A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. BSG Characterization
2.2. Reactors Setup
2.2.1. BSG Hydrolysate as Feedstock
2.2.2. Batch Assays
2.2.3. Expanded Granular Sludge Bed Reactor (EGSB)
2.3. Analytical Methods
2.4. Calculations
2.4.1. BSG Characterization and Hydrolysis
2.4.2. Acidogenic Fermentation
3. Results and Discussion
3.1. Raw BSG Characterization and Pretreatment
3.2. Acidogenic Fermentation
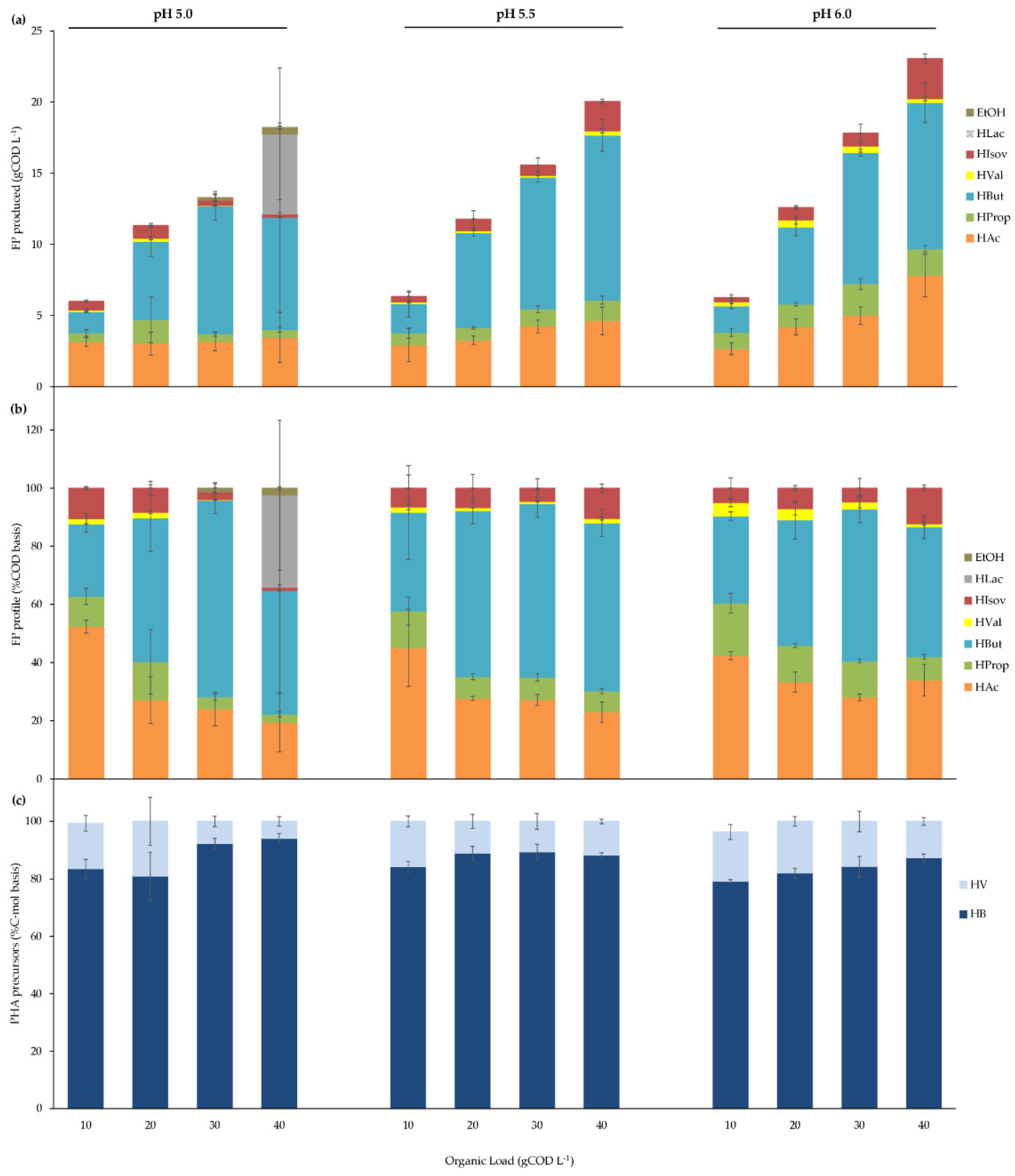
3.2.1. pH and Organic Load Effects on BSG Acidogenic Batch Fermentation
3.2.2. Continuous Acidogenic Fermentation of BSG Hydrolysate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.; Dragone, G.; Roberto, I. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Liang, S.; Wan, C. Carboxylic acid production from brewer’s spent grain via mixed culture fermentation. Bioresour. Technol. 2015, 182, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.R.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef]
- Caetano, N.S.; Moura, R.F.; Meireles, S.; Mendes, A.M.; Mata, T.M. Bioethanol from brewer’s spent grains: Acid pretreatment optimization. Chem. Eng. Trans. 2013, 35, 1021–1026. [Google Scholar] [CrossRef]
- Liguori, R.; Soccol, C.R.; Vandenberghe, L.P.D.S.; Woiciechowski, A.L.; Faraco, V. Second Generation Ethanol Production from Brewers’ Spent Grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Gírio, F.; Fonseca, C.; Carvalheiro, F.; Duarte, L.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Apha, A. WEF Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Lim, S.J.; Kim, T.-H. Applicability and trends of anaerobic granular sludge treatment processes. Biomass Bioenergy 2014, 60, 189–202. [Google Scholar] [CrossRef]
- Duque, A.F.; Oliveira, C.S.; Carmo, I.T.; Gouveia, A.R.; Pardelha, F.; Ramos, A.M.; Reis, M.A. Response of a three-stage process for PHA production by mixed microbial cultures to feedstock shift: Impact on polymer composition. New Biotechnol. 2014, 31, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.; Lemos, P.C.; Oehmen, A.; Reis, M.A. Denitrifying phosphorus removal: Linking the process performance with the microbial community structure. Water Res. 2007, 41, 4383–4396. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a Functional Germinated Barley Foodstuff from Brewer’s Spent Grain for the Treatment of Ulcerative Colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Gouveia, A.R.; Freitas, E.B.; Galinha, C.F.; Carvalho, G.; Duque, A.F.; Reis, M.A. Dynamic change of pH in acidogenic fermentation of cheese whey towards polyhydroxyalkanoates production: Impact on performance and microbial population. New Biotechnol. 2017, 37, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Pires, E.J.; Ruiz, H.A.; Teixeira, J.A.; Vicente, A.A. A New Approach on Brewer’s Spent Grains Treatment and Potential Use as Lignocellulosic Yeast Cells Carriers. J. Agric. Food Chem. 2011, 60, 5994–5999. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.P.; Trivedi, R.K. Acid and alkaline pretreatment of lignocellulosic biomass to produce ethanol as biofuel. Int. J. Chem.Tech. Res. 2013, 5, 727–734. [Google Scholar]
- Paz, A.; Sabo, S.D.S.; Vallejo, M.; Marguet, E.; Oliveira, R.P.D.S.; Domínguez, J.M. Using brewer’s spent grain to formulate culture media for the production of bacteriocins using Patagonian strains. LWT 2018, 96, 166–174. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.; Rooney, D.; Walker, G. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Seong, H.A.; Lee, J.S.; Yoon, S.Y.; Song, W.-Y.; Shin, S.-J. Fermentation characteristics of acid hydrolysates by different neutralizing agents. Int. J. Hydrogen Energy 2016, 41, 16365–16372. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Sonochemical synthesis of hydroxyapatite from H3PO4 solution with Ca(OH)2. Ultrason. Sonochem. 2001, 8, 85–88. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Serrano, A.; Chica, A.F.; Martín, M.A. Effect of variation in the C/[N+P] ratio on anaerobic digestion. Environ. Prog. Sustain. Energy 2019, 38, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Purwadi, R.; Niklasson, C.; Taherzadeh, M.J. Kinetic study of detoxification of dilute-acid hydrolyzates by Ca(OH)2. J. Biotechnol. 2004, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Rodriguez, M.E.; York, S.W.; Preston, J.F.; Ingram, L.O. Effects of Ca(OH)2 treatments(“overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol. Bioeng. 2000, 69, 526–536. [Google Scholar] [CrossRef]
- Roberto, I.; Lacis, L.; Barbosa, M.; de Mancilha, I. Utilization of sugar cane bagasse hemicellulosic hydrolysate by pichia stipitis for the production of ethanol. Process. Biochem. 1991, 26, 15–21. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Eklund, R.; Gustafsson, L.; Niklasson, A.C.; Lidén, G. Characterization and Fermentation of Dilute-Acid Hydrolyzates from Wood. Ind. Eng. Chem. Res. 1997, 36, 4659–4665. [Google Scholar] [CrossRef]
- Tamis, J.; Joosse, B.; Van Loosdrecht, M.; Kleerebezem, R. High-rate volatile fatty acid (VFA) production by a granular sludge process at low pH. Biotechnol. Bioeng. 2015, 112, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Temudo, M.F.; Mato, T.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Xylose anaerobic conversion by open-mixed cultures. Appl. Microbiol. Biotechnol. 2009, 82, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Vergine, P.; Sousa, F.; Lopes, M.; Silva, F.; Gameiro, T.; Nadais, H.; Capela, I. Synthetic soft drink wastewater suitability for the production of volatile fatty acids. Process. Biochem. 2015, 50, 1308–1312. [Google Scholar] [CrossRef]
- Temudo, M.F.; Kleerebezem, R.; Van Loosdrecht, M. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef]
- Lemos, P.C.; Serafim, L.S.; Reis, M.A. Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J. Biotechnol. 2006, 122, 226–238. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.; Kleerebezem, R. Butyrate as preferred substrate for polyhydroxybutyrate production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef]
- Shi, H.; Shiraishi, M.; Shimizu, K. Metabolic flux analysis for biosynthesis of poly(β-hydroxybutyric acid) in Alcaligenes eutrophus from various carbon sources. J. Ferment. Bioeng. 1997, 84, 579–587. [Google Scholar] [CrossRef]
- Kourmentza, C.; Kornaros, M. Biotransformation of volatile fatty acids to polyhydroxyalkanoates by employing mixed microbial consortia: The effect of pH and carbon source. Bioresour. Technol. 2016, 222, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Components (%, w/w) | This Study | Carvalheiro et al. [20] | Pires et al. [21] | Kanauchi et al. [18] |
|---|---|---|---|---|
| Moisture | 72.2 ± 0.1 | 80 | n.a. | n.a. |
| Asha | 3.40 ± 0.04 | 1.2 | 2.2 | 2.4 |
| Proteina | 26.9 ± 0.1 | 24.6 | 39.1 | 24.0 |
| Lipidsa | 11.5 ± 0.03 | n.a. | 10.5 | 10.6 |
| Carbohydratesa | 46.2 ± 0.06 b | 51.5 | 36.2 | 47.2 |
| Lignin a | 12 b | 21.7 | 25.6 | 11.9 |
| [Sugars] (g L−1) | Hydrolysis Efficiency (%) | Overall Hydrolysis Efficiency a (%) | |
|---|---|---|---|
| 1st hydrolysis | 32.4 ± 2.6 | 56.0 | 84.5 |
| 2nd hydrolysis | 16.4 ± 2.9 | 64.6 |
| Before Neutralization | After Neutralization | |
|---|---|---|
| Calcium (g L−1) | 1.12 ± 0.04 | 4.60 ± 0.33 |
| Magnesium (g L−1) | 1.76 ± 0.03 | 0.38 ± 0.04 |
| Sodium (mg L−1) | 5.06 ± 0.07 | 4.90 ± 0.01 |
| Sulfate (g L−1) | 66.6 ± 2.9 | 4.61 ± 1.8 |
| Furfural (g L−1) | 0.71 ± 0.07 | 0.26 ± 0.05 |
| 5-HMF (mg L−1) | 70 ± 30 | 30 ± 5 |
| Acetic acid (g L−1) | 1.13 ± 0.14 | 1.52 ± 0.001 |
| Glucose (g L−1) (gCOD L−1) | 3.47 ± 0.19 | 3.35 ± 0.31 |
| 3.68 ± 0.20 | 3.55 ± 0.33 | |
| Xylose (g L−1) (gCOD L−1) | 12.4 ± 2.8 | 9.20 ± 0.23 |
| 13.2 ± 2.9 | 9.75 ± 0.24 | |
| Arabinose (g L−1) (gCOD L−1) | 6.53 ± 0.60 | 4.94 ± 0.06 |
| 6.92 ± 0.64 | 5.23 ± 0.06 | |
| Total protein (g L−1) | 29.6 ± 1.9 | 19.7 ± 2.1 |
| NH4+ (gN L−1) | 0.69 ± 0.05 | 0.64 ± 0.001 |
| PO43− (mgP L−1) | 90 ± 10 | < 2 |
| COD (gO2 L−1) | 56.8 ± 0.7 | 54.6 ± 0.8 |
| C:N:P ratio (C-mol:N-mol:P-mol) | 100:31:60 | 100:33:2 |
| pH | 5.0 | 5.5 | 6.0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic Load (OL) (gCOD L−1) | 12.3 (0.4) | 22.6 (0.6) | 32.4 (0.6) | 35.8 (2.9) | 11.9 (0.3) | 22.6 (0.4) | 32.0 (1.5) | 39.2 (1.3) | 11.9 (0.5) | 23.7 (0.7) | 33.0 (0.1) | 39.9 (1.2) |
| FP produced (FP) (gCOD-FP L−1) | 6.01 (0.70) | 11.3 (1.7) | 13.3 (0.9) | 18.2 (0.5) | 6.34 (0.81) | 11.8 (0.9) | 15.6 (0.6) | 20.0 (1.8) | 6.27 (0.79) | 12.6 (0.5) | 17.8 (2.1) | 23.1 (2.2) |
| YFP/S (gCOD-FP gCOD−1) | 0.66 (0.11) | 0.69 (0.04) | 0.55 (0.04) | 0.60 (0.04) | 0.63 (0.04) | 0.57 (0.07) | 0.60 (0.05) | 0.44 (0.01) | 0.63 (0.03) | 0.60 (0.03) | 0.55 (0.02) | 0.52 (0.05) |
| rFP (mgCOD-FP L−1 h−1) | 42.2 (5.9) | 79.3 (14.9) | 93.2 (7.5) | 300 (76) | 45.0 (7.2) | 82.8 (8.4) | 170 (54) | 141 (15) | 44.5 (7.1) | 112 (36) | 125 (18) | 162 (19) |
| rFPmax (mgCOD-FP L−1 h−1) | 270 (34) | 531 (26) | 730 (58) | 915 (85) | 194 (15) | 502 (29) | 555 (14) | 745 (76) | 158 (15) | 383 (38) | 626 (47) | 1096 (82) |
| −rsmax (mgCOD L−1 h−1) | 405 (82) | 785 (50) | 1323 (128) | 1529 (119) | 300 (30) | 869 (251) | 916 (299) | 1692 (152) | 245 (24) | 609 (83) | 1146 (61) | 2104 (179) |
| qFP (mgCOD-FP gVSS−1 h−1) | 2.72 (0.44) | 4.45 (0.98) | 5.11 (0.83) | 16.2 (7.2) | 2.89 (0.51) | 4.29 (0.68) | 9.15 (3.07) | 9.78 (2.83) | 2.85 (0.73) | 6.45 (2.20) | 6.73 (1.42) | 10.3 (4.0) |
| qFPmax (mgCOD-FP gVSS−1 h−1) | 17.1 (3.4) | 29.8 (4.7) | 37.3 (7.4) | 50.2 (15.8) | 12.4 (1.8) | 24.5 (6.7) | 28.4 (9.8) | 48.0 (5.3) | 9.40 (1.70) | 20.2 (3.6) | 32.4 (6.6) | 71.6 (24) |
| −qsmax (mgCOD gVSS−1 h−1) | 25.5 (3.5) | 44.1 (1.4) | 67.3 (0.9) | 83.8 (7.2) | 19.1 (0.4) | 42.5 (5.8) | 46.8 (7.7) | 108 (4) | 14.7 (1.6) | 32.7 (1.2) | 59.5 (2.5) | 133 (13) |
| Degree of fermentation (DF) (gCOD-FP gCOD−1) | 0.49 (0.04) | 0.50 (0.06) | 0.41 (0.03) | 0.51 (0.06) | 0.53 (0.06) | 0.52 (0.04) | 0.49 (0.03) | 0.51 (0.03) | 0.52 (0.05) | 0.53 (0.01) | 0.54 (0.07) | 0.58 (0.05) |
| OLR (gCOD L−1 d−1) | 8.64 ± 0.60 |
| FP produced (FP) (gCOD L−1) | 9.00 ± 1.59 |
| rFP (mgCOD-FP L−1 h−1) | 147 ± 24 |
| -rS (mgCOD L−1 h−1) | 360 ± 22 |
| qFP (mgCOD-FP gVSS−1 h−1) | 13.8 ± 1.7 |
| -qS (mgCOD gVSS−1 h−1) | 34.2 ± 5.5 |
| FP profile [HAc/HProp/ETOH /HBut/HIsov/HVal] (%COD basis) | 22/1.2/4/3/69/2 |
| HB:HV a (%C-mol basis) | 49:51 |
| YFP/S (gCOD-FP gCOD−1) | 0.58 ± 0.1 |
| Degree of Fermentation (DF) (gCOD-FP gCOD−1) | 0.41 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarda, E.C.; Oliveira, A.C.; Antunes, S.; Freitas, F.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Appl. Sci. 2021, 11, 3222. https://doi.org/10.3390/app11073222
Guarda EC, Oliveira AC, Antunes S, Freitas F, Castro PML, Duque AF, Reis MAM. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Applied Sciences. 2021; 11(7):3222. https://doi.org/10.3390/app11073222
Chicago/Turabian StyleGuarda, Eliana C., Ana Catarina Oliveira, Sílvia Antunes, Filomena Freitas, Paula M. L. Castro, Anouk F. Duque, and Maria A. M. Reis. 2021. "A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation" Applied Sciences 11, no. 7: 3222. https://doi.org/10.3390/app11073222









