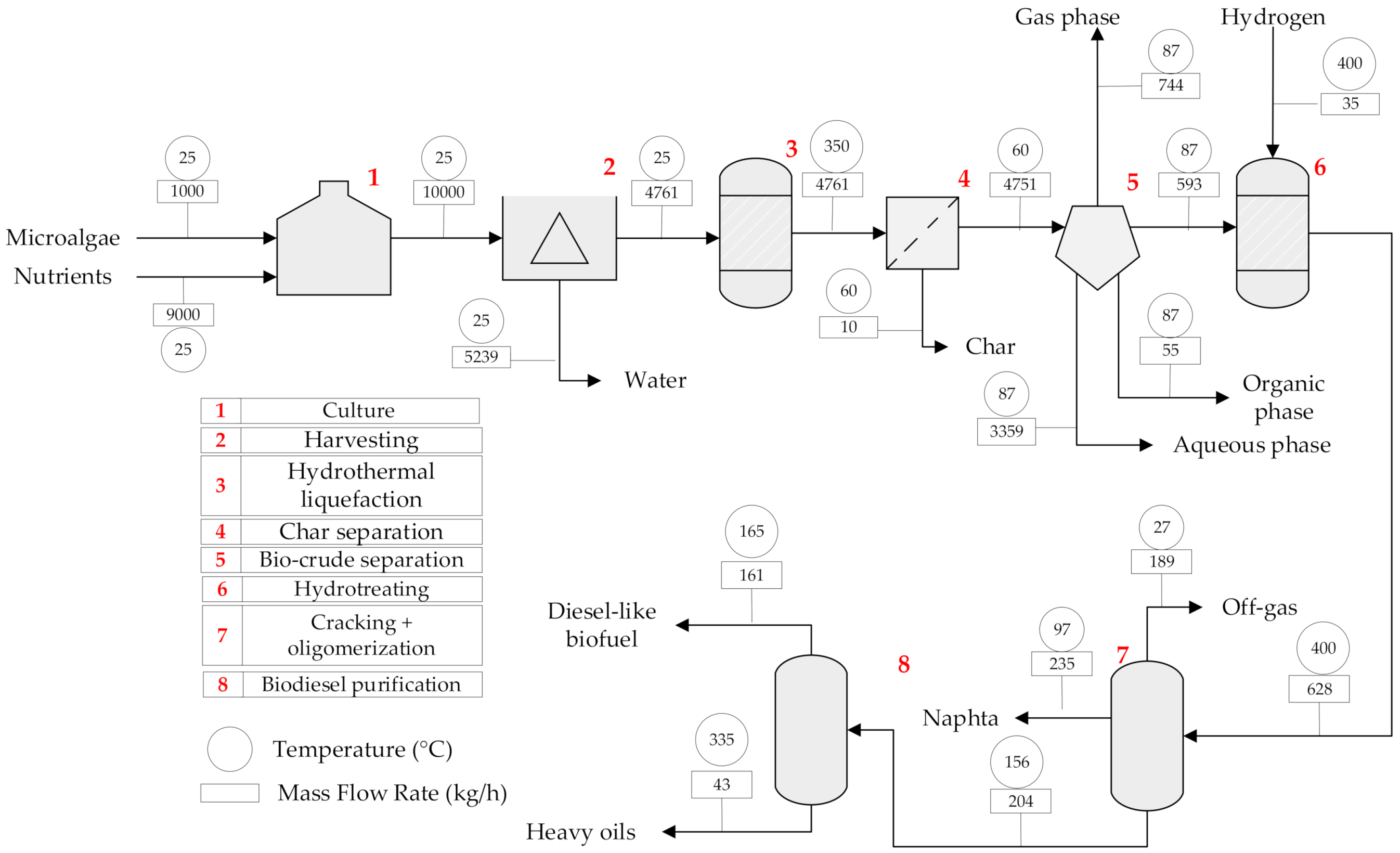
2.1.3. Topology 3. Hydrothermal Liquefaction (HTL)
2.2. Inherent Safety Analysis
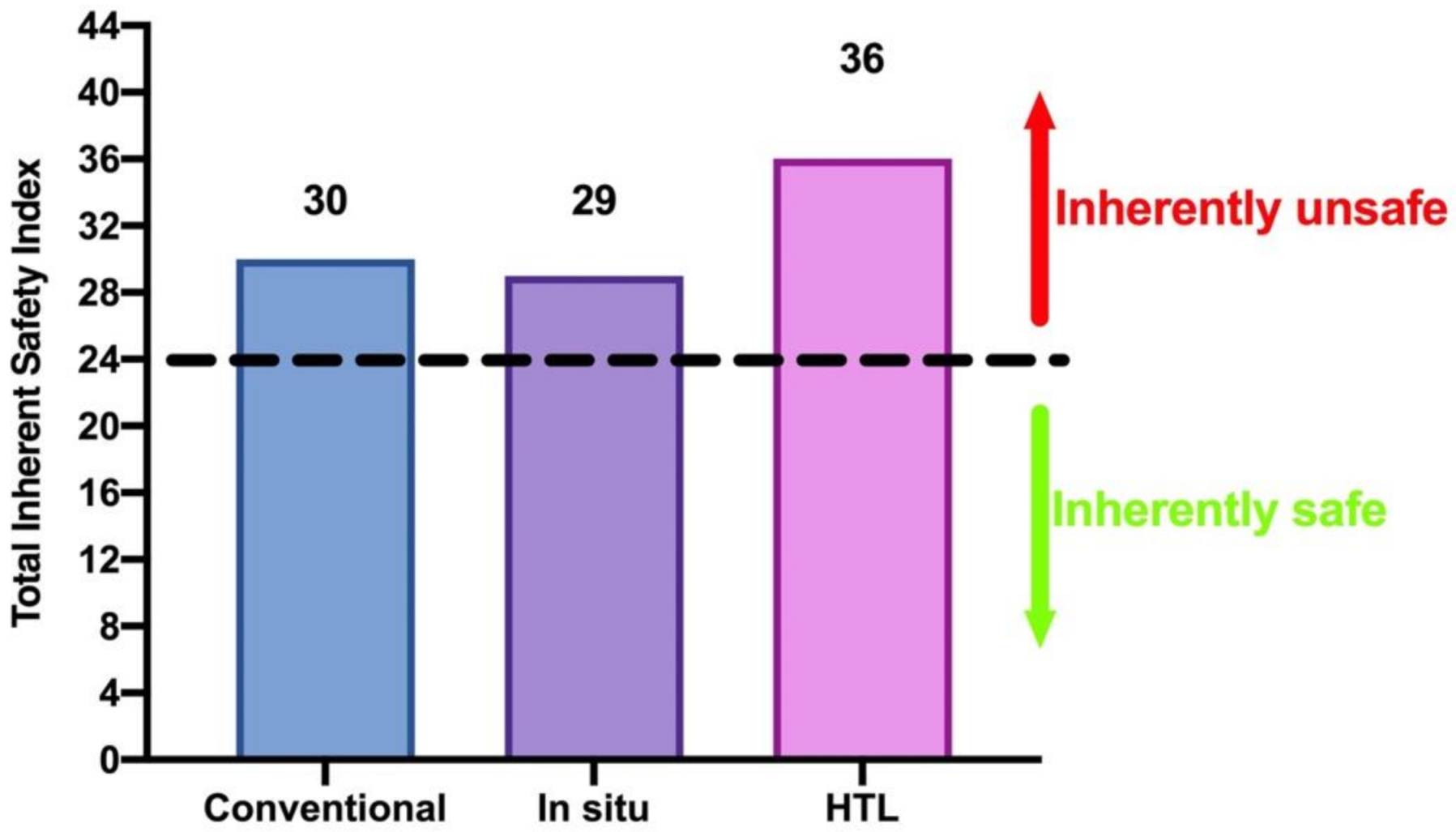
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, H.C.; Tiong, Y.W.; Hoe Goh, G.; Yang Gan, Y.; Mofijur, M.; Rizwanul, I.M.; Tung, C.; Asraful, M.; Voon Lee, H.; Dilitong, A.S.; et al. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2020. [Google Scholar] [CrossRef]
- Tejada Carbajal, E.M.; Martínez Hernández, E.; Fernández Linares, L.; Novelo Maldonado, E.; Limas Ballesteros, R. Techno-economic analysis of Scenedesmus dimorphus microalgae biorefinery scenarios for biodiesel production and glycerol valorization. Bioresour. Technol. Rep. 2020, 12. [Google Scholar] [CrossRef]
- Chowdhury, H.; Loganathan, B. Third-generation biofuels from microalgae: A review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Azadi, P.; Malina, R.; Barrett, S.R.H.; Kraft, M. The evolution of the biofuel science. Renew. Sustain. Energy Rev. 2016, 76, 1479–1484. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Ali, M.; Sultana, R.; Tahir, S.; Watson, I.; Saleem, M. Prospects of microalgal biodiesel production in Pakistan—A review. Renew. Sustain. Energy Rev. 2017, 80, 1588–1596. [Google Scholar] [CrossRef] [Green Version]
- Rutz, D.; Janssen, R. Biofuel Technology Handbook, in Sylvensteinstr. 2; WIP Renewable Energies: München, Germany, 2007. [Google Scholar]
- Kumar Pathaka, P.; Rajb, J.; Saxenab, G.; Shankar Sharmac, U. A Review on Production of Biodiesel by Transesterification using Heterogeneous Nanocatalyst. Int. J. Sci. Res. Dev. 2018, 5, 631–636. [Google Scholar]
- Deng, X.; Li, Y.; Fei, X. Microalgae: A promising feedstock for biodiesel. Afr. J. Microbiol. Res. 2009, 3, 1008–1014. [Google Scholar]
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Huete-Ortega, M.; Okurowska, K.; Kapoore, R.V.; Johnson, M.P.; Gilmour, D.J.; Vaidyanathan, S. Effect of ammonium and high light intensity on the accumulation of lipids in Nannochloropsis oceanica (CCAP 849/10) and Phaeodactylum tricornutum (CCAP 1055/1). Biotechnol. Biofuels 2018, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Rosenberg, J.N.; Betenbaugh, M.J.; Wang, F. Optimization of one-step in situ transesterification method for accurate quantification of epa in nannochloropsis gaditana. Appl. Sci. 2016, 6, 343. [Google Scholar] [CrossRef] [Green Version]
- Hena, S.; Znad, H.; Heong, K.T.; Judd, S. Dairy Farm Wastewater Treatment and Lipid Accumulation by Arthrospira Platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.D.; Ruiz, J.; van den Broek, L.A.M.; Hesselink, T.; Peters, S.; Kleinegris, D.M.M.; Smith, A.G.; van der Veen, D.; Barbosa, M.J.; Wijffels, R.H. Botryococcus Braunii Strains Compared for Biomass Productivity, Hydrocarbon, and Carbohydrate Content. J. Biotechnol. 2017, 248, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Solano, A.F.; Guzmán-Monsalve, A.; Kafarov, V. Effect of Carbon-Nitrogen Ratio for the Biomass Production, Hydrocarbons and Lipids on Botryoccus Braunii UIS 003. Chem. Eng. Trans. 2016, 49, 247–252. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi Mediated Phosphoenolpyruvate Carboxylase Regulation to Enhance the Production of Lipid in Chlamydomonas Reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted Knockout of Phospholipase A2 to Increase Lipid Productivity in Chlamydomonas Reinhardtii for Biodiesel Production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.-L.; Li, C.; Peng, Y.-Y.; Lu, M.-M.; Jin, W.-H.; Bao, J.-J.; Guo, Y.-M. Effect of Organic Carbon to Nitrogen Ratio in Wastewater on Growth, Nutrient Uptake and Lipid Accumulation of a Mixotrophic Microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth Stimulation and Synthesis of Lipids, Pigments and Antioxidants with Magnetic Fields in Chlorella Kessleri Cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the Dominant Chlorella Pyrenoidosa for Biofilm Attached Culture and Feed Production While Treating Swine Wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella Vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Estévez-Landazábal, L.L.; Barajas-Solano, A.F.; Barajas-Ferreira, C.; Kafarov, V. Improvement of Lipid Productivity on Chlorella vulgaris Using Waste Glycerol and Sodium Acetate. CT&F Cienc. Tecnol. Futuro 2013, 5, 113–126. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-53832013000100009 (accessed on 29 November 2020).
- Guiza-Franco, L.; Orozco-Rojas, L.G.; Sanchez-Galvis, M.; Garcia-Martinez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Production of Chlorella vulgaris Biomass on UV-Treated Wastewater as an Alternative for Environmental Sustainability on High-Mountain Fisheries. Chem. Eng. Trans. 2018, 64, 517–522. [Google Scholar] [CrossRef]
- Garcia-Martinez, B.; Ayala-Torres, E.; Reyes-Gomez, O.; Zuorro, A.; Barajas-Solano, A.; Barajas-Ferreira, C. Evaluation of a Two-Phase Extraction System of Carbohydrates and Proteins from Chlorella vulgaris UTEX 1803. Chem. Eng. Trans. 2016, 49, 355–360. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Gonzalez-Delgado, A.D.; Kafarov, V. Effect of Thermal Pre-Treatment on Fermentable Sugar Production Of Chlorella Vulgaris. Chem. Eng. Trans. 2014, 37, 655–660. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of cell wall degrading enzymes to improve the recovery of lipids from Chlorella sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Priharto, N.; Ronsse, F.; Prins, W.; Carleer, R.; Heeres, H.J. Experimental Studies on a Two-Step Fast Pyrolysis-Catalytic Hydrotreatment Process for Hydrocarbons from Microalgae (Nannochloropsis Gaditana and Scenedesmus Almeriensis). Fuel Process. Technol. 2020, 206, 106466. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Garcia-Minguillan, A.M.; Valverde, J.L. Pyrolysis, Combustion and Gasification Characteristics of Nannochloropsis Gaditana Microalgae. Bioresour. Technol. 2013, 130, 321–331. [Google Scholar] [CrossRef]
- Gautam, R.; Vinu, R. Non-Catalytic Fast Pyrolysis and Catalytic Fast Pyrolysis of Nannochloropsis Oculata Using Co-Mo/γ-Al2O3 Catalyst for Valuable Chemicals. Algal Res. 2018, 34, 12–24. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zeng, J.; Liu, M.; Zhang, Y.; Liu, Z. Influence of Fe/HZSM-5 Catalyst on Elemental Distribution and Product Properties during Hydrothermal Liquefaction of Nannochloropsis sp. Algal Res. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Aysu, T.; Sanna, A. Nannochloropsis Algae Pyrolysis with Ceria-Based Catalysts for Production of High-Quality Bio-Oils. Bioresour. Technol. 2015, 194, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic Cultivation of Green Microalgae Scenedesmus Obliquus on Cheese Whey Permeate for Biodiesel Production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Urbina-Suarez, N.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A. Lipids production from Scenedesmus obliquus through carbon/nitrogen ratio optimization. J. Phys. Conf. Ser. 2019, 1388, 012043. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A.; Urbina-Suarez, N.A. Towards the production of microalgae biofuels: The effect of the culture medium on lipid deposition. BioTechnologia 2019, 100, 273–278. [Google Scholar] [CrossRef]
- Srivatsa, S.C.; Li, F.; Bhattacharya, S. Optimization of Reaction Parameters for Bio-Oil Production by Catalytic Pyrolysis of Microalga Tetraselmis Suecica: Influence of Ni-Loading on the Bio-Oil Composition. Renew. Energy 2019, 142, 426–436. [Google Scholar] [CrossRef]
- Aysu, T.; Abd Rahman, N.A.; Sanna, A. Catalytic Pyrolysis of Tetraselmis and Isochrysis Microalgae by Nickel Ceria Based Catalysts for Hydrocarbon Production. Energy 2016, 103, 205–214. [Google Scholar] [CrossRef]
- Eboibi, B.E.; Lewis, D.M.; Ashman, P.J.; Chinnasamy, S. Influence of Process Conditions on Pretreatment of Microalgae for Protein Extraction and Production of Biocrude during Hydrothermal Liquefaction of Pretreated Tetraselmis sp. RSC Adv. 2015, 5, 20193–20207. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.S.; Felix, V.; JoselynMonica, M.; Malolan, R. A conceptual review on microalgae biorefinery through thermochemical and biological pathways: Bio-circular approach on carbon capture and wastewater treatment. Bioresour. Technol. Rep. 2020, 11. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The application of catalytic processes on the production of algae-based biofuels: A review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Ranganathan, P.; Savithri, S. Techno-economic analysis of microalgae-based liquid fuels production from wastewater via hydrothermal liquefaction and hydroprocessing. Bioresour. Technol. 2019, 284, 256–265. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; González-Delgado, A.D.; Kafarov, V. Evaluation of alternatives for microalgae oil extraction based on exergy analysis. Appl. Energy 2013, 101, 226–236. [Google Scholar] [CrossRef]
- Pardo-Cardenas, Y.; Herrera- Orozco, I.; González-Delgado, A.D.; Kafarov, V. Environmental Assessment of Microalgae Biodiesel Production in Colombia: Comparison of Three oil Extraction Systems. Latinoam. J. Oil Gas Altern. Energy 2013, 5, 85–100. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Vicente, G.; Bautista, L.F.; Gutiérrez, F.J.; Sádaba, I.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 2009, 48, 22–27. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Ehimen Ehiaze, A. An Investigation on the Co-Production of Biodiesel and Methane from Microalgae; University of Otago: Otago, New Zealand, 2010. [Google Scholar]
- Veljković, V.B.; Lakićević, S.H.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L. Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 2006, 85, 2671–2675. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, M.S.; Lee, Y.C.; Yang, J.W. Advances in direct transesterification of algal oils from wet biomass. Bioresour. Technol. 2015, 184, 267–275. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.; Lay, G.; Nguyen, D.; Pugazhendhi, A.; Woong, S.; Humar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Jones, S.; Zhu, Y.; Anderson, D.; Hallen, R.T.; Elliott, D.C. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading; PNNL: Richland, WA, USA, 2014; pp. 1–69. [Google Scholar]
- Heikkilä, A. Inherent Safety in Process Plant Design; VTT Publications: Espoo, Finland, 1999; pp. 1–132. [Google Scholar]
- Khan, F.I.; Amyotte, P.R. A comprehensive quantitative tool for inherent safety and cost evaluation. J. Loss Prev. Process Ind. 2005, 18, 310–326. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, A.A.C.; Ukogu, K.; Ibemesi, A. Catalysis in Biodiesel Production by Transesterification Process-An Insight. Egypt. J. Pet. 1998, 9, 332–337. [Google Scholar]
- Meramo-Hurtado, S.I.; Ojeda, K.A.; Sanchez-Tuiran, E. Environmental and Safety Assessments of Industrial Production of Levulinic Acid via Acid-Catalyzed Dehydration. ACS Omega 2019, 4, 22302–22312. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.I.; Hashim, H.; Hassim, M.H.; Muis, Z.A. Inherent Safety Assessment of Biodiesel Production: Flammability Parameter. Procedia Eng. 2016, 148, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Salzano, E.; Di Serio, M.; Santacesaria, E. Emerging risks in the biodiesel production by transesterification of virgin and renewable oils. Energy Fuels 2010, 24, 6103–6109. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Sanchez-Tuiran, E.; Ponce-Ortega, J.M.; El-Halwagi, M.M.; Ojeda-Delgado, K.A. Synthesis and Sustainability Evaluation of a Lignocellulosic Multifeedstock Biorefinery Considering Technical Performance Indicators. ACS Omega 2020, 5, 9259–9275. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, B.K.; Sharma, M.P. Prospects of biodiesel production from vegetable oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Sam Mannan, M.; Wang, Y.; Zhang, C.; West, H.H. Application of inherently safer design principles in biodiesel production process. Inst. Chem. Eng. Symp. Ser. 2006, 151, 982–989. [Google Scholar]

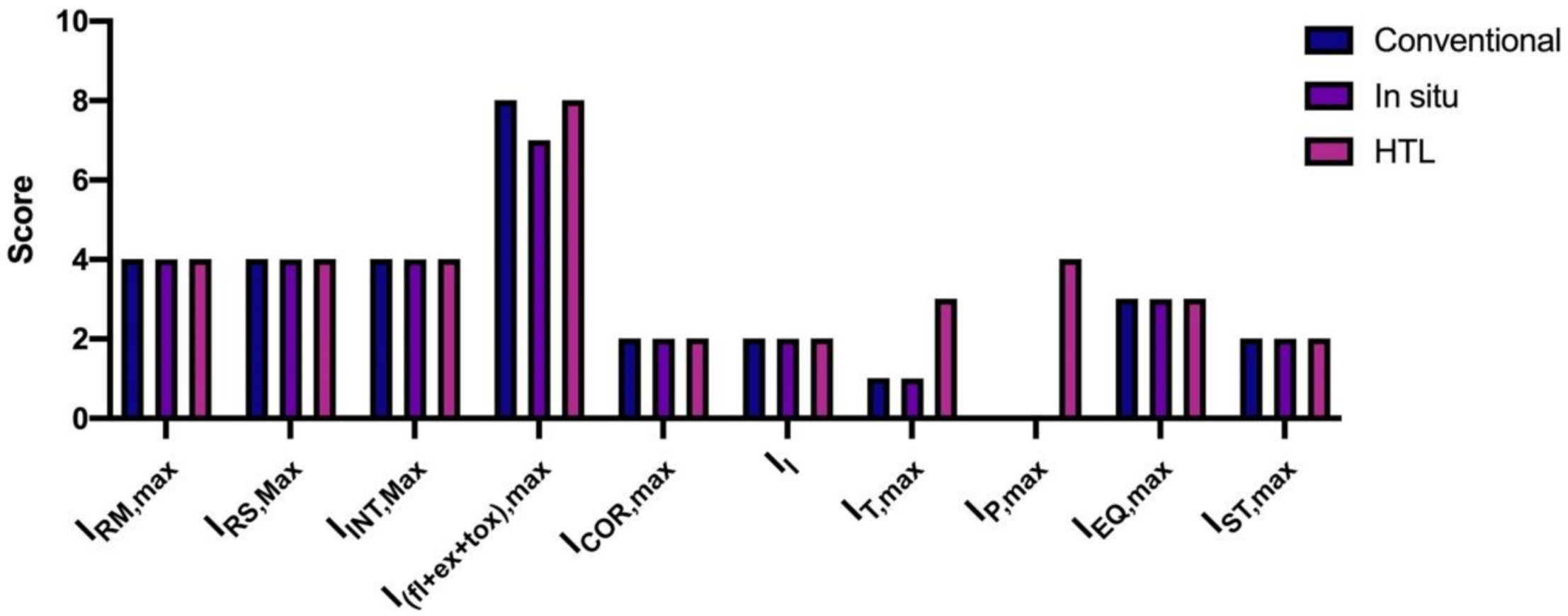
| ICI | Symbol | Score |
|---|---|---|
| Mean heat of reaction | IRM | 0–4 |
| Side heat of reaction | IRS | 0–4 |
| Chemical interaction | IINT | 0–4 |
| Flammability | Ifl | 0–4 |
| Explosiveness | Iex | 0–4 |
| Toxicity | Itox | 0–6 |
| Corrosiveness | ICOR | 0–2 |
| IPI | Symbol | Score |
|---|---|---|
| Inventory | II | 0–5 |
| Process temperature | IT | 0–4 |
| Process pressure | IP | 0–4 |
| Equipment safety | IEQ | 0–4 (ISBL); 0–3 (OSBL) |
| Safe process structure | IST | 0–5 |
| Topology 1 | Topology 2 | Topology 3 | |||
|---|---|---|---|---|---|
| Substances | Hexane | Methanol | Hexane | Piperidine | CO |
| Flash point (°C) | −22 | 9.7 | −22 | 4 | Flammable |
| Ifl | 4 | 3 | 4 | 3 | 2 |
| UEL-LEL (v/v%) | 7.1 | 38.5 | 7.1 | 9 | 62 |
| Iex | 1 | 2 | 1 | 1 | 3 |
| TLV (ppm) | 50 | 200 | 50 | 1 | 50 |
| Itox | 3 | 2 | 3 | 4 | 3 |
| I(fl+ex+tox)max | 8 | 7 | 8 | 8 | 8 |
| Unit | Type of Unit | Temperature (°C) | Pressure (bar) | Inventory (t) | Material |
|---|---|---|---|---|---|
| Culture | Tank | 25 | 1.013 | 10.00 | Stainless steel |
| Harvesting | Centrifuge | 25 | 1.013 | 10.00 | Stainless steel |
| Drying | Dryer | 71.38 | 1.013 | 6.06 | Stainless steel |
| Lipid extraction | Column | 40 | 1.013 | 5.48 | Stainless steel |
| Hexane recovery | Distillation column | 94.64 | 1.013 | 4.70 | Stainless steel |
| Transesterification | Reactor | 60 | 1.013 | 3.31 | Hastelloy-c 200 |
| Neutralization | Reactor | 55 | 1.013 | 3.31 | Hastelloy-c 200 |
| Methanol recovery | Distillation column | 81.24 | 1.013 | 3.13 | Stainless steel |
| Washing | Tank | 70 | 1.013 | 0.44 | Stainless steel |
| Biodiesel purification | Flash separator | 138.61 | 0.10 | 0.21 | Stainless steel |
| Glycerol purification | Distillation column | 100.70 | 1.013 | 0.23 | Stainless steel |
| Unit | Type of Unit | Temperature (°C) | Pressure (bar) | Inventory (t) | Material |
|---|---|---|---|---|---|
| Culture | Tank | 25 | 1.013 | 10.00 | Stainless steel |
| Harvesting | Centrifuge | 25 | 1.013 | 10.00 | Stainless steel |
| Drying | Dryer | 71.38 | 1.013 | 1.06 | Stainless steel |
| Transesterification | Reactor | 60 | 1.013 | 2.75 | Hastelloy-c 200 |
| Neutralization | Reactor | 55 | 1.013 | 2.15 | Hastelloy-c 200 |
| Methanol recovery | Distillation column | 99.53 | 1.013 | 1.84 | Stainless steel |
| Washing | Tank | 70 | 1.013 | 0.41 | Stainless steel |
| Biodiesel purification | Flash separator | 134.87 | 0.10 | 0.24 | Stainless steel |
| Glycerol purification | Distillation column | 100.04 | 1.013 | 0.17 | Stainless steel |
| Unit | Type of Unit | Temperature (°C) | Pressure (bar) | Inventory (t) | Material |
|---|---|---|---|---|---|
| Culture | Tank | 25 | 1.013 | 10.00 | Stainless steel |
| Harvesting | Centrifuge | 25 | 1.013 | 10.00 | Stainless steel |
| HTL | Reactor | 350 | 200 | 4.76 | Hastelloy-c 200 |
| Char separation | Separator | 60 | 0.9 | 4.76 | Hastelloy-c 200 |
| Bio-crude separation | Separator | 87.22 | 0.9 | 4.75 | Hastelloy-c 200 |
| Hydrotreating | Reactor | 400 | 100 | 0.63 | Hastelloy-c 200 |
| Fractioning | Tank | 155.60 | 1.013 | 0.63 | Stainless steel |
| Biodiesel purification | Flash separator | 165.09 | 1.43 | 0.20 | Stainless steel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Delgado, A.D.; García-Martínez, J.B.; Barajas-Solano, A.F. Evaluation of Algae-Based Biodiesel Production Topologies via Inherent Safety Index (ISI). Appl. Sci. 2021, 11, 2854. https://doi.org/10.3390/app11062854
González-Delgado AD, García-Martínez JB, Barajas-Solano AF. Evaluation of Algae-Based Biodiesel Production Topologies via Inherent Safety Index (ISI). Applied Sciences. 2021; 11(6):2854. https://doi.org/10.3390/app11062854
Chicago/Turabian StyleGonzález-Delgado, Angel Darío, Janet B. García-Martínez, and Andrés F. Barajas-Solano. 2021. "Evaluation of Algae-Based Biodiesel Production Topologies via Inherent Safety Index (ISI)" Applied Sciences 11, no. 6: 2854. https://doi.org/10.3390/app11062854
APA StyleGonzález-Delgado, A. D., García-Martínez, J. B., & Barajas-Solano, A. F. (2021). Evaluation of Algae-Based Biodiesel Production Topologies via Inherent Safety Index (ISI). Applied Sciences, 11(6), 2854. https://doi.org/10.3390/app11062854








