Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA composites
2.3. Characterization
2.3.1. Differential Scanning Calorimetry
2.3.2. Polarizing Light Microscopy (PLM)
2.3.3. Thermogravimetric Analysis (TGA)
3. Results and Discussion
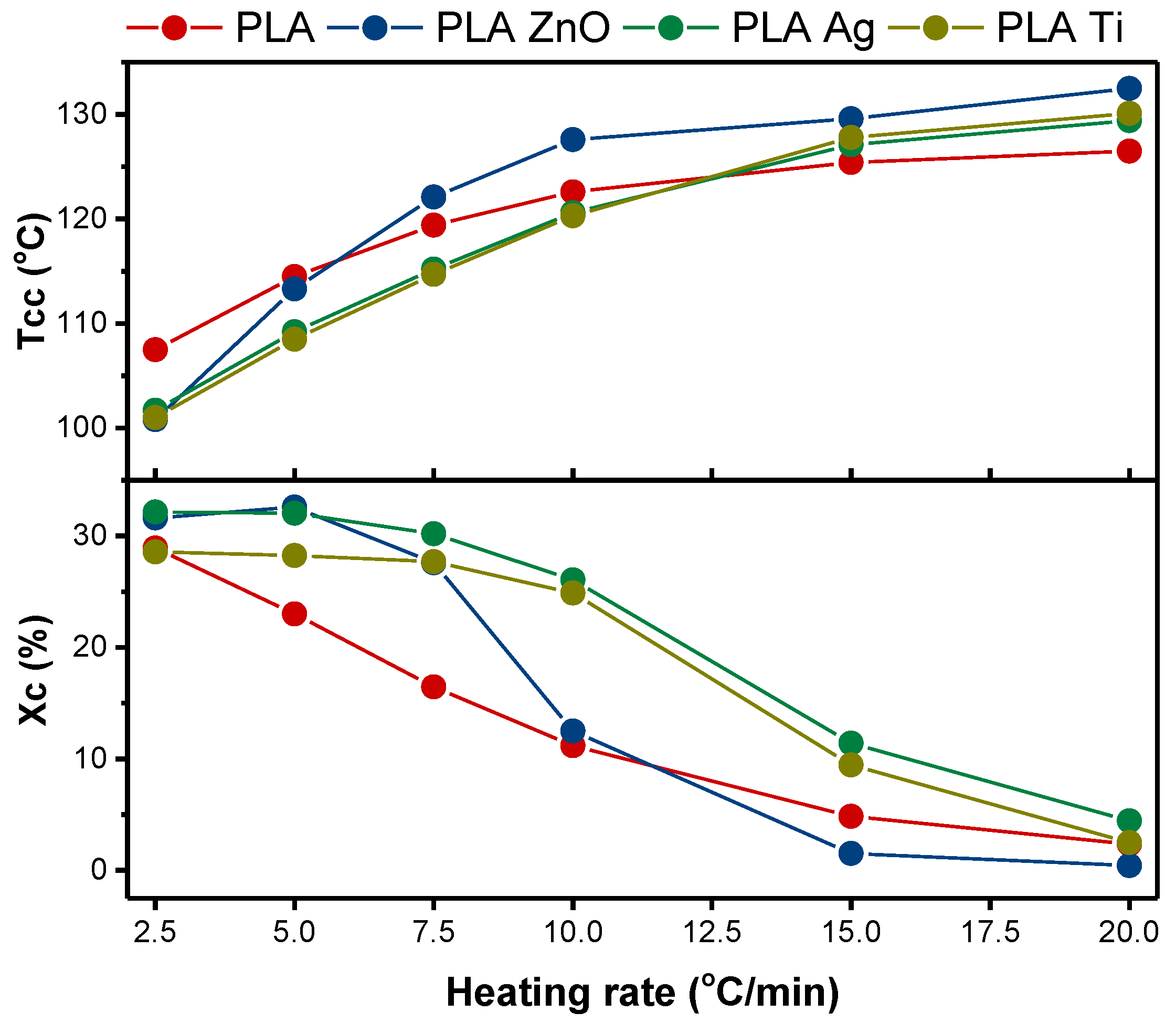
3.1. Non-Isothermal Cold Crystallization
3.1.1. Ozawa Theory
3.1.2. Mo Theory
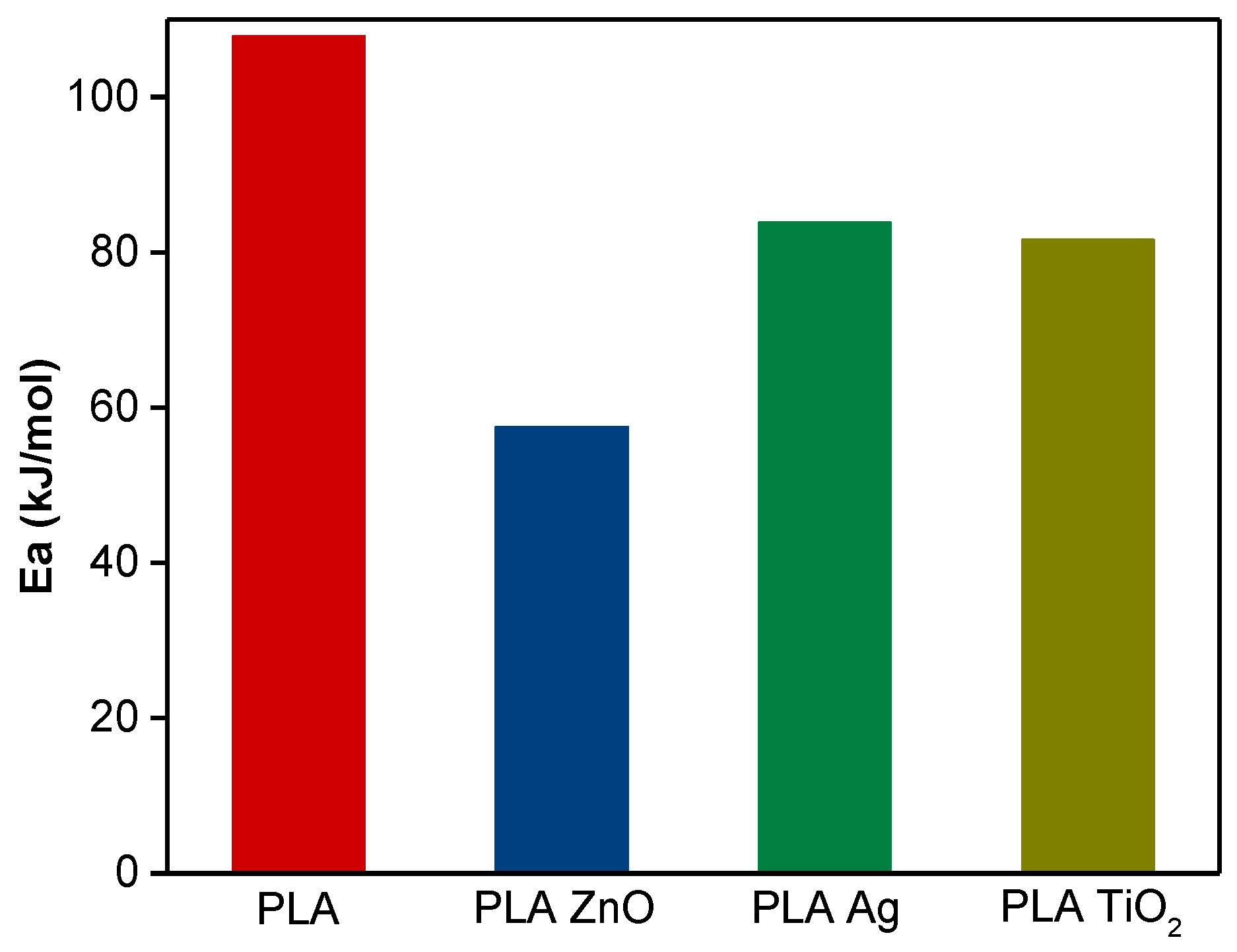
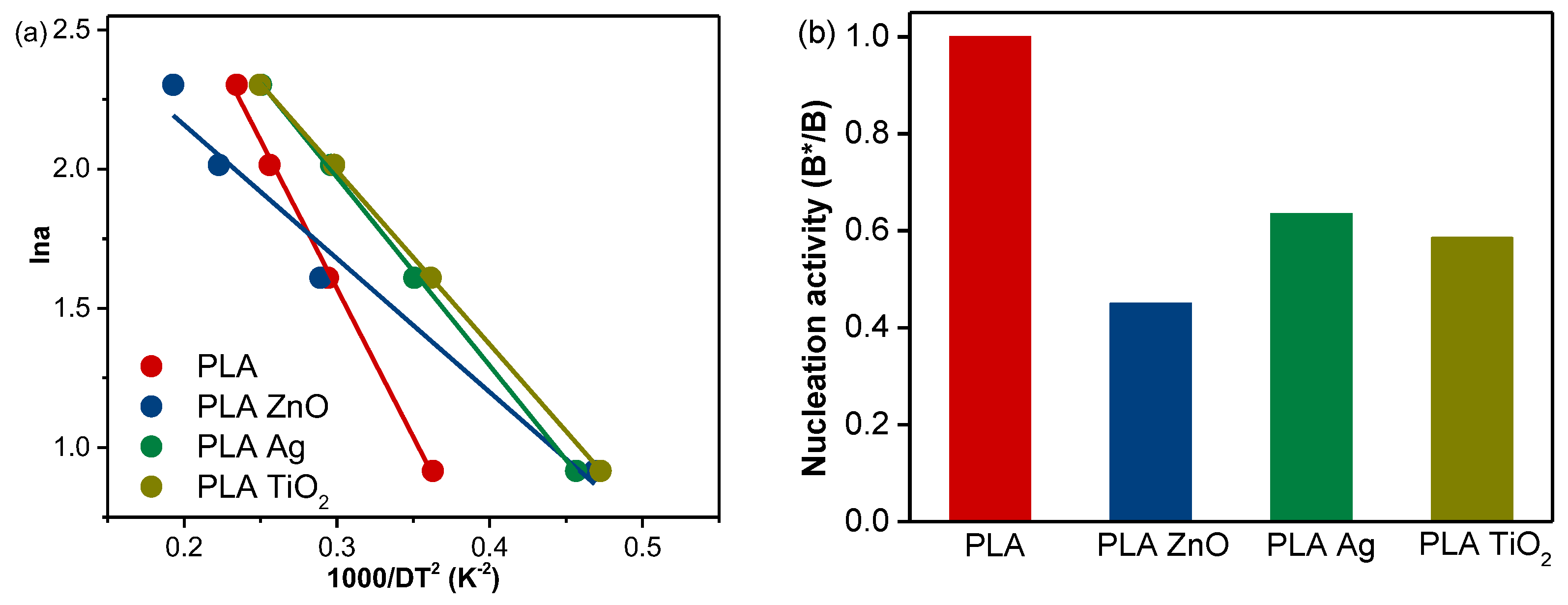
3.2. Activation Energy of Non-Isothermal Cold Crystallization
3.3. Nucleation Activity
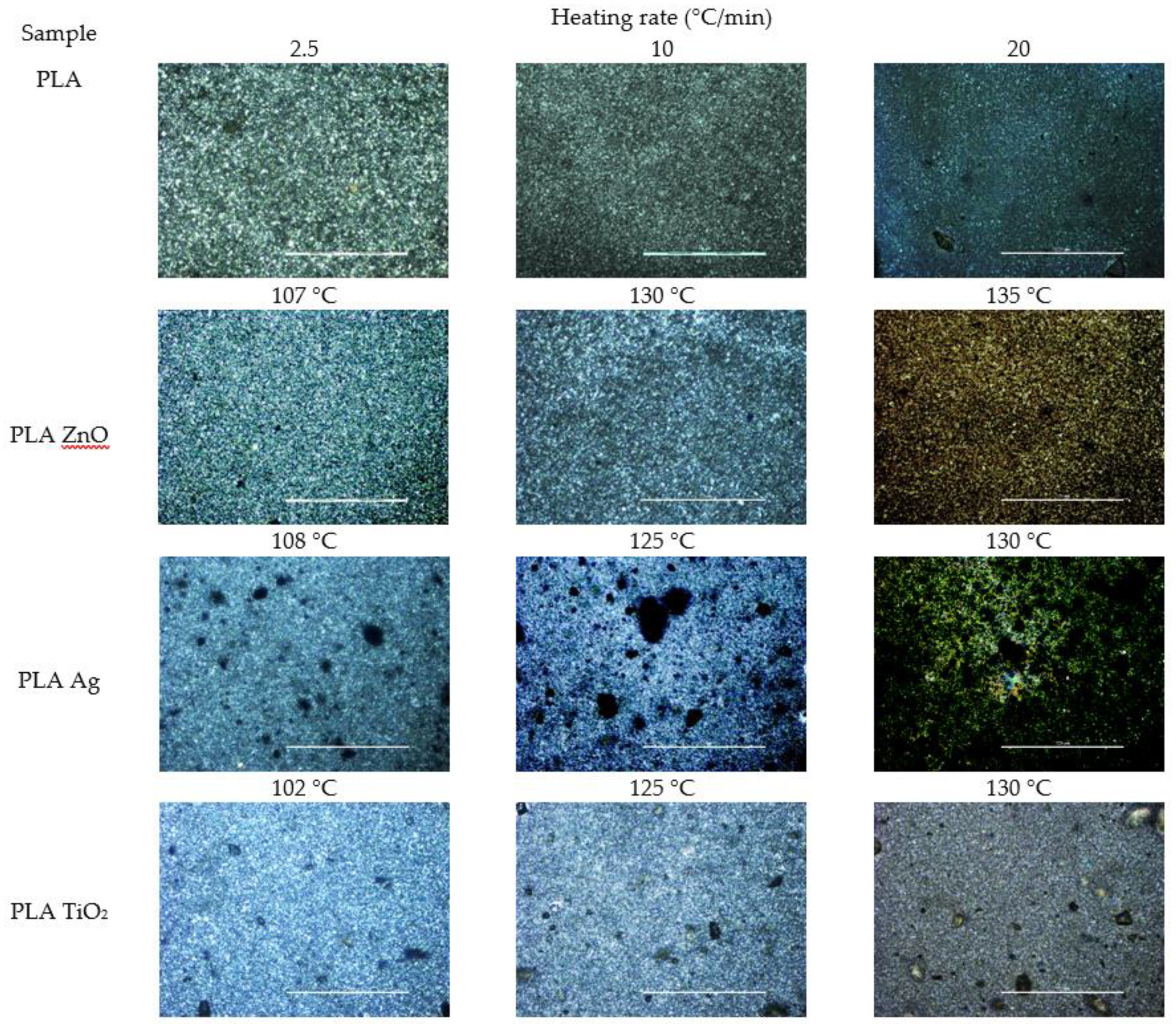
3.4. PLM Observations
3.5. Thermal Degradation Study of PLA Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Bioplastics e.V. (EUBP), Bioplastics Market Development Update 2020. 2020. Available online: https://www.european-bioplastics.org/news/publications/#MarketData (accessed on 10 February 2021).
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; De Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Biobased Building Blocks and Polymers—Global Capacities, Production and Trends, 2018–2023. Ind. Biotechnol. 2019, 15, 237–241. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Aït Hocine, N. Effect of rigid nanoparticles and preparation techniques on the performances of poly(lactic acid) nanocomposites: A review. Polym. Adv. Technol. 2021, 32, 444–460. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid amorphous fraction and segmental dynamics in nanocomposites based on poly(L–lactic acid) and nano-inclusions of 1–3D geometry studied by thermal and dielectric techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- Klonos, P.; Kripotou, S.; Kyritsis, A.; Papageorgiou, G.Z.; Bikiaris, D.; Gournis, D.; Pissis, P. Glass transition and segmental dynamics in poly(l-lactic acid)/graphene oxide nanocomposites. Thermochim. Acta 2015, 617, 44–53. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Prokopiou, L.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Klonos, P.; Kyritsis, A.; Pissis, P. In situ prepared poly(DL-lactic acid)/silica nanocomposites: Study of molecular composition, thermal stability, glass transition and molecular dynamics. Thermochim. Acta 2018, 669, 16–29. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Q.; Kim, J.K. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 100708. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for food packaging applications: An overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Antibacterial activities of aliphatic polyester nanocomposites with silver nanoparticles and/or graphene oxide sheets. Nanomaterials 2019, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.d. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- González, E.A.S.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2 for antibacterial packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Zhao, X.; Wang, X.; Zhou, L.; Yang, Y.; Liao, F.; Ju, Y. Poly(lactic acid)/graphene oxide-ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J. Chem. Technol. Biotechnol. 2015, 90, 1677–1684. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Erem, A.D.; Ozcan, G.; Erem, H.H.; Skrifvars, M. Antimicrobial activity of poly(l-lactide acid)/silver nanocomposite fibers. Text. Res. J. 2013, 83, 2111–2117. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Lee, S.M.; Kit, A.Y. ZnO deposited/encapsulated halloysite-poly (lactic acid) (PLA) nanocomposites for high performance packaging films with improved mechanical and antimicrobial properties. Appl. Clay Sci. 2015, 111, 10–20. [Google Scholar] [CrossRef]
- Sirotkin, N.A.; Gurina, D.L.; Khlyustova, A.V.; Costerin, D.Y.; Naumova, I.K.; Titov, V.A.; Agafonov, A.V. Experimental and computational investigation of polylactic acid/silver-NP nanocomposite with antimicrobial activity prepared by plasma in liquid. Plasma Process. Polym. 2020. [Google Scholar] [CrossRef]
- Wang, X.J.; Huang, Z.; Wei, M.Y.; Lu, T.; Nong, D.D.; Zhao, J.X.; Gao, X.Y.; Teng, L.J. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. [Google Scholar] [CrossRef]
- Petchwattana, N.; Narupai, B. Synergistic Effect of Talc and Titanium Dioxide on Poly(lactic acid) Crystallization: An Investigation on the Injection Molding Cycle Time Reduction. J. Polym. Environ. 2019, 27, 837–846. [Google Scholar] [CrossRef]
- Mendoza, G.; Peña-Juárez, M.G.; Gonzalez-Calderon, J.A.; Pérez, E. Use of chemically modified titanium dioxide particles to mediate the non-isothermal cold crystallization of poly(latic acid). J. Mex. Chem. Soc. 2020, 64, 44–63. [Google Scholar] [CrossRef]
- Nomai, J.; Suksut, B.; Karl Schlarb, A. Crystallization Behavior of Poly(lactic acid)/Titanium Dioxide Nanocomposites. KMUTNB Int. J. Appl. Sci. Technol. 2015, 1–8. [Google Scholar] [CrossRef]
- Luyt, A.S.; Gasmi, S. Influence of TiO2 Nanoparticles on the Crystallization Behaviour and Tensile Properties of Biodegradable PLA and PCL Nanocomposites. J. Polym. Environ. 2018, 26, 2410–2423. [Google Scholar] [CrossRef]
- Bussiere, P.O.; Therias, S.; Gardette, J.L.; Murariu, M.; Dubois, P.; Baba, M. Effect of ZnO nanofillers treated with triethoxy caprylylsilane on the isothermal and non-isothermal crystallization of poly(lactic acid). Phys. Chem. Chem. Phys. 2012, 14, 12301–12308. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- NatureWorks, Crystallizing and Drying of PLA, Tech. Resour. 2007. Available online: http://www.natureworksllc.com/Technical-Resources/4-Series.aspx (accessed on 23 March 2021).
- Makovec, D.; Sajko, M.; Selišnik, A.; Drofenik, M. Magnetically recoverable photocatalytic nanocomposite particles for water treatment. Mater. Chem. Phys. 2011, 129, 83–89. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Kinetics Neo; How Fast Are Your Chemical Reactions? Available online: https://kinetics.netzsch.com/en/ (accessed on 18 February 2021).
- Su, Z.; Liu, Y.; Guo, W.; Li, Q.; Wu, C. Crystallization behavior of poly(lactic acid) filled with modified carbon black. J. Macromol. Sci. Part B Phys. 2009, 48, 670–683. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Puglia, D.; Terenzi, A.; Berglund, L.A.; Kenny, J.M. Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym. Degrad. Stab. 2012, 97, 2027–2036. [Google Scholar] [CrossRef]
- Trasi, N.S.; Taylor, L.S. Effect of polymers on nucleation and crystal growth of amorphous acetaminophen. CrystEngComm 2012, 14, 5188–5197. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Terzopoulou, Z.; Bikiaris, D.; Triantafyllidis, K.S.; Diamanti, E.; Gournis, D.; Klonos, P.; Giannoulidis, E.; Pissis, P. Evaluation of the formed interface in biodegradable poly(l-lactic acid)/graphene oxide nanocomposites and the effect of nanofillers on mechanical and thermal properties. Thermochim. Acta 2014, 597, 48–57. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, H.; Liu, W.; Zhang, M.; Du, Z.; Wang, X. The synergistic effect of zinc oxide and phenylphosphonic acid zinc salt on the crystallization behavior of poly (lactic acid). Polym. Degrad. Stab. 2015, 122, 25–35. [Google Scholar] [CrossRef]
- Ries, A.; Canedo, E.L.; Souto, C.R.; Wellen, R.M.R. Non-isothermal cold crystallization kinetics of poly(3-hydoxybutyrate) filled with zinc oxide. Thermochim. Acta 2016, 637, 74–81. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Vyazovkin, S. Nonisothermal crystallization of polymers: Getting more out of kinetic analysis of differential scanning calorimetry data. Polym. Cryst. 2018, 1, e10003. [Google Scholar] [CrossRef]
- Li, M.; Hu, D.; Wang, Y.; Shen, C. Nonisothermal crystallization kinetics of poly(lactic acid) formulations comprising talc with poly(ethylene glycol). Polym. Eng. Sci. 2010, 50, 2298–2305. [Google Scholar] [CrossRef]
- Somsunan, R.; Mainoiy, N. Isothermal and non-isothermal crystallization kinetics of PLA/PBS blends with talc as nucleating agent. J. Therm. Anal. Calorim. 2020, 139, 1941–1948. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Yu, Y.; Xiao, L.; Shao, Y. Isothermal and nonisothermal cold crystallization kinetics of poly(l-lactide)/functionalized eggshell powder composites. J. Therm. Anal. Calorim. 2018, 131, 2213–2223. [Google Scholar] [CrossRef]
- dos Santos Silva, I.D.; Schäfer, H.; Jaques, N.G.; Siqueira, D.D.; Ries, A.; de Souza Morais, D.D.; Haag, K.; Koschek, K.; Carvalho, L.H.; Ramos Wellen, R.M. An investigation of PLA/Babassu cold crystallization kinetics. J. Therm. Anal. Calorim. 2020, 141, 1389–1397. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Wu, L.; Xu, B.; Zhang, Y.; Zhang, M. Nonisothermal cold crystallization behavior and kinetics of polylactide/clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1100–1113. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Isothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone) (PEEKK). Eur. Polym. J. 1997, 33, 1405–1414. [Google Scholar] [CrossRef]
- Durmus, A.; Ercan, N.; Soyubol, G.; Deligöz, H.; Kaşgöz, A. Nonisothermal crystallization kinetics of poly (ethylene terephthalate)/clay nanocomposites prepared by melt processing. Polym. Compos. 2010, 31, 1056–1066. [Google Scholar] [CrossRef]
- Xiong, H.; Gao, Y.; Li, H.M. Non-isothermal crystallization kinetics of syndiotactic polystyrene–polystyrene functionalized SWNTs nanocomposites. Lett 2007, 1, 416–426. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Canedo, E.L. On the Kissinger equation and the estimate of activation energies for non-isothermal cold crystallization of PET. Polym. Test. 2014, 40, 33–38. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Cheng, Q.; Zhang, N.; Cai, S.; Ren, J. Non-isothermal crystallization kinetics of ramie fiber-reinforced polylactic acid biocomposite. RSC Adv. 2017, 7, 46014–46021. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S. Activation energies and temperature dependencies of the rates of crystallization and melting of polymers. Polymers 2020, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- El-Taweel, S.H.; Abboudi, M. Nonisothermal crystallization kinetics of PLA/nanosized YVO4 composites as a novel nucleating agent. J. Appl. Polym. Sci. 2020, 137, 48340. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Beslikas, T.; Gigis, J.; Christoforides, J.; Bikiaris, D.N. Crystallization and enzymatic hydrolysis of PLA grade for orthopedics. Adv. Polym. Technol. 2010, 29, 280–299. [Google Scholar] [CrossRef]
- Patwa, R.; Singh, M.; Kumar, A.; Katiyar, V. Kinetic modelling of thermal degradation and non-isothermal crystallization of silk nano-discs reinforced poly (lactic acid) bionanocomposites. Polym. Bull. 2019, 76, 1349–1382. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Z.; Yan, S.; Yang, W. Crystallization behavior of biodegradable poly (L-lactide)/multiwalled carbon nanotubes nanocomposites from the amorphous state. Polym. Eng. Sci. 2011, 51, 1564–1573. [Google Scholar] [CrossRef]
- Bosq, N.; Guigo, N.; Aht-Ong, D.; Sbirrazzuoli, N. Crystallization of poly (butylene succinate) on rapid cooling and heating: Toward enhanced nucleation by graphene nanosheets. J. Phys. Chem. C 2017, 121, 11915–11925. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S. Effect of molecular weight on the cold-crystallization of biodegradable poly (ethylene succinate). Thermochim. Acta 2007, 457, 41–54. [Google Scholar] [CrossRef]
- Bosq, N.; Guigo, N.; Persello, J.; Sbirrazzuoli, N. Melt and glass crystallization of PDMS and PDMS silica nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 7830–7840. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Approach to Evaluating the Hoffman–Lauritzen Parameters (U* and Kg) from the Overall Rates of Nonisothermal Crystallization. Macromol. Rapid Commun. 2004, 25, 733–738. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Thermal properties and cold crystallization kinetics of surface-treated banana fiber (BF)-reinforced poly (lactic acid)(PLA) nanocomposites. J. Therm. Anal. Calorim. 2013, 114, 1265–1278. [Google Scholar] [CrossRef]
- Boonying, S.; Sutapun, W.; Suppakarn, N.; Ruksakulpiwat, Y. Crystallization behavior of vetiver grass fiber-polylactic acid composite. Adv. Mater. Res. 2012, 410, 55–58. [Google Scholar] [CrossRef]
- Lee, S.-H.; Wang, S.; Teramoto, Y. Isothermal crystallization behavior of hybrid biocomposite consisting of regenerated cellulose fiber, clay, and poly(lactic acid). J. Appl. Polym. Sci. 2008, 108, 870–875. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Non-isothermal Crystallization Kinetics of Biobased Poly(ethylene 2,5-furandicarboxylate) Synthesized via the Direct Esterification Process. Macromol. Chem. Phys. 2014, 215, 2065–2074. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Achilias, D.S.; Nanaki, S.; Beslikas, T.; Bikiaris, D. PLA nanocomposites: Effect of filler type on non-isothermal crystallization. Thermochim. Acta 2010, 511, 129–139. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Cyras, V.P.; Manfredi, L.B. Non-isothermal crystallization kinetics from the melt of nanocomposites based on poly(3-hydroxybutyrate) and modified clays. Thermochim. Acta 2014, 594, 80–88. [Google Scholar] [CrossRef]
- Tarani, E.; Papageorgiou, G.Z.; Bikiaris, D.N.; Chrissafis, K. Kinetics of crystallization and thermal degradation of an isotactic polypropylene matrix reinforced with graphene/glass-fiber filler. Molecules 2019, 24, 1984. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. II. Experimental evidence. J. Non. Cryst. Solids 1993, 162, 13–25. [Google Scholar] [CrossRef]
- Dobreva, A.; Gutzow, I. Activity of substrates in the catalyzed nucleation of glass-forming melts. I. Theory. J. Non. Cryst. Solids 1993, 162, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Dou, Q.; Bai, Z.; Lu, Q. Non-isothermal crystallization behaviors and spherulitic morphology of poly(lactic acid) nucleated by a novel nucleating agent. J. Therm. Anal. Calorim. 2015, 122, 407–417. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of surface functionalization of halloysite nanotubes on synthesis and thermal properties of poly(ε-caprolactone). J. Mater. Sci. 2018, 53, 6519–6541. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tarani, E.; Kasmi, N.; Papadopoulos, L.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal decomposition kinetics and mechanism of in-situ prepared bio-based poly(propylene 2,5-furan dicarboxylate)/graphene nanocomposites. Molecules 2019, 24, 1717. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Tarani, E.; Terzopoulou, Z.; Bikiaris, D.N.; Kyratsi, T.; Chrissafis, K.; Vourlias, G. Thermal conductivity and degradation behavior of HDPE/graphene nanocomposites: Pyrolysis, kinetics and mechanism. J. Therm. Anal. Calorim. 2017, 129, 1715–1726. [Google Scholar] [CrossRef]
- Chrissafis, K. Detail kinetic analysis of the thermal decomposition of PLA with oxidized multi-walled carbon nanotubes. Thermochim. Acta 2010, 511, 163–167. [Google Scholar] [CrossRef]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]


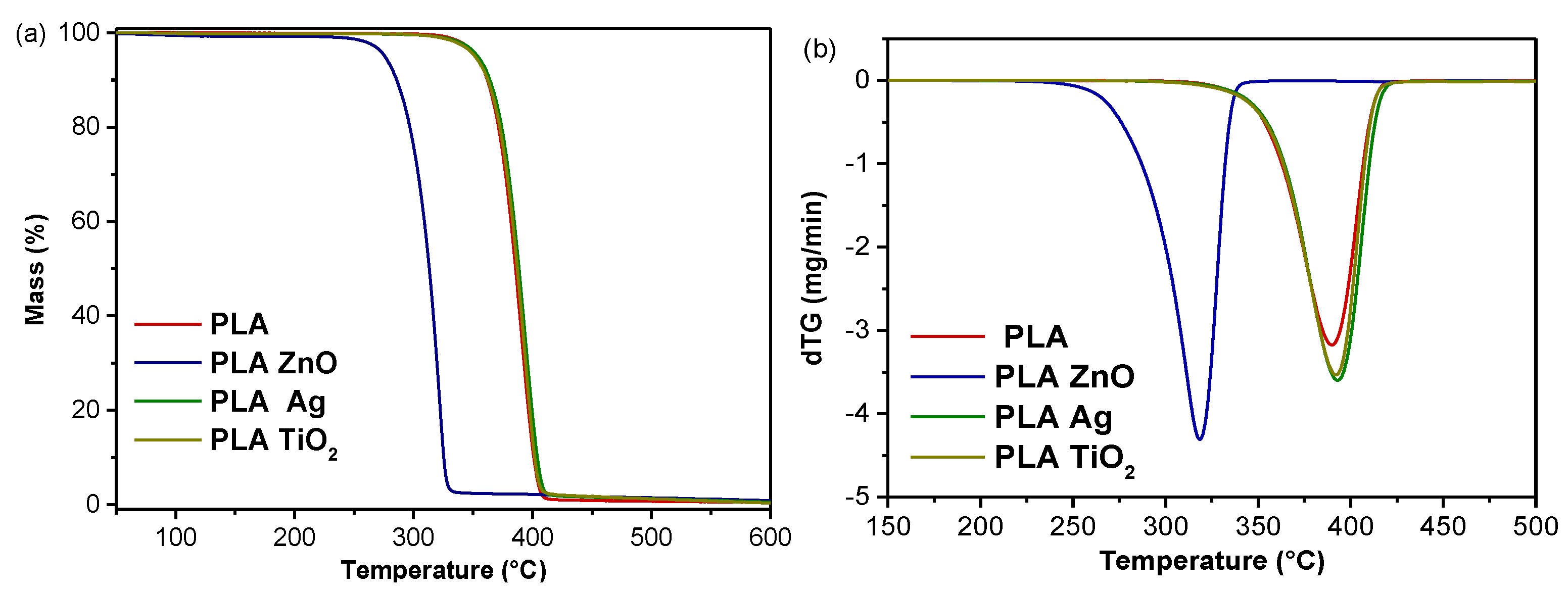
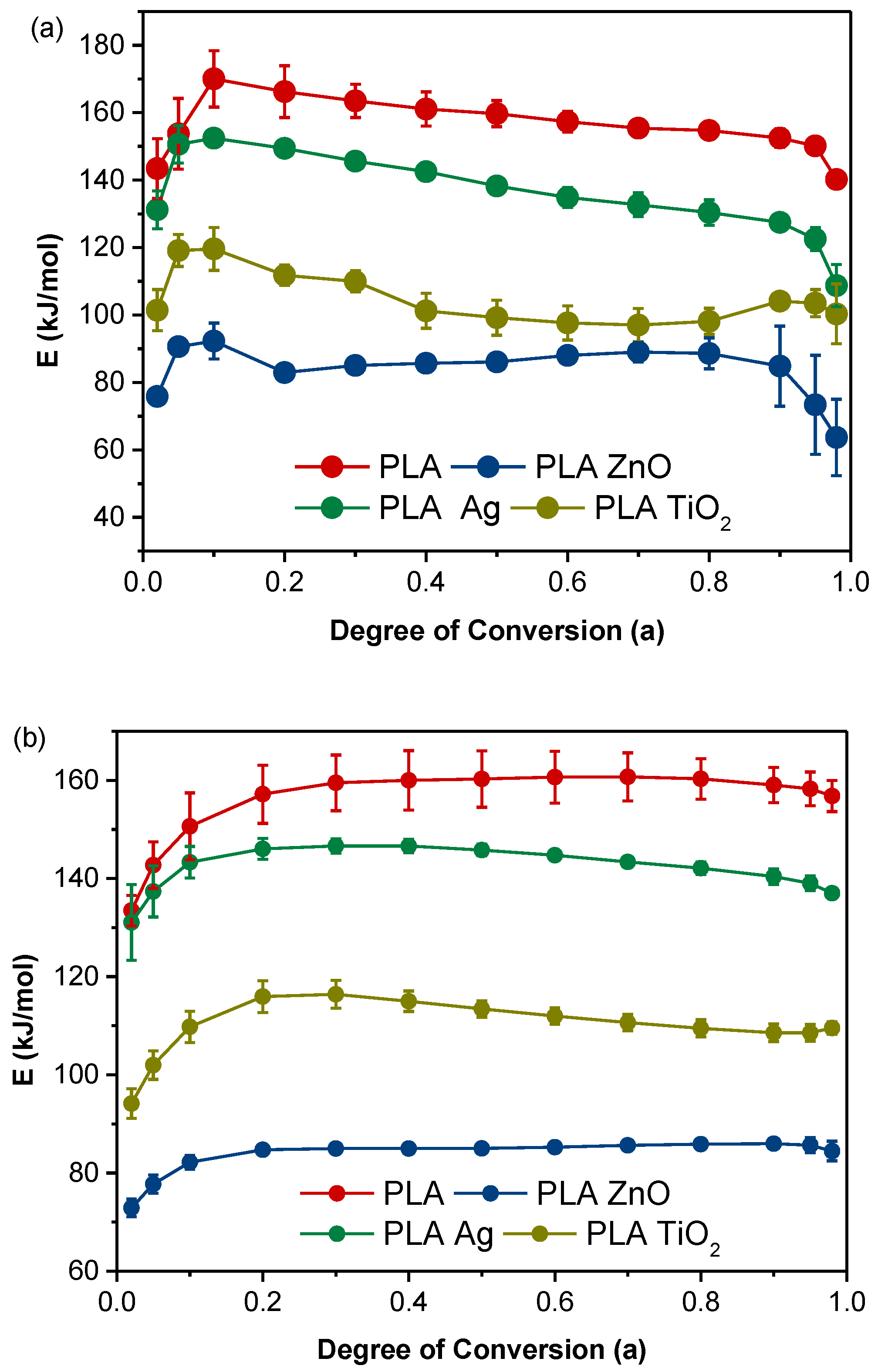

| Temperature (°C) | 100 | 105 | 110 | 115 | 120 | 125 | 130 |
|---|---|---|---|---|---|---|---|
| m | 2.10 | 2.28 | 1.70 | 1.33 | 1.09 | 0.92 | 0.90 |
| K(T) | 0.59 | 3.21 | 4.15 | 5.28 | 6.73 | 8.56 | 13.93 |
| R2 | 0.95359 | 0.97803 | 0.99907 | 0.99898 | 0.99793 | 0.99684 | 0.97468 |
| Sample | Kg (K2) | U* (kJ/mol) |
|---|---|---|
| PLA | 3.07 × 105 ± 5.9 × 104 | 5.52 ± 0.21 |
| PLA ZnO | 1.98 × 105 ± 4.8 × 104 | 2.56 ± 0.25 |
| PLA Ag | 2.96 × 105 ± 5.6 × 104 | 4.45 ± 0.25 |
| PLA TiO2 | 2.41 × 105 ± 5.2 × 104 | 3.76 ± 0.19 |
| Sample | Mechanism | Activation Energy/(kJ/mol) | Pre-Exponential Factor/s−1 | Reaction Order/n | Log Kcat | Regression Coefficient |
|---|---|---|---|---|---|---|
| PLA | Cn | 159.7 | 11.49 | 0.98 | 0.80 | 0.9997 |
| PLA ZnO | Cn | 84.5 | 4.86 | 0.64 | 1.17 | 0.9996 |
| PLA Ag | Cn | 143.5 | 8.96 | 0.88 | 0.92 | 0.9998 |
| PLA TiO2 | Cn | 110.9 | 6.01 | 0.92 | 1.33 | 0.9998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarani, E.; Pušnik Črešnar, K.; Zemljič, L.F.; Chrissafis, K.; Papageorgiou, G.Z.; Lambropoulou, D.; Zamboulis, A.; N. Bikiaris, D.; Terzopoulou, Z. Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers. Appl. Sci. 2021, 11, 3004. https://doi.org/10.3390/app11073004
Tarani E, Pušnik Črešnar K, Zemljič LF, Chrissafis K, Papageorgiou GZ, Lambropoulou D, Zamboulis A, N. Bikiaris D, Terzopoulou Z. Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers. Applied Sciences. 2021; 11(7):3004. https://doi.org/10.3390/app11073004
Chicago/Turabian StyleTarani, Evangelia, Klementina Pušnik Črešnar, Lidija Fras Zemljič, Konstantinos Chrissafis, George Z. Papageorgiou, Dimitra Lambropoulou, Alexandra Zamboulis, Dimitrios N. Bikiaris, and Zoi Terzopoulou. 2021. "Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers" Applied Sciences 11, no. 7: 3004. https://doi.org/10.3390/app11073004
APA StyleTarani, E., Pušnik Črešnar, K., Zemljič, L. F., Chrissafis, K., Papageorgiou, G. Z., Lambropoulou, D., Zamboulis, A., N. Bikiaris, D., & Terzopoulou, Z. (2021). Cold Crystallization Kinetics and Thermal Degradation of PLA Composites with Metal Oxide Nanofillers. Applied Sciences, 11(7), 3004. https://doi.org/10.3390/app11073004












