1. Introduction
Engineered landfills are among the most common and frequently used methods for the disposal of municipal solid waste (MSW) due to their simplicity, reliability, and cost-effectiveness [
1,
2,
3]. Nonetheless, due to three major bioprocesses (hydrolysis, aerobic, and anaerobic degradation), a large amount of leachate with high concentrations of ammonia, heavy metals, organic and inorganic compounds that are harmful to the environment and human health are generated in landfills [
4,
5]. Besides the production of leachate, landfills also generate a substantial amount of carbon dioxide (CO
2) resulting from the decomposition of the wastes deposited in the fill, which accounts for approximately 40–60%
v/v of total landfill gas [
6,
7,
8], thus constituting an urgent environmental issue [
7]. These generated by-products (leachate and CO
2), therefore, have the potential of contaminating the environment, groundwater, and soil if not adequately managed. Consequently, the prevention of soil and groundwater contamination and environmental degradation by the generated leachate and CO
2 is still a critical issue associated with engineered landfills.
To isolate the waste in the landfill from the surrounding environment, thereby minimizing soil and groundwater contamination, as well as environmental degradation, various types of hydraulic barriers have been proposed and used in engineered landfills [
1,
9,
10,
11,
12,
13,
14]. Over the past years, although landfill technology has seen major advancements, such as the development of synthetic liner materials, compacted clay liners (CCLs), however, still remain a critical component of liner systems in engineered landfills [
1,
3,
12,
15]. CCLs are commonly employed in barrier systems owing to their availability, low hydraulic conductivity, excellent compatibility with the permeating leachate, high attenuation capacity, and cost-effectiveness [
8,
9,
16]. However, in the field, CCLs can be damaged during installation. Likewise, after installation, failure mechanisms and environmental conditions including day-night temperature variations, freeze/thaw and dry/wet cycling’s, intrusion of biota, piping, hydraulic fracturing, unequal settlement, and desiccation can cause cracks in the CCLs [
12,
17,
18,
19]. These cracks can result in a decrease in the liner strength and an increase in hydraulic conductivity, leading to an increased possibility of groundwater and soil contamination. To improve the liner properties, natural, artificial, and waste materials, as well as advanced techniques, have been of great interest to researchers in recent decades.
Consequently, several studies have been conducted to address the aforementioned problem, among them include the modification of liner properties with various natural, synthetic, and waste additives such as lime, sand, bentonite, fly ash, fuel ash, zeolite, red mud, sepiolite, shale, silica fume, and pozzolan, cement, bagasse ash, etc., [
1,
18,
20,
21,
22,
23,
24,
25,
26,
27,
28]. A review of the above studies indicates that the various materials can be used to minimize the use of clays as liner materials and improve the liner’s engineering and hydraulic properties. Despite these efforts, there is still no sustainable and cost-effective way to seal cracks and maintain a low permeability throughout the liner’s required operational span. Therefore, developing innovative techniques to overcome this challenge has become of the utmost necessity. Under such circumstances, self-repairing or self-sealing of liner cracks without onerous labor and capital requirement is of great attraction and a state-of-the-art solution for addressing this challenge.
A self-sealing or self-repairing liner is one with the ability to lessen the increased hydraulic conductivity by forming precipitates of low permeability to fill up the pores resulting from the developed cracks [
29]. As the precipitates fill the pores, they reduce the hydraulic conductivity and consequently increase the liner’s strength. In recent years, a novel technique employed for minimizing industrial greenhouse emissions involves capturing CO
2 by reactive minerals to produce stable products such as carbonates and bicarbonates [
30,
31]. Studies by Cai et al. [
32], Yi et al. [
33], Wang et al. [
34] indicate that the stable carbonates and bicarbonates produced during this process can minimize the soil voids and increase the strength of soils if such reactive minerals are utilized as additives.
CO
2, the most common component of landfill gas constituting about 40–60%
v/v after methane, is considered the predominant greenhouse gas on earth due to its adverse effects on the environment. Engineered landfills are generally designed with gas collection systems to capture the generated CO
2. Nonetheless, complete capture is not feasible (approximately 84%
v/v gas capture for closed landfills, and 67%
v/v for open landfills) [
6]. Therefore, utilization of this adverse gas by reactive soil liners to improve their strength and hydraulic performance as well as their self-sealing efficiency through mineral carbonation seems a logical option and an innovative technique for counteracting the anthropogenic emissions of CO
2 and simultaneously preventing the contamination of groundwater and soil, as well as environmental degradation.
According to ASTM D5370, olivine is a sustainable, cost-effective, and widely available pozzolanic material (material with the ability to form cementitious compounds in the presence of water after reacting) with the potential to enhance soil strength characteristics owing to its strong affinity for CO
2 adsorption leading to the formation of stable carbonates with strength enhancement properties [
10,
35,
36]. Olivine carbonation has been and is still a leading mineral for the sequestration process, which involves converting magnesium-rich minerals to stable magnesium carbonate precipitates after reacting with CO
2 in the presence of water [
37]. The carbonated products may potentially bond the soil particles together, increase strength and reduce hydraulic conductivity. Recently, studies on carbonated olivine-admixed soils focus mainly on the strength characteristic [
38,
39,
40]. However, few if any studies have evaluated the hydraulic conductivity of carbonated olivine soils, which is one of the most critical parameters in assessing the suitability of a material for liner application. Moreover, knowledge about the required olivine particle size to be utilized for carbonation to achieve significant hydraulic conductivity reduction is also lacking. Furthermore, no study has attempted to employ a global optimization technique to optimize the control factors (olivine content, carbonation time, and carbonation pressure) so as to achieve maximum hydraulic conductivity reduction for the carbonated olivine-treated soil. As a result, the right combinations of the control factors for optimum reduction in carbonated-olivine treated soil’s hydraulic conductivity are not obvious.
In view of this, the purpose of the current study was to assess the suitability of CO2 carbonated olivine-admixed marine clay for use as a bottom liner material in engineered landfills to reduce leachate migration while sequestering CO2 from the landfill. The influence of carbonation time, carbonation pressure, and olivine content on the hydraulic conductivity of CO2-carbonated olivine-admixed marine clay was assessed using the attainable region (AR) and response surface methodology (RSM) techniques. The AR method was used to optimize the olivine particle size during the grinding process to get olivine particles in a defined particle size class (−75 µm) before treating the marine clay. The RSM approach was used to design the experiments, analyze the performance, and optimize the factors responsible for achieving significant hydraulic conductivity reduction of the CO2-carbonated olivine-admixed clay. The findings of this study will assist geotechnical and geoenvironmental engineers in designing a smart liner material that would be economically viable, socially acceptable, and environmentally sustainable with the potential to sequester landfill CO2, thus making it superior to the current conventional compacted clay liners. The findings of the study would also promote the use of marine clay typically considered as construction waste to value-added application as a liner material in engineered landfills. The results of the study would also open up a promising perspective for the use of AR and RSM to define the optimal olivine particle size and olivine content in targeting optimum hydraulic conductivity of the olivine-admixed soil during field application.
4. Summary and Conclusions
This work investigates the hydraulic conductivity of CO2-carbonated olivine-admixed marine clay for potential use as a liner material in engineered landfills. The AR technique was used to optimize the olivine particle size during the grinding process to get olivine particles in a defined particle size class before treating the marine clay, while RSM was used in designing the experiments, evaluating the results, and optimizing the variables responsible for reducing the hydraulic conductivity of the carbonated olivine-admixed clay. The effects of various factors, including olivine content, carbonation time, and carbonation pressure on the hydraulic conductivity, were studied by ANOVA. The major findings from the study are summarized as follows:
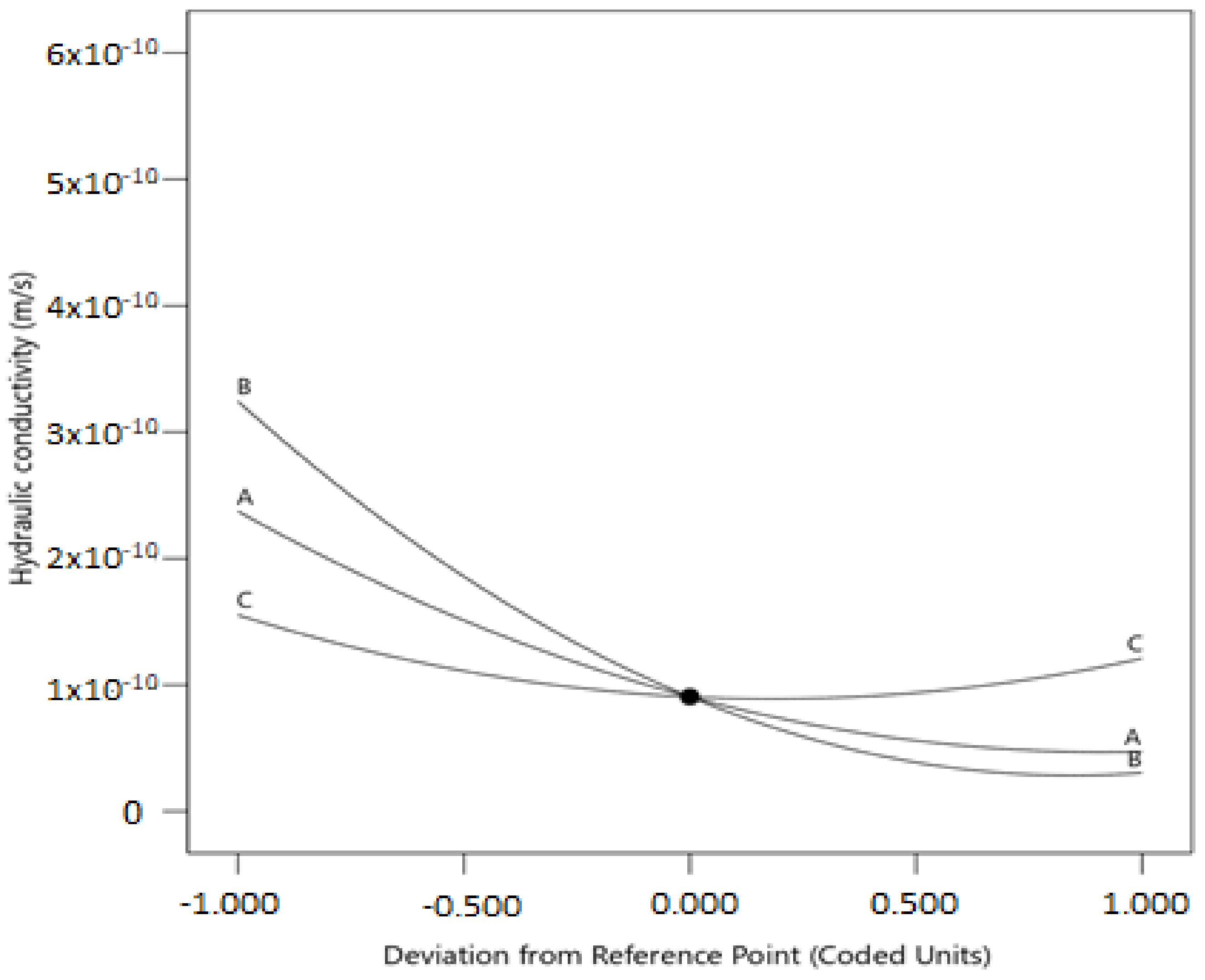
With increasing olivine content, carbonation time, and pressure, the hydraulic conductivity of the treated-clay decreased. The decrease in the hydraulic conductivity is due to the hydration reaction that resulted in the formation of M-S-H and M-A-H phases. Further hydraulic conductivity reduction is attributed to the formation of more carbonates, including nesquehonite, hydromagnesite, and dypingite.
The quadratic model was considered the best fitted and non-aliased model between the control factors and response. ANOVA analyses revealed that the main and quadratic effects of olivine content, carbonation time, and carbonation pressure significantly affected the hydraulic conductivity.
It was found that the predicted values from the model are in strong agreement with their experimental counterparts. Within the set of conditions applied in the current study, the model can be used for prediction.
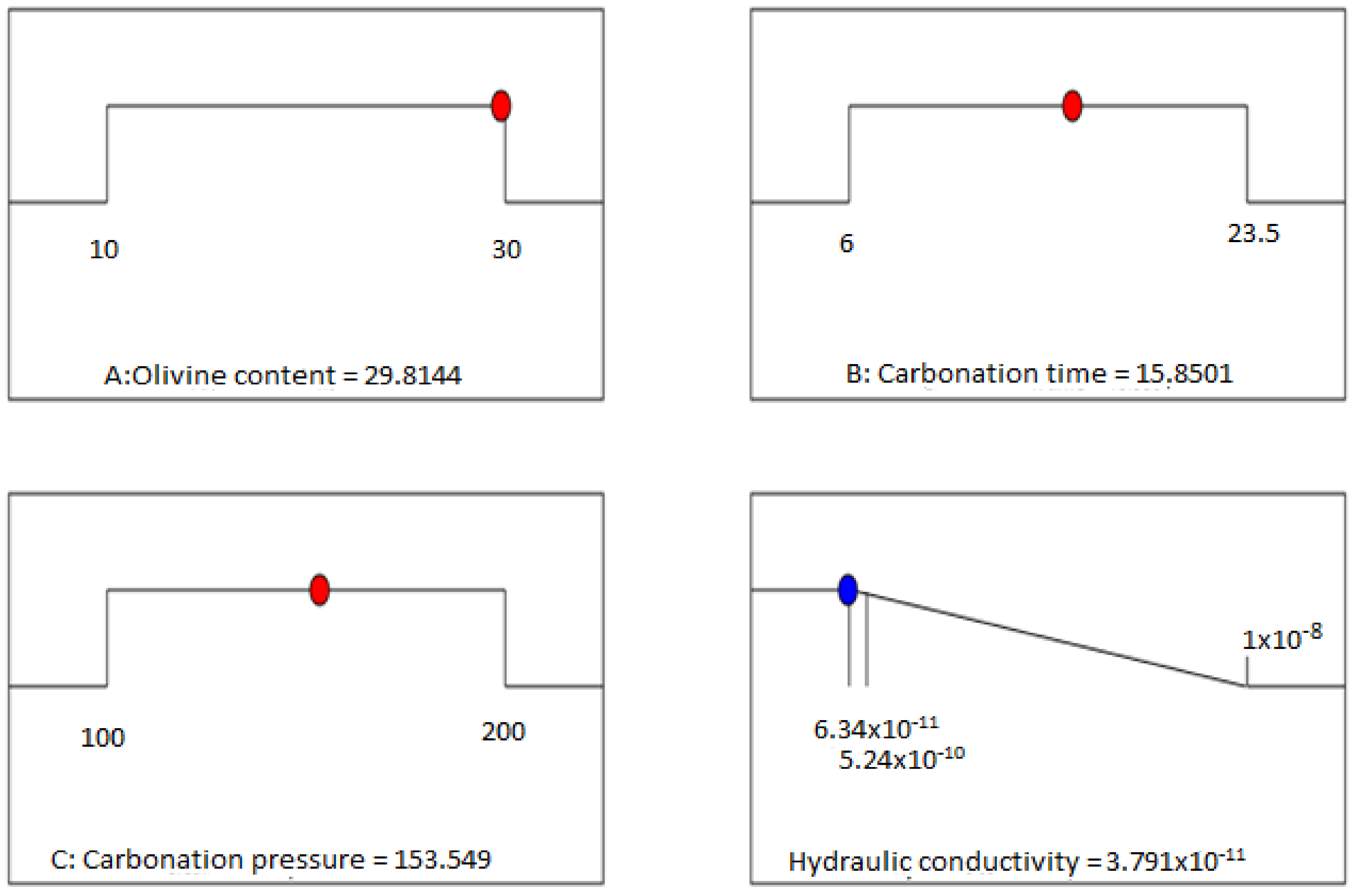
Based on the optimization study, the optimum value of the hydraulic conductivity was suggested as 2.0 × 10−11 m/s and was attained under the following operating conditions: 24.7% olivine content, 20.1 h carbonation time, and 161 kPa carbonation pressure, with a designated maximum desirability function (D = 1.0).
Comparative analyses of the hydraulic conductivity reduction of the carbonated samples and cured specimens demonstrate that CO2-carbonated olivine-admixed soils have a great capacity to sequestrate CO2 and minimize CO2 emissions while rapidly decreasing the hydraulic conductivity of soils significantly relative to cured specimens.
In summary, CO2-carbonation and olivine blend have proven to be a sustainable technique to reduce the hydraulic conductivity of marine clay, while employing the AR technique to optimize the olivine particle size and RSM to design the experiment and obtain optimum operating conditions in achieving maximum hydraulic conductivity reduction. The results indicate that the carbonated olivine-admixed clay can be used as a liner material in engineered landfills with the potential of sequestering landfill CO2. However, the above results are based mainly on the outcome of laboratory experiments under limited operating conditions. Hence, future studies are recommended to extend the tests to a broader range of conditions and to carry out prototype and field trials to establish techniques for its in-situ application. Consequently, it might also provide a clue on the cost-estimate of the technique. Moreover, studies are also recommended considering the application of this carbonation technique to soil specimens prone to cracking and using leachate as the permeating fluid. Finally, future studies are also recommended to elucidate this hydraulic conductivity reduction through microstructural investigations.