Abstract
Dental caries (decay) is caused by pathogenic bacterial species, which afflicts nearly a third of the world’s population from early childhood to old age. Treatment of tooth decay often involves the use of filling materials to restore the cavity; however, if untreated, it can cause pain, infection and eventually lead to tooth loss. Since the oral environment is colonised by many different microorganisms, bacterial biofilms can form on these filling materials, contributing to secondary caries that can eventually lead to the failure of the dental restoration. Thus, preventing the formation of bacterial biofilms is an important strategy in the management of caries, which has led to research enabling antimicrobial capabilities in dental materials. Materials and pharmaceutical sciences are in a continuous race against microbial resistance but are trying to balance between beneficial biota associated with the oral cavity, and, of course, avoiding a harmful effect on tissues is challenging. This has, therefore, stemmed a substantial interest in both preventive and restorative measures that would enable limiting the formation of secondary caries, oral microbial biofilms, and the retention of tooth mineral. Thus, innovative strategies are being explored and here we present a review with a focus on strategies that can inhibit or limit the formation of bacterial biofilms.
1. Introduction
Antimicrobial resistance (AMR) is now recognized as a global burden by the World Health Organization [1]. AMR occurs when microorganisms such as bacteria, fungi, viruses, and parasites evolve because of exposure to antimicrobial drugs. Consequently, the antimicrobial drugs become ineffective and infections persist in the body, increasing the risk of spread to others, thereby threatening our ability to treat common infectious diseases [2]. It is estimated that AMR could cause 10 million deaths per year by the year 2050 and 300 million people are expected to die prematurely because of drug resistance over the next 35 years and the world’s GDP will be 2 to 3.5% lower than it otherwise would be in 2050 [3]. These statistics are alarming and clearly emphasize the importance of not only treating infections, but also reducing, controlling, and finding alternative solutions.
The major challenges associated with AMR can be attributed to the lack of new classes of antibiotics reaching the market since 1987 [4]. Today, there are very few novel antibiotics in advanced stages of drug development, especially against the most troublesome pathogens, resistant Gram-negative bacteria. Moreover, no single antibiotic can provide a solution, as multi-drug-resistant bacteria have emerged around the world, and different bacterial infections require different antibiotics, with the development of resistance making constant innovation necessary [5]. The absence of efficient and low-cost rapid diagnostics is also an obstacle, as uncertainty about the cause of infection drives the inappropriate use of antibiotics. Furthermore, resistant bacteria do not respect international borders, especially in the current interconnected world of trade and travel. Resistance originating in one place can spread rapidly worldwide, in some cases in a matter of weeks. Travelers to areas with high AMR prevalence are likely to be exposed to resistant bacteria and return to their countries colonised. This means that misuse of antibiotics anywhere in the world is enough to overturn achievements in containing resistance elsewhere [6].
2. Antimicrobials Used in Dentistry
The oral cavity encounters a host of microorganisms that cause biofilms to form, leading to plaque formation that subsequently results in tooth decay. Dental caries is a highly prevalent non-communicable disease that occurs when plaque-associated bacteria generate acid that causes damage to tooth tissue. Active dental caries irreversibly damage and eventually kill teeth; hence, combatting this disease has been one of the priorities in improving global oral health. Despite significant measures being implemented that range from preventive to restorative procedures, this disease continues to affect approximately a third of the world’s population [7]. To prevent dental caries, it is vital to control the formation of oral microbial biofilms, and for dental restorations specifically it is important that it not only resists biofilm formation but is able to maintain tooth mineral and/or promote mineralisation [8]. Advances in the field of biomaterials have led to excellent materials that are clinically used to repair and restore the function of tooth tissue due to loss or disease. Among others, natural polymers such as chitosan, peptides and some biobased polyesters show promising potential for the reconstruction of damaged or lost tissue, especially when infections impair their regenerative processes [9]. However, the propensity of the formation of biofilms in the oral environment necessitates designing synthetic materials with complex functionalities such as antimicrobial and remineralising capacities. Since dental caries have been recognized as a disease induced by cariogenic bacteria, significant research has focused on developing restorative systems possessing antibacterial properties in dental materials science. Thus, controlling bacterial contamination around and beneath restorations is expected to decrease the risk of further demineralisation and cavitation and help prevent forming secondary caries [10,11]. Many different approaches have been investigated to minimise the burden of the effects of oral biofilms, such as preventive measures including antibacterial agents in restorative systems, polymeric materials with antibacterial properties and their release.
Preventive measures to improve general oral health that are commercially available range from fluoride-containing toothpastes, dentifrices and mouthwashes with antibacterial agents [12]. However, there are many different polymeric systems used in dentistry for the restoration of teeth that are affected by caries; therefore, significant research has focused on rendering these with antibacterial properties. Dental resin composite restorations are the most widely used restorative materials and are bonded to teeth using adhesive systems. The tooth tissue is prepared using an acid that demineralises it to render a porous structure that enables the interlocking of the resin either in the enamel etched pits or the exposed collagen lattice in dentine. The adhesive systems typically comprise photopolymerisable methacrylate monomers that are used following the preparation of the tooth surface. One of the main reasons for failure is the breakdown of the tooth-restorative adhesive interface, which can occur due to the hydrolytic cleavage of the bond, collagenolytic activity that destabilises the bond and ingress of salivary enzymes and microorganisms in micro-gaps formed at the interface leading to the formation of secondary caries due to bacterial activity [13,14,15]. Hence, research and clinical applications have focused on developing dental composites and adhesive systems that can reduce both recurrent caries and degradation of the adhesive interface [16,17]. However, if the carious lesion is left untreated or the restoration of choice is unable to provide an effective seal against further bacterial ingress, the infection can persist to involve the dental pulp and the tooth’s root canal system.
Root canal treatment involves the removal of an infected root canal through procedures such as mechanical debridement or disinfection through chemical treatments such as irrigation with sodium hypochlorite that can subsequently be hermetically sealed with appropriate filling materials. The preparation of the root canal is important and the complete removal of bacterial species from the canal is a vital step towards minimising reinfection and current approaches consider that antimicrobial agents introduced during the root canal preparation can facilitate the removal of residual bacteria within this complex anatomical area [18,19]. This review highlights the current antimicrobials and research in the field of dentistry, especially as the direct depot release of antibacterial agents from restorative materials is not effective in the long term and, in addition, some additives can be detrimental to the properties of the restoratives and to the tooth tissue.
2.1. Chlorhexidine
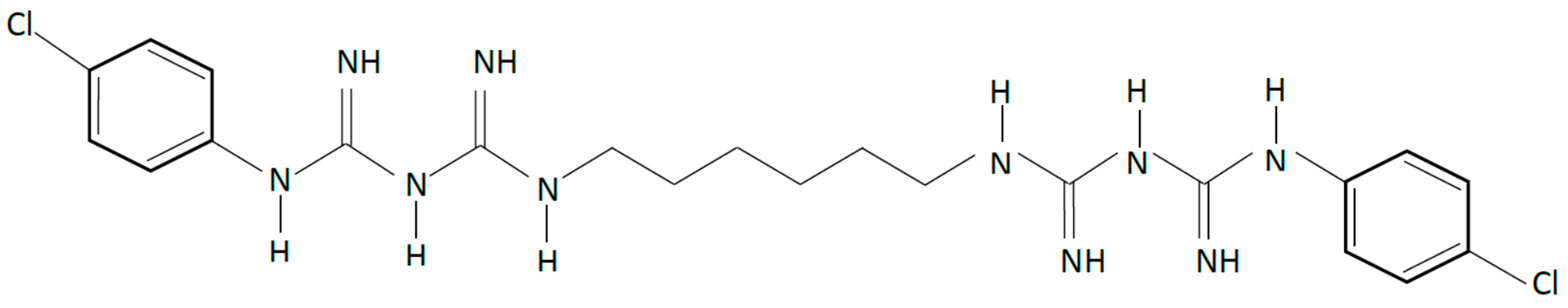
Chlorhexidine (CHX) is a cationic compound with broad-spectrum antibacterial activity (Figure 1). It causes disruption of cellular membranes and has been used successfully as an antimicrobial agent in dentistry for the management of oral biofilms [20,21,22]. It is a key component in oral formulations such as mouthwashes and oral gels that can be used topically in the management of oral biofilms. Combining CHX in polymeric matrices have been attempted for sustained delivery specifically in polyalkenoate cements, commonly known as glass ionomer cements (GIC) that contain water soluble polymers such as poly (acrylic acid), poly (maleic) and poly(itaconic acid), which undergo an acid-base reaction to yield a cement. Although incorporation of CHX in GIC enables better protection against caries, it results in cements with inferior mechanical properties that exhibit a less than optimal period of sustained release [22,23]. Other derivatives of CHX such as CHX diacetate and chloride have also been incorporated in GICs; however, using CHX diacetate exhibits release over a few days, whilst a very rapid release occurs with CHX chloride. Barbour et al. [24] reported a CHX hexametaphosphate salt that exhibited lower solubility, and laboratory studies showed that incorporation and release from GICs could be sustained over 48 months [23], this being dependent on dose. This compound was also explored for release from elastomeric ligatures [25] used in orthodontic treatments, and the experimental results showed that functionalisation of these elastomeric polymers with CHX hexametaphosphate under controlled conditions did not have detrimental effects on the mechanical properties; however, further work will be required to ascertain its appropriate function. Although there is clear evidence that CHX is effective for the control of specific conditions such as controlling plaque and gingivitis [26,27], it is important to consider antimicrobial resistance and adverse reactions when designing drug delivery systems despite the fact that controlled release from polymeric systems is potentially more efficacious, especially for targeted release.
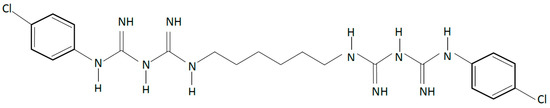
Figure 1.
Chemical structure of chlorhexidine (CHX), a cationic compound with broad-spectrum antibacterial activity.
Apart from its antimicrobial activity, CHX has been shown to inhibit the activity of matrix metalloproteinases (MMPs) 2, 8 and 9 as well as cysteine cathepsins (CPs) [28,29]. At concentrations as low as 0.2%, CHX has been reported to preserve the collagen matrix in hybrid layers and reduce the degradation of resin–dentine bonds [30,31]. Since MMPs depend on calcium to maintain their tertiary structure and zinc to perform their catalytic activity [32,33], it is hypothesized that CHX acts by inhibiting the binding of calcium and zinc ions to the MMPs, thereby reducing their catalytic activity [34]. Since establishing the role of CHX as an efficient enzyme inhibitor, several efforts have been made to incorporate it into the components of dental adhesive systems [35,36]. However, since the binding of the cationic CHX to the anionic sites in mineralised and demineralised dentine (phosphate group in hydroxyapatite and carboxyl group in collagen) is electrostatic, this is found to be reversible within 18 months [37,38]. Deterioration is further accelerated when ʺwet bondingʺ is performed with etch-and-rinse adhesives or hydrophilic self-etch adhesives, as CHX may leach out due to the hydrolytic medium created by the incompletely removed water [39].
2.2. Quaternary Salts
Quaternary ammonium compounds (QACs) form a group of cationic antimicrobials that possess a positively charged head with a hydrophobic tail, which is usually a chain of alkyl groups, and the number of units governs the hydrophobicity of the molecule [40]. Although the mechanism of the antibacterial activity of these molecules is not clearly understood, it is broadly associated with the adsorption of QACs on the bacterial cell wall that then penetrates it, leading to the disruption of the cell structure, eventually causing disorganisation of the cells [41]. Since monomers with quaternary ammonium salts are not dependent on the release of an active agent to impart antibacterial activity, these systems are considerably more efficacious than systems that rely on the release of an active agent. Thus, employing QACs in dental materials to inhibit microbial activity led to polymerisable quaternary ammonium methacrylates, as integrating methacrylates into these compounds seemed to improve their efficiency, and this efficacy was directly related to the alkyl chain length of the quaternary ammonium groups [40]. 12-methacryloyloxydodecylpyridinium bromide [42], an analogue of cetylpyridinium chloride, methacryloxylethyl cetyl dimethyl ammonium chloride, and poly(2-methyloxazoline) [43] are some examples of monomeric methacrylates with pendant quaternary ammonium salt species that have been incorporated in dental composite/adhesive systems to impart intrinsic antibacterial properties in dental composite restorations. Furthermore, improved antimicrobial performance has been reported by combining two quaternary ammonium groups with one polymerisable group [44], and by crosslinking the antimicrobial monomers through two polymerisable groups [45]. QACs have since been incorporated into various dental materials, including composite resins [37,38,46,47], adhesive systems [39,40,41,48,49,50], acrylic resins [50], and bone cements [51], that have demonstrated effective antimicrobial properties. Among these tested compounds, the polymerisable QAC, 12-methacryloyloxydodecylpyridinium bromide (MDPB), well known for its potent antimicrobial activity, has been incorporated into a commercially available adhesive system. Although MDPB has demonstrated a good ability to inhibit MMP activity [52] as well as the preservation of bond strengths for up to one year [53], some studies report overall lower bond strengths compared to other adhesive systems [54,55].
2.3. Tetracyclines
Antibiotics are commonly prescribed by dentists to either prevent infection or to treat infections, which also adds to the burden of antibiotic resistance. Most antibiotics are prescribed for systemic use; however, local delivery of tetracyclines as an antimicrobial and cationic chelating agent has been explored. Tetracyclines, a family of broad-spectrum antibiotics with cationic chelating properties, have shown collagenase- and gelatinase-inhibiting properties [56,57,58]. In addition to their antimicrobial activity, tetracyclines can chelate the catalytic zinc ions found within MMP enzymes [59,60]. Of the tetracyclines and their semi-synthetic analogues doxycycline and minocycline, doxycycline is the most potent, due to its greater affinity for zinc ions, which led to the incorporation of this antibiotic into restorative materials [61,62]. Although results demonstrated successful antibiofilm effects, bacterial resistance against tetracyclines is common, and due to their photo-oxidative potential, tetracyclines can cause purple staining of teeth, limiting their use clinically [57,63]. However, their anti-inflammatory, antibacterial, anticollagenase, and immunosuppressive properties have seen the use of doxycyclines in bone scaffolds to promote bone growth and reduce peri-implantitis [64,65].
2.4. Antibacterial Monomers in Dental Restorative Systems
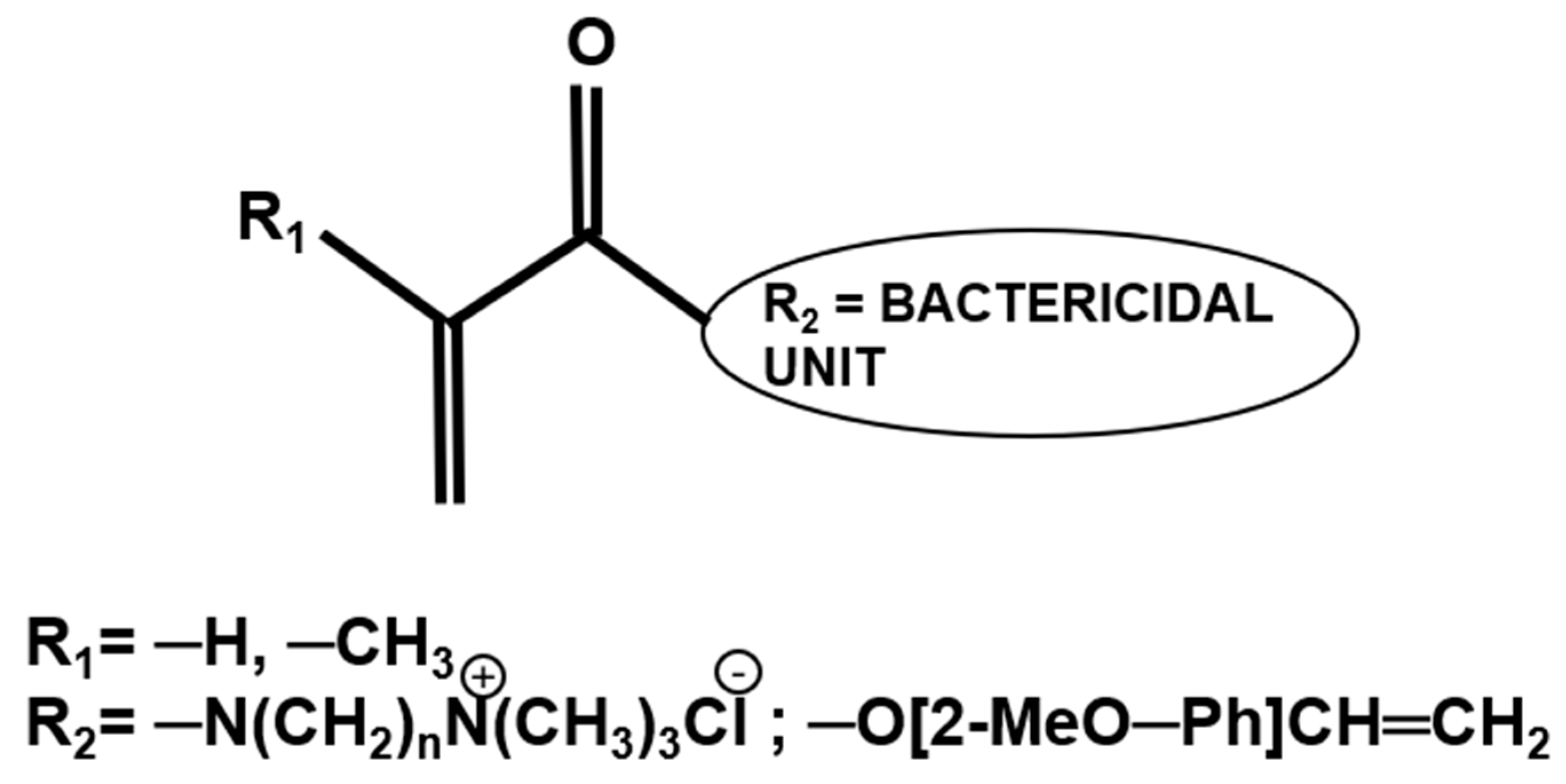
Dental adhesive systems that exhibit intrinsic antibacterial properties are not reliant on the release of any antibacterial agents and either function through contact killing as in the case of quaternary ammonium salts or the presence of charged species [66,67,68,69,70]. There are a few QACs that have been investigated in experimental adhesive formulations (Section 2.2); however, the balance of bactericidal effects and properties of the polymerised systems needs further evaluation. Another approach, which has shown promising results but so far is limited to laboratory evaluation, is utilising (meth-)acrylate derivatives of molecules such as eugenol or N,N-dimethylammonium derivatives that have an established bactericidal effect and has been used as polymer therapeutics in dental applications (Figure 2) [71].
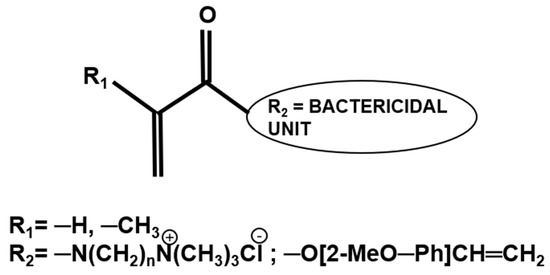
Figure 2.
Chemical structure of (meth)acrylate (R1) monomers bearing antibacterial pendant groups (R2).
A number of methacrylate derivatives based on mono, di and polysaccharide methacrylate monomers synthesised from glucose, sucrose and chitosan, respectively, with glycidyl methacrylate added to dental adhesive formulations have been reported to exhibit promising antibacterial action [72,73], with the sucrose-methacrylate derivative outperforming the others [74]. A decrease in bacterial metabolism was reported with sucrose-methacrylate that exhibited low toxicity and increased the bond strength after 6 months of storage. More recently, 5-(methacryloxy-2-ethoxyaminocarbonyl)methyl-thiazole (MEMT) [75] and ester-free quaternary ammonium monomers based on methacrylamides with side alkyl chain lengths of 9 or 14 carbons have been reported with antimicrobial properties [76] for potential use in dentistry. The antibacterial activity of MEMT-containing polymers was mainly associated with the release of unreacted monomer and surface adhesion on the immobilised MEMT units. Similarly, the methacrylamides exhibited their antimicrobial activity by virtue of the charge due to quaternary ammonium species. Another approach has also been recently reported that applies a dual strategy of bacterial gene modification with dimethyl amino hexadecyl methacrylate as an antibacterial monomer [77]. These systems have the potential to imbibe antimicrobial properties in dental materials; however, the following section focuses specifically on intrinsically antibacterial methacrylate derivatives of eugenol, which form comonomers in dental formulations that exhibit promising potential for imbibing antimicrobial properties.
2.4.1. Eugenol and Eugenyl Methacrylate
Eugenol (4-allyl-2-methoxyphenol), a member of the allylbenzene class of chemical compounds, is a major constituent (72–90%) of the essential oil extracted from cloves. Cloves are the flower buds found on the tree in the family Myrtaceae, Syzygium aromaticum. Eugenol possesses analgesic, anti-inflammatory, antifungal and antibacterial activity. It has been used in dentistry since the 19th century due to its ability to relieve pain in irritated or diseased tooth pulp. Currently, it is available as a pulp capping agent, root canal sealer and temporary filling material in combination with zinc oxide in the form of zinc oxide eugenol (ZOE). Eugenol inhibits the growth of several microorganisms, including facultative anaerobes commonly isolated from infected root canals, and produces a soothing effect on the pulp [78]. However, unreacted eugenol in ZOE cements may produce tissue irritation and induce an inflammatory reaction in the oral mucous membranes. Another drawback of ZOE cements is their weak mechanical properties, as demonstrated by their low strength, poor resistance to abrasion, and disintegration in the oral cavity with time [79,80]. Moreover, ZOE cements interfere with the polymerisation reaction of methacrylate-based restorative resins due to the remaining free eugenol [81,82]. Eugenol is a strong antioxidant and a potent free-radical inhibitor, which can cause a detrimental effect on the physical and mechanical properties if an overlying permanent dental resin-based composite material is used. Thus, complete cleaning of the tooth cavity of any residual eugenol is necessary if a restoration that cures via free-radical polymerisation is to be applied. However, modifying the chemical structure of eugenol by incorporating a polymerisable group, i.e., a methacrylate group, allows the eugenol derivative to participate in polymerisation reactions rather than inhibit them [83,84]. Hence, the incorporation of this new derivative can be advantageous in the field of dental materials or bone cements [84], whilst benefitting from the bactericidal effects of eugenol.
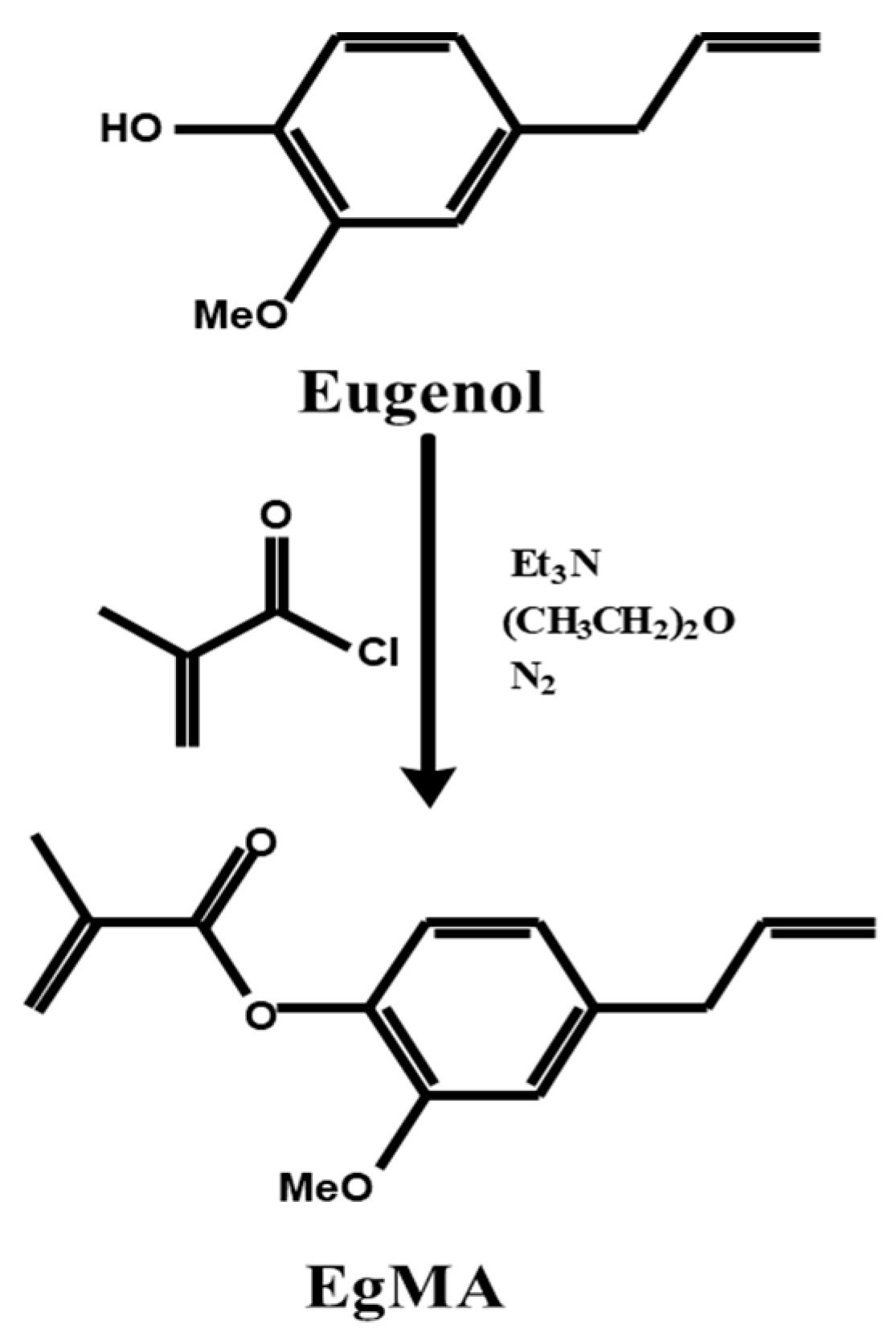
An acrylic derivative of eugenol was synthesised through Fischer esterification [83] using stoichiometric amounts of eugenol and triethylamine dissolved in diethyl ether and reacted with an excess of methacryloyl chloride at room temperature under nitrogen atmosphere (Figure 3). The triethylamine chlorhydrate formed was removed from the reaction medium and unreacted reagents eliminated through successive extraction and purified via flash distillation and column chromatography to yield eugenyl methacrylate. The acrylic derivative of eugenol, in which the acrylic residue is directly bonded to the aromatic ring of eugenol, was successfully synthesised directly from eugenol with a product yield of 80%. The esterification reaction was confirmed by NMR and FTIR spectroscopies showing presence of the bands corresponding to the methacrylic residue [83].
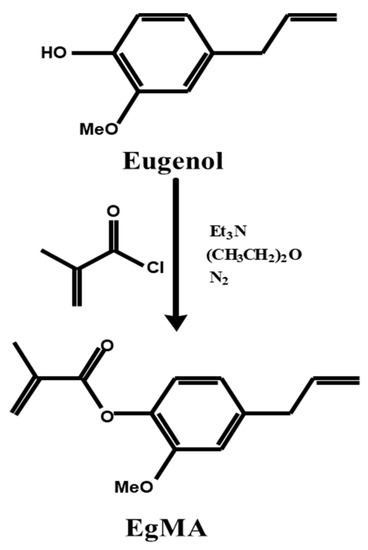
Figure 3.
Synthetic scheme of the Fisher esterification of the eugenol molecule yielding its derivative eugenyl methacrylate [80].
The antibacterial activity of EgMA was initially tested against Streptococcus mutans and Escherichia coli, the most common species found in medical and dental surgical sites and devices. The data suggest that the conjugated double bond in the aromatic structure of EgMA and the allylic side chain are responsible for the strong inhibitory effects of this compound [84]. In addition, the alkyl substitution into the phenol nucleus alters the distribution ratio between the aqueous and the non-aqueous bacterial phases by reducing the surface tension (hydrophobicity of the compounds) or altering the species selectivity [85]. Thus, modification of the chemical structure of the eugenol molecule yielding an α-unsaturated ester, EgMA, resulted in a good hydrophobic balance with a certain proton exchange capacity. This, together with the presence of the 4-allyl group and the presence of the hydroxylic group coupled to a delocalised electron system, allows the monomer to maintain the ability to alter the cytoplasmic membrane permeability and the inhibitory capacity of colony growing with a consequent block of ionic pumps as proton and potassium ions [84,86,87]. The data also indicated that the disruptive effect on cytoplasmic membranes is maintained after the polymerisation reaction, indicating that EgMA-based materials render bioactive bacteriostatic surfaces that reduce microbial resistance and biofilm formation. Moreover, eugenyl-derivative copolymers showed a clear growing inhibition against E. coli and S. mutans, but no halo formation, suggesting that the bacteriostatic effect is caused by immobilised agents. It was observed that the reduction in colonies’ growth on the agar zone was only found beneath the polymer samples, indicating that the immobilised molecules showed their activity only against bacteria which came into contact with them, and that the effect does not reach distant areas from the material. Accordingly, dental resins and other restorative materials containing eugenyl derivatives would eventually contribute to the reduction or even the inhibition of bacteria growing at the interface between the materials and the surrounding tissues, possibly inhibiting secondary caries formation between the dentine surface and the restorative material.
Antibacterial agents whilst inhibiting bacterial growth should also be minimally cytotoxic to mammalian cells. As is known, low concentrations of eugenol exert anti-inflammatory and local anaesthetic effects on the dental pulp; however, high concentrations can exert some cytotoxic effects [88]. Eugenol and related compounds have been shown to have a high affinity for plasma membranes because of their lipid solubility, which may contribute to cell damage [89]. However, both EgMA monomer and PEgMA polymer were found to be cytocompatible when tested in vitro with human fibroblasts [90]. It should also be noted that the eugenol derivatives are only added in small amounts to high-molecular-weight monomers, with the derivatives being incorporated in a cross-linked network, leading to only traces of any unreacted monomer. The cell morphology and cell-material interaction of fibroblasts with polymeric systems showed cells with normal morphology, well spread and flattened over the materials, indicating the formation of stable adhesive contacts and showing good cytocompatibility of the polymer [84]. All these results open a novel approach to achieve biofunctionalised acrylic systems with potential applications in dentistry, orthopaedic surgery and ophthalmology. The novelty of these systems in comparison with other eugenol-containing materials lies in the covalent anchorage of the eugenol molecule to the macromolecular structure, avoiding its migration to the surrounding tissues and improving its hydrolytic stability, whilst still maintaining the beneficial properties of eugenol [71,80,81].
2.4.2. Applications of Eugenyl Methacrylate
Eugenyl Methacrylate in Polyacrylic Acid for Glass-Ionomer Cements
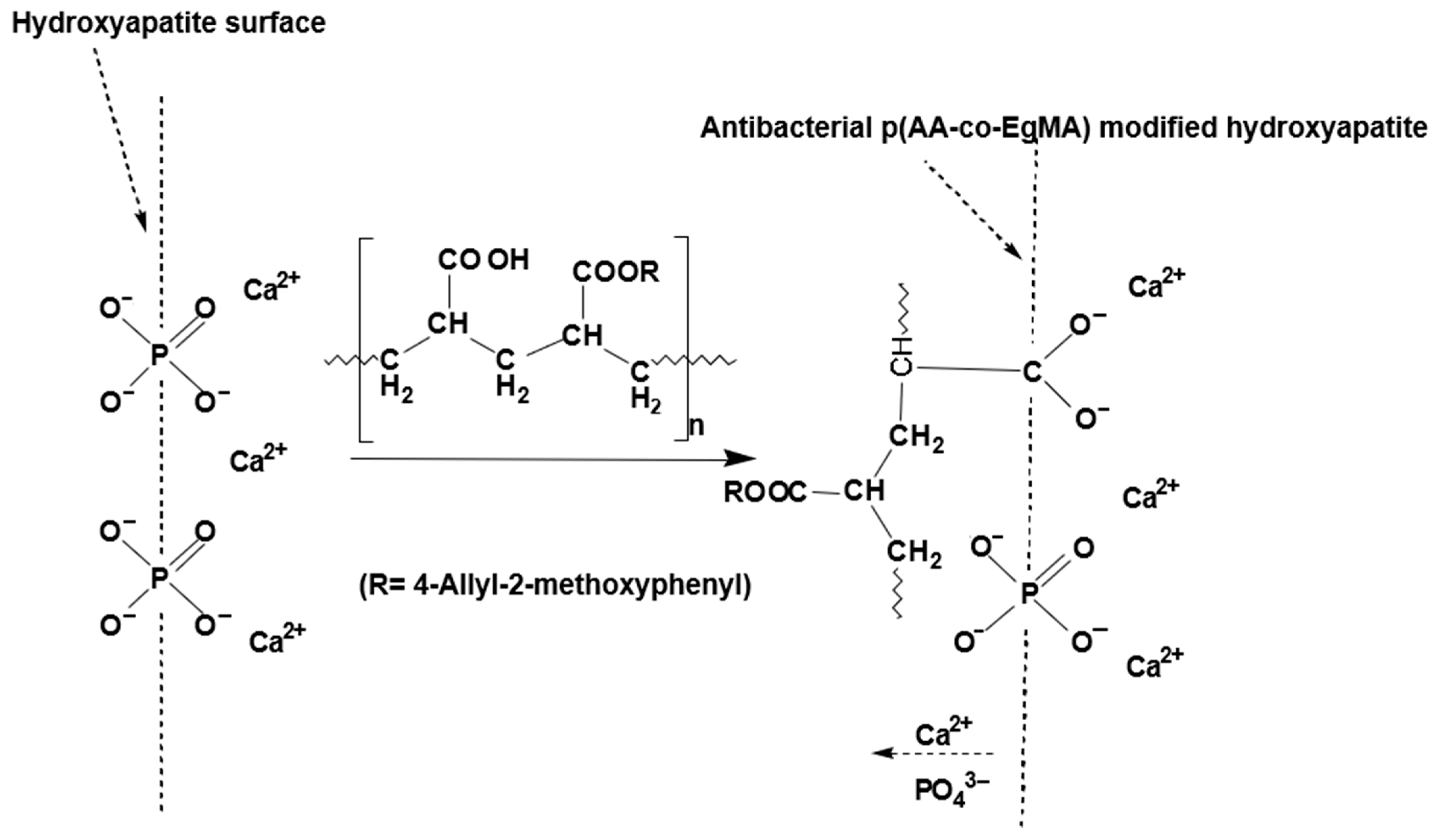
Glass ionomer cements (GICs) are an important class of dental materials that set by an acid–base reaction of an ion leachable calcium fluoro-aluminosilicate glass powder and a polyalkenoic acid, which is usually either a homopolymer of polyacrylic acid or a copolymer of acrylic and maleic acids in the presence of water [91]. The setting of GIC involves neutralisation of the acid groups available from the water-soluble polymer and the glass powder base. As these cements harden, they are associated with various changes, one of which is an increase in the proportion of tightly bound water within their structure. Therefore, GICs are sensitive to moisture in the early stages of placement and can lose matrix-forming ions in the presence of excessive moisture or desiccation [92]. Different acrylic acid blends and copolymers have been reported not only imbibing anti-inflammatory [93] and antibacterial [94] effects, but can also alter the moisture sensitivity of composites [95] such as GICs. The modified GIC with a mixture of acrylic acid and EgMA resulted in the experimental GICs exhibiting physical and mechanical properties in compliance with ISO standard requirements and is not significantly different than the commercial GIC [96]. Furthermore, the eugenyl residue was associated with an increase in the fraction of the volume surrounding the macromolecules, providing more flexible materials. This balance resulted in the experimental GICs having a marked increase in the flexural strength values, whilst maintaining the other mechanical properties over the standard requirements and additionally lowering the net water uptake. An additional advantage of these experimental GICs was the higher pH of the initial mix that was attributed to the lower net amount of −COOH groups in the copolymer and essentially these being neutralised in the early stages of the reaction and during the maturation process of the ionically crosslinked cement (Figure 4) [97]. The higher pH is expected to reduce irritation of the tooth pulp, when placed near it [98]. Although the incorporation of EgMA molecules in the polyacrylic acid copolymer resulted in a net decrease in the amount of −COOH groups in the matrix that retain the fluoride ions, there was no difference in the fluoride release between the commercial and experimental GICs. As the pH of the experimental cements was higher than the commercial cement, it was expected to release higher amounts of fluoride in the initial stage, which may improve the anticariogenic effects [99]. Based on these detailed studies, it can be concluded that GICs constituting acrylic copolymers with immobilised eugenol moieties improve physical properties as well as exhibit anticariogenic behaviour.
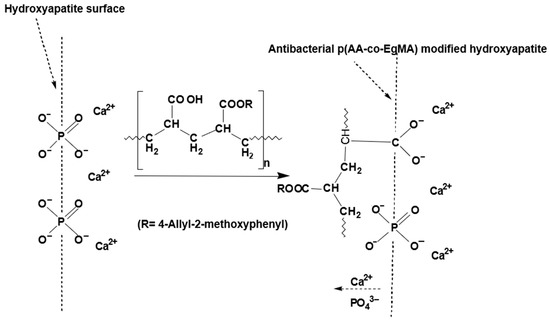
Figure 4.
Adhesion mechanism between the hydroxyapatite surface and polymeric component of antibacterial glass ionomers formulated with acrylic acid and eugenol-derived copolymer p(AA-co-EgMA) [94].
Eugenyl Methacrylate in Resin Composites
Resin composites were specifically developed for the cementation of endodontic posts and core build-up restorations that would benefit from possessing bactericidal properties; thus, dual cure (chemical and photopolymerisation) resin composites based on EgMA monomer and Bis-GMA/TEGDMA resin systems were formulated. The addition of EgMA monomer resulted in the enhancement of the viscoelastic properties, the mechanical response of the composites and imparted antibacterial properties to the resin system by virtue of the EgMA residues, specifically against a range of oral bacteria commonly associated with failure of coronal and endodontic restorations. The experimental composites also exhibited outstanding properties in comparison to currently used dental composite materials [100,101]. The low viscosity of the EgMA monomer functioned as an excellent diluent for the viscous Bis-GMA monomer and enhanced the initial handling viscosity. This effect is desirable as it can facilitate injection into the root canal system through the delivery devices [102]. It also reduced the polymerisation shrinkage stress within the material during early setting [103], and the stress relief via resin flow relaxation decreases the possibility of gap formation [104] and marginal leakage [105], which consequently can enhance the longevity of the restorations.
The experimental resin composites with EgMA also exhibited significantly higher depth of cure and lower polymerisation exotherm compared to commercial composites. The reduction in exothermic polymerisation constitutes an additional advantage preventing thermal damage of the adjacent root dentine whilst curing occurs within the endodontic cavity. The addition of EgMA into the composite formulations also increased both flexural and compressive strengths. This confirms the effect of EgMA, which allows for both crosslinking and forming branched structures. The crosslinked network leads to strengthening whilst an increase in ductility is observed due to the branching [106]. Water sorption and solubility of polymeric composites are also of importance for dental applications since the physical and mechanical properties of resin composite materials may be significantly altered by fluid uptake in the oral environment, which also leads to the harbouring of bacteria within these composites that eventually leads to discolouration and failure. The water sorption and diffusion coefficient values of the EgMA resin composites were significantly reduced with increasing content of EgMA due to the hydrophobicity and ability to form slightly crosslinked structures. The increasing surface contact angle and decrease in surface free energy of the EgMA resin composites were expected due to the presence of the aromatic ring in the structure of EgMA. As the antibacterial activity of the experimental composites is derived from the pendent eugenyl residue, the ability of these composites to reduce or inhibit bacterial growth is highly dependent on the direct contact between eugenyl residues of the composite surface and the bacteria [107,108,109]. It was noted that the greater hydrophobic character of EgMA-containing composites improved the total interaction energy with the bacteria and resulted in a higher accessibility to the eugenyl moieties responsible for the bacteriostatic activity.
The antibacterial activity of the EgMA-containing resin composites determined against E. faecalis, S.mutans and P. acnes showed surface-inhibition properties using the agar diffusion test [101]. The lack of inhibition zones around the composite discs containing EgMA confirmed their non-releasing behaviour, as the inhibition zones were formed by the diffusion of antimicrobial material, indicating that the antibacterial activity of the composites is not through the release of agents to the medium but is instead associated with surface contact. Secondly, the surface antibacterial activity test against the adherence and growth of E. faecalis, S. mutans and P. acnes was performed. There was a significant reduction in the number of colony-forming units (CFUs) of the bacteria tested, indicating that the chemically bound monomer has the capability to reduce or inhibit the colonisation of these bacteria that come into contact with the composite surface. Therefore, incorporation of EgMA monomers as immobilised bactericidal moieties within polymerisable formulations provides a novel approach to develop resin composite materials with intrinsically antibacterial activity against common coronal and endodontic oral bacteria.
Eugenyl Methacrylate in Dental Adhesive Systems
Dental adhesives play an important role in restorative dentistry. Enabling polymerisable monomers with antibacterial properties in dental adhesives is of value since the interface between the restorative and tooth tissue is susceptible to the ingress of microbial species.
EgMA is a compatible comonomer with methacrylate monomers present in dental adhesives; thus, post curing immobilises the antibacterial eugenol moieties in the polymer backbone without the inhibitory effect characteristic of the phenol group. The antibacterial property of the dental adhesive is expected to help eliminate residual bacteria, which often remains in dentinal tubules, and lower the risk of reinfection and secondary caries. The efficacy of EgMA as a comonomer in single-component self-etch systems and the adhesive and catalyst components of a total-etch system were reported by Almaroof et al. [103]. The curing ability of a bonding system is a major factor that governs the formation of a strong and durable bond with the dentinal substrate, and the degree of cure is influenced by any compositional modifications to the system. Almaroof et al. [106] showed that despite the significantly lower initial degree of cure of the modified adhesives than their corresponding control, they were increased when measured at 24 h post curing, with no significant differences [106]. As with the EgMA-modified resin composites, the initial reduction in the degree of conversion was attributed to the bi-functional nature of acrylic and allylic double bonds in the EgMA moiety that led to the formation of crosslinked structures with unreacted allylic bonds. An additional advantage of the EgMA formulations in adhesives was attributed to the significantly lower polymerisation exotherm, which would decrease the potential of thermal damage to the host tissue.
EgMA in bonding formulations significantly increased the contact angle of the polar and dispersive liquids tested and decreased surface free energy indicating the higher hydrophobicity of the bonding surfaces. Although the effect of surface free energy of substrates on bacterial adhesion has been critically discussed in the literature with no clear consensus, our previous findings for polymers and resin composites incorporating EgMA reported a greater hydrophobic character of these materials, presented a lower surface free energy, and ultimately impacted the total interaction energy with the bacteria, as higher accessibility to the eugenyl moieties was responsible for improved bacteriostatic activity [100,104]. The chemical composition of adhesive resins and their net hydrophilicity influence the water sorption, solubility and water diffusion of these polymers. The hydrophobic nature of EgMA substituted methacrylates and its ability to form slightly crosslinked structures of the modified adhesives resulted in a significant reduction in their water sorption and solubility in comparison to bonding systems containing acidic, highly polar functional groups in newer formulations of bonding agents. The reduction in solubility indicates the limited extraction of any unreacted monomers into the surrounding environment, potentially increasing the stability and durability of the resin–dentine bonds [105].
The incorporation of EgMA into self-etch and total etch dental adhesives showed potent antibacterial activity, before and after light polymerisation. The EgMA-modified adhesives produced significantly greater inhibition zones than their corresponding controls, indicating that the eugenyl residue maintained the bactericidal effect of eugenol and the sensitivity of P. acnes., S. mutans and E. faecalis to this monomer within their formulations. Moreover, the inclusion EgMA reduced biofilm viability, evidenced by a decrease in the number of CFUs of the total species, live biofilm volume and the percentage of live bacteria (Figure 5). It is well known that Gram-positive bacteria are more sensitive to essential oils such as eugenol, as the hydrophilic cell wall structures of Gram-negative bacteria block the penetration of hydrophobic components into the cell membrane [91]. The respective virulence of each species and the differences in the chemical composition and structure of the bacteria cell walls resulted in different bacterial sensitivities towards EgMA. E. faecalis is the most resistant bacterium to a wide range of antibiotics; however, the effectiveness of EgMA-modified adhesives against E. faecalis offers a considerable advantage over the commercial dental adhesives. It is also known that eugenol at high concentrations can exert some toxic effects on the dental pulp [80]. However, EgMA-modified adhesives showed good biocompatibility and a high percentage of cell viability. The cytocompatibility of EgMA-modified adhesives exhibited human gingival fibroblast viability matching that of their commercial non-antibacterial controls, as well as that of the negative control without any resin eluent.
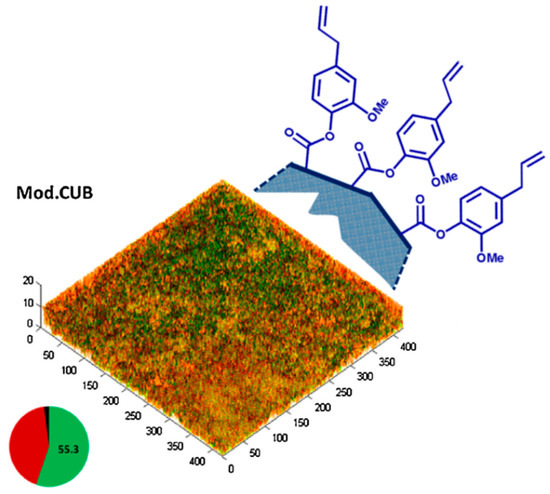
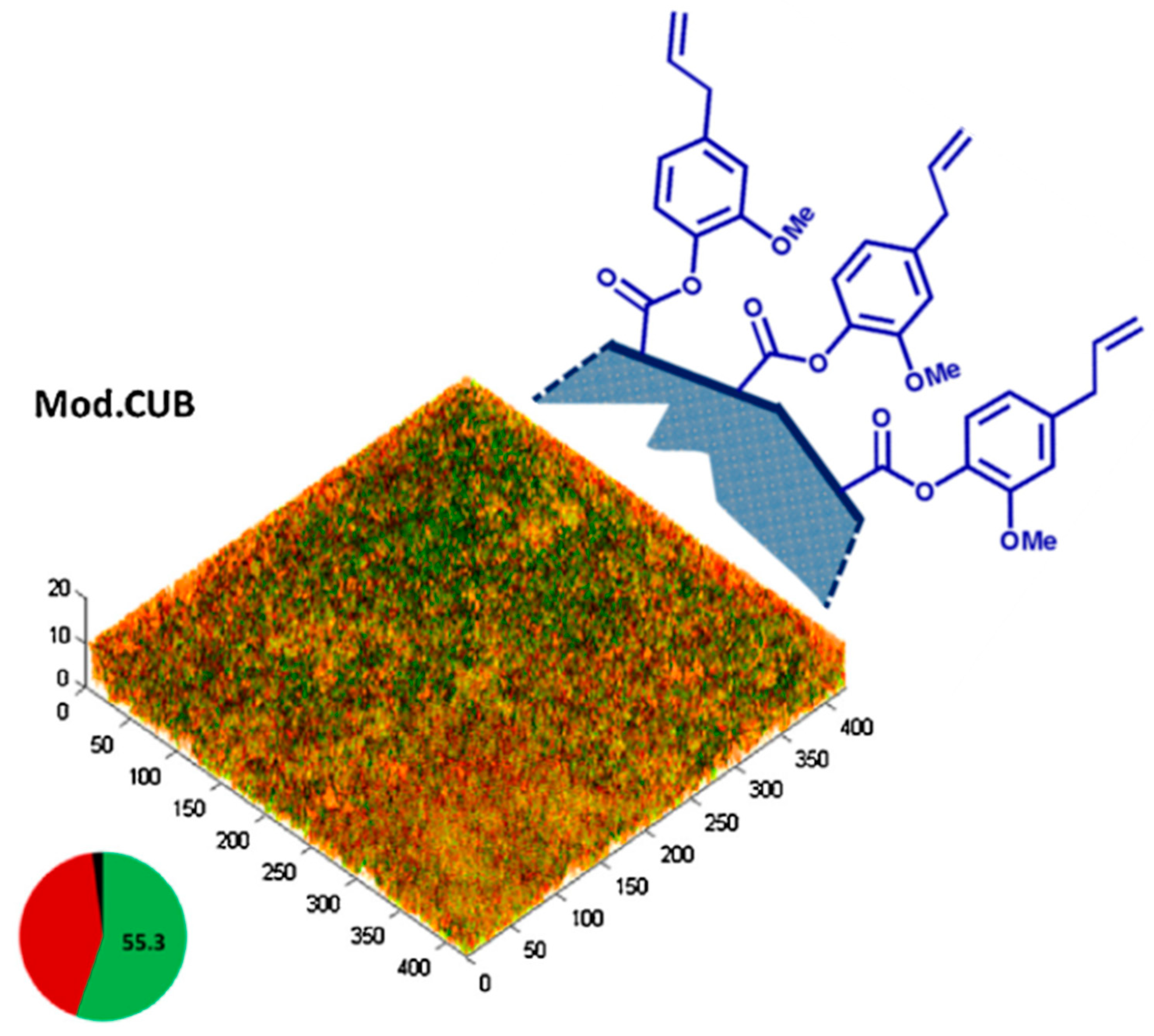
Figure 5.
Schematic illustration of pendent eugenyl residues from the polymeric surface of the EgMA-containing resin composites (Mod.CUB) and microbial biofilms grown on cured resin discs tested with live/dead staining. The pie chart shows the effect of EgMA incorporation on the mean percentages of dead (red), live (green) and unknown (black) biovolumes. Adapted from Almaroof et al., 2017, with permission from Elsevier.
Finally, the incorporation of EgMA did not adversely affect the root dentine bonding ability of both parent adhesive systems evaluated by push-out bond strength testing. The EgMA-modified adhesives presented bond strength values similar to their corresponding controls. In addition, confocal laser scanning microscopy assessment of the resin–dentine interface showed that EgMA-modified self-etch and total-etch adhesives were able to create a resin diffusion zone within the coronal and middle root dentin, forming a clear, continuous, gap-free hybrid layer located underneath a thick adhesive layer. The presence of resin tags was also evident with both adhesives. The incorporation of EgMA produced an effective bond to root canal dentin and high compatibility in vitro, indicating a potential application to achieve successful post-endodontic restorations [101].
2.5. Chitosan
Chitin is a natural biopolymer sourced from crustacean shells, which on deacetylation yields chitosan. The monomeric units of chitosan contain a primary amine and two hydroxyl groups and in acidic medium the protonation of the amine groups leads to a charged polymer. Chitosan is a cationic polysaccharide and the degree of deacetylation governs both solubility and the availability of the free amino groups [110]. It possesses broad-spectrum antimicrobial activity and has an effective killing rate against Gram-positive and Gram-negative bacteria; however, the efficacy depends on the properties of the chitosan used such as the physical state (solid or water soluble form), molecular weight, charge density, concentration, hydrophilicity, chelating capacity, pH of the medium, temperature, interaction time and factors that relate to the microbial species [111,112,113,114]. The favourable biocompatibility, biodegradability, hydrophilicity, haemostatic and antiantigenic properties of chitosan have thus led to its use in a variety of biomedical applications [115,116]. Chitosan has been used in different aspects of clinical dentistry, whilst many experimental research studies highlight the potential of application in other dental applications [117]. It has been used in dentifrices, toothpastes and chewing gums that confer antimicrobial activity and reportedly limit enamel demineralisation by forming a protective layer on enamel surfaces in the presence of mucin present in saliva [118,119]. This polysaccharide has also been used as a carrier for the delivery of drugs and compounds such as naringin, chlorhexidine, statins, metronidazole, doxycycline and other antibiotics for the treatment of periodontal disease, endodontic therapy and preventive dentistry. The incorporation of chitosan in glass ionomer cements was reported to not only enhance the antibacterial activity, but also to improve flexural and compressive strength of the cement. A methacrylate derivative of chitosan [120] has been reported as a potential component in dental adhesives that is able to reduce microbial ingress, which is beneficial towards the longevity of the restoration. Similarly, chitosan-coated dental implants have been reported to assist in bone regeneration whilst maintaining their antibacterial properties [121].
Chitosan has been used in gels and oral formulations for periodontal treatment. Furthermore, its haemostatic properties have been exploited to form sponges, films and membranes for use in oral wound dressings and to curb excessive bleeding such as Axiostat®, Celox™ and Hemcon® for clinical use post tooth extraction [122,123].
2.6. Graphene and Graphene Oxide
The nanomaterials, graphene and graphene oxide (GO), possess unique properties, which have stemmed enormous interest in their use as nanofillers in composites, drug delivery and other biomedical applications [121,122]. Nanosheets of GO are two-dimensional structures with a honeycombed carbon framework. The potential application of graphene and its derivatives [124,125] in oral healthcare have highlighted the antimicrobial properties and low cytotoxicity of graphene and its derivatives. GO has been reported to exhibit antibacterial properties, and GO nanosheets inhibit the growth of dental pathogens [126]; thus, it is a promising alternative in minimising antibiotic resistance and reducing the prevalence of biofilm formation. GO and graphene have also been used to form polymer nanocomposites and evaluated for their antimicrobial activity. Poly (methylmethacrylate), a widely used polymer in dental applications, was developed into a composite with GO, which exhibited superior mechanical properties and antibacterial activity (Figure 6) [116,127,128]. The use of GO in coating titanium implants is another potential application of interest that minimises the adhesion of bacterial species on the surface. In addition, silver nanoparticles (Ag-NPs) deposited onto functionalised hybrid graphene demonstrated an increase in antimicrobial activity against S. aureus and E. coli. Ioannidis et al. [129] used a similar system and developed a silver-graphene oxide system as a root canal irrigant for microbial killing and biofilm disruption with the preliminary results indicating that Ag-GO can be potentially used as an auxiliary disinfectant.
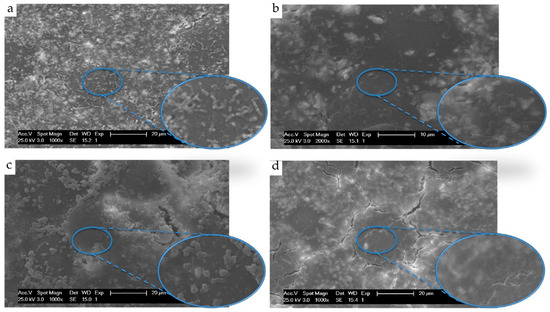
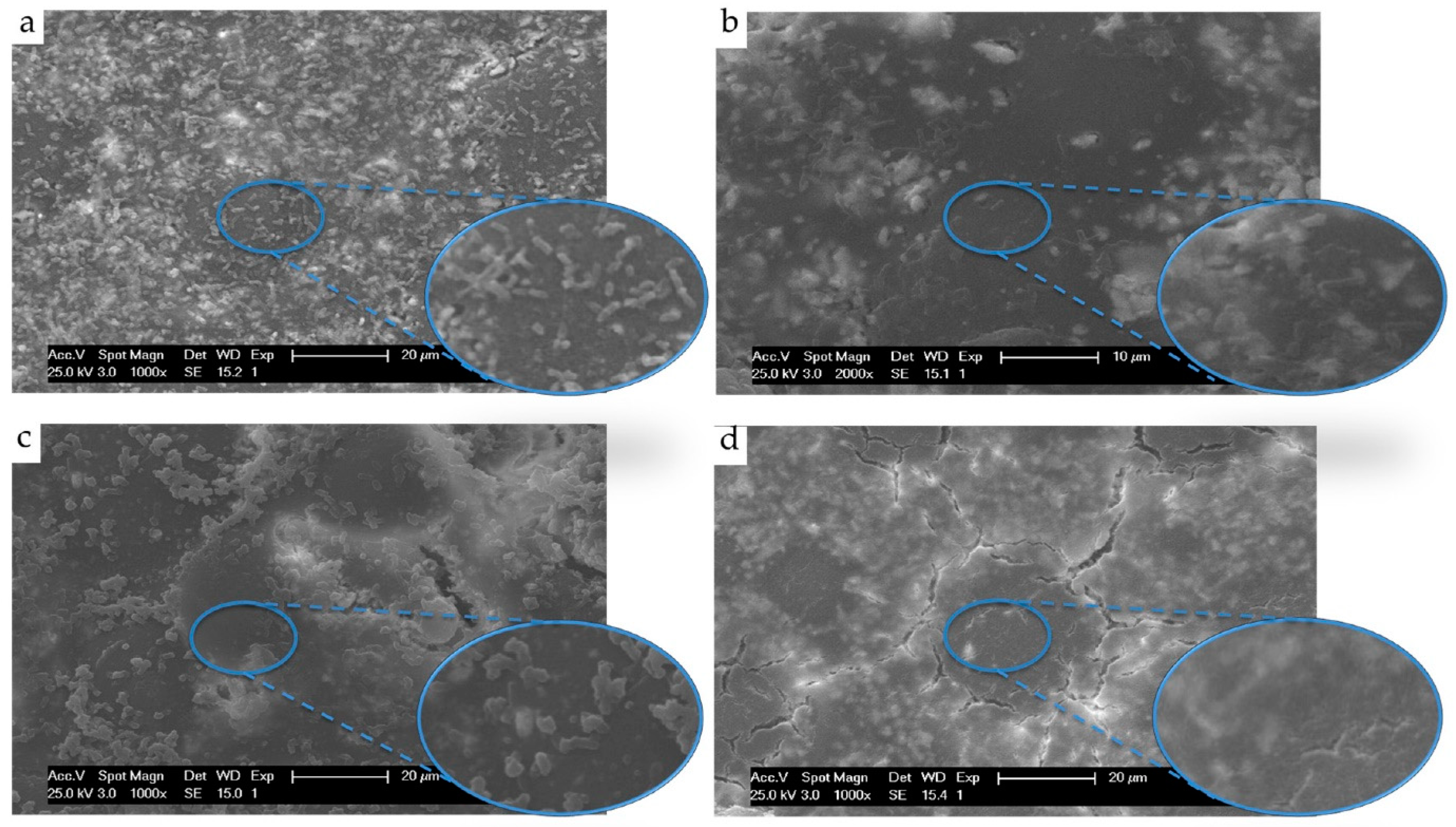
Figure 6.
SEM observation of attached E. coli adhered onto (a) Poly(methylmethacrylate) (PMMA) acrylic surfaces, (b) PMMA containing 0.3% graphene oxide, (c) PMMA containing 15% chitosan and (d) PMMA containing 0.3% graphene oxide and 15% chitosan. Reproduced from Zapata et al. [116] with permission from the authors.
2.7. Antimicrobial Peptides
Antimicrobial peptides (AMPs) are low-molecular-weight proteins that exhibit a wide spectrum of antibacterial activity [130]. Antimicrobial peptides are predominantly cationic and amphiphilic in nature. They can bind to the negatively charged bacterial cell membrane and cause bacterial cell lysis and the potency depends on the cationicity and amphipathicity, and different models have been used to explain the mechanism of action [130]. The antibacterial activity of AMPs is of huge interest [131] as they exhibit superior pharmacokinetics, offer effective treatment and potentially can provide solutions to overcome the burden of antibiotic resistance developing swiftly around the globe. This promising approach is naturally being explored actively in dentistry to both prevent and treat dental caries [132]. Several natural and synthetic AMPs have been shown to have bactericidal effects [132] on several bacterial species present in the oral cavity, especially S. mutans. Although these findings are predominantly based on in vitro laboratory studies, they provide valuable information on the biomemis of synthetic AMPs that can be developed for clinical applications. Several synthetic AMPs have been synthesised and specifically used for studying caries prevention and treatment, with several exhibiting sustained activity against S. mutans [132,133,134,135]. Long acting amphipathic AMPs targeting the oral plaque microbiome were also reported by Moussa et al. [134] and used as coatings on dentine–restoration interfaces. The study showed selective antimicrobial potency, and ex vivo tests indicated potential protection against recurrent caries. The use of tooth-binding antimicrobial AMPs to inhibit plaque biofilm formation is another approach that has been used as a potential pathway against recurrent caries [94].
3. Conclusions
This review indicates that it is important to consider the risks of antimicrobial resistance whilst designing drug delivery systems for application in the oral environment, especially as it concerns a large proportion of the global population. The strategy of employing intrinsically antibacterial compounds is likely to be more beneficial than the incorporation of antimicrobial agents within dental materials to function via the release of therapeutic molecules. It is evident that targeted antimicrobial activity at sites prone to the formation and accumulation of biofilms such as on restorative materials, the tooth-restoration interface or a root canal is expected to be an efficacious pathway in limiting the detrimental effects caused by pathogenic bacteria. Although the results of most of the studies reviewed here are largely laboratory based, there is evidence that polymerisable moieties with intrinsic antimicrobial properties and the application of antimicrobial peptides are future directions that should be considered in designing systems with clinical translation as a focus for applications in the oral environment.
Author Contributions
Writing—original draft preparation, R.A., L.R., S.D.; Writing—review and editing, R.A., L.R., S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). High Levels of Antibiotic Resistance Found Worldwide, New Data Shows; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Wellcome Collection: London, UK, 2014. [Google Scholar]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Martín-Del-campo, M.; Fernández-Villa, D.; Cabrera-Rueda, G.; Rojo, L. Antibacterial bio-based polymers for cranio-maxillofacial regeneration applications. Appl. Sci. 2020, 10, 8371. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Ten Cate, J.M. Contemporary perspective on the use of fluoride products in caries prevention. Br. Dent. J. 2013, 214, 161–167. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.P.; Laurence, J.S. Proteins, pathogens, and failure at the composite-tooth interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.R.; De Oliveira Da Rosa, W.L.; Da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Clinical Implications and Microbiology of Bacterial Persistence after Treatment Procedures. J. Endod. 2008, 34, 1291–1301.e3. [Google Scholar] [CrossRef] [PubMed]
- Zandi, H.; Petronijevic, N.; Mdala, I.; Kristoffersen, A.K.; Enersen, M.; Rôças, I.N.; Siqueira, J.F.; Ørstavik, D. Outcome of Endodontic Retreatment Using 2 Root Canal Irrigants and Influence of Infection on Healing as Determined by a Molecular Method: A Randomized Clinical Trial. J. Endod. 2019, 45, 1089–1098.e5. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bruhn, G.; Richter, S.; Netuschil, L.; Brecx, M. Clinical controlled study on plaque and gingivitis reduction under long-term use of low-dose chlorhexidine solutions in a population exhibiting good oral hygiene. Clin. Oral Investig. 2001, 5, 89–95. [Google Scholar] [CrossRef]
- Barnett, M.L. The rationale for the daily use of an antimicrobial mouthrinse. J. Am. Dent. Assoc. 2006, 137, S16–S21. [Google Scholar] [CrossRef]
- Bellis, C.A.; Addison, O.; Nobbs, A.H.; Duckworth, P.F.; Holder, J.A.; Barbour, M.E. Glass ionomer cements with milled, dry chlorhexidine hexametaphosphate filler particles to provide long-term antimicrobial properties with recharge capacity. Dent. Mater. 2018, 34, 1717–1726. [Google Scholar] [CrossRef]
- Bellis, C.A.; Nobbs, A.H.; O’Sullivan, D.J.; Holder, J.A.; Barbour, M.E. Glass ionomer cements functionalised with a concentrated paste of chlorhexidine hexametaphosphate provides dose-dependent chlorhexidine release over at least 14 months. J. Dent. 2016, 45, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, Y.; Skeats, M.K.; Ireland, A.J.; Barbour, M.E. Chlorhexidine hexametaphosphate as a coating for elastomeric ligatures with sustained antimicrobial properties: A laboratory study. Am. J. Orthod. Dentofac. Orthop. 2020, 158, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.C.; Slot, D.E.; Van Der Velden, U.; Van Der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D. Chlorhexidine mouthwash reduces plaque and gingivitis. Evid. Based Dent. 2013, 14, 17–18. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Scaffa, P.M.C.; Vidal, C.M.P.; Barros, N.; Gesteira, T.F.; Carmona, A.K.; Breschi, L.; Pashley, D.H.; Tjäderhane, L.; Tersariol, I.L.S.; Nascimento, F.D.; et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J. Dent. Res. 2012, 91, 420–425. [Google Scholar] [CrossRef]
- Carrilho, M.R.O.; Geraldeli, S.; Tay, F.; De Goes, M.F.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.F.; Hebling, J.; Mazzoni, A.; Breschi, L.; et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Hass, V.; Gutierrez, M.F.; Luque-Martinez, I.V.; Szezs, A.; Stanislawczuk, R.; Bandeca, M.C.; Reis, A. Five-year effects of chlorhexidine on the in vitro durability of resin/dentin interfaces. J. Adhes. Dent. 2016, 18, 35–42. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- De Munck, J.; Van Den Steen, P.E.; Mine, A.; Van Landuyt, K.L.; Poitevin, A.; Opdenakker, G.; Van Meerbeek, B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J. Dent. Res. 2009, 88, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, J.; Chen, L.; Li, D.; Tan, Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J. Dent. 2009, 37, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.M.; De Sá Rodrigues, C.U.F.; De Oliveira Matos, M.P.; De Carvalho, T.R.; Dos Santos, G.B.; Amaral, C.M. Experimental etch-and-rinse adhesive systems containing MMP-inhibitors: Physicochemical characterization and resin-dentin bonding stability. J. Dent. 2015, 43, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ricci, H.A.; Sanabe, M.E.; de Souza Costa, C.A.; Pashley, D.H.; Hebling, J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur. J. Oral Sci. 2010, 118, 411–416. [Google Scholar] [CrossRef]
- Kim, J.; Uchiyama, T.; Carrilho, M.; Agee, K.A.; Mazzoni, A.; Breschi, L.; Carvalho, R.M.; Tjäderhane, L.; Looney, S.; Wimmer, C.; et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent. Mater. 2010, 26, 771–778. [Google Scholar] [CrossRef]
- Sadek, F.T.; Braga, R.R.; Muench, A.; Liu, Y.; Pashley, D.H.; Tay, F.R. Ethanol wet-bonding challenges current anti-degradation strategy. J. Dent. Res. 2010, 89, 1499–1504. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef]
- Fik, C.P.; Konieczny, S.; Pashley, D.H.; Waschinski, C.J.; Ladisch, R.S.; Salz, U.; Bock, T.; Tiller, J.C. Telechelic Poly (2-oxazoline)s with a biocidal and a polymerizable terminal as collagenase inhibiting additive for long-term active antimicrobial dental materials. Macromol. Biosci. 2014, 14, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Caillier, L.; de Givenchy, E.T.; Levy, R.; Vandenberghe, Y.; Géribaldi, S.; Guittard, F. Synthesis and antimicrobial properties of polymerizable quaternary ammoniums. Eur. J. Med. Chem. 2009, 44, 3201–3208. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Vallittu, P.K.; Lassila, L.V.J. Investigation of double bond conversion, mechanical properties, and antibacterial activity of dental resins with different alkyl chain length quaternary ammonium methacrylate monomers (QAM). J. Biomater. Sci. Polym. Ed. 2013, 24, 565–573. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial activity of a modified unfilled resin containing a novel polymerizable quaternary ammonium salt MAE-HB. Sci. Rep. 2016, 6, 33858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90 B, 813–817. [Google Scholar] [CrossRef]
- Pesci-Bardon, C.; Fosse, T.; Serre, D.; Madinier, I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodontology 2006, 23, 111–116. [Google Scholar] [CrossRef]
- Deb, S.; Doiron, R.; DiSilvio, L.; Punyani, S.; Singh, H. PMMA bone cement containing a quaternary amine comonomer with potential antibacterial properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Agee, K.A.; Mazzoni, A.; Carvalho, R.M.; Carrilho, M.; Tersariol, I.L.; Nascimento, F.D.; Imazato, S.; Tjäderhane, L.; Breschi, L.; et al. Can quaternary ammonium methacrylates inhibit matrix MMPs and cathepsins? Dent. Mater. 2015, 31, e25–e32. [Google Scholar] [CrossRef]
- Jaberi Ansari, Z.; Sadr, A.; Moezizadeh, M.; Aminian, R.; Ghasemi, A.; Shimada, Y.; Tagami, J.; Jaberi Ansari, S.; Moayedi, S. Effects of one-year storage in water on bond strength of self-etching adhesives to enamel and dentin. Dent. Mater. J. 2008, 27, 266–272. [Google Scholar] [CrossRef][Green Version]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simão, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int. J. Mol. Sci. 2014, 15, 8998–9015. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Mine, A.; Van den Steen, P.E.; Van Landuyt, K.L.; Poitevin, A.; Opdenakker, G.; Van Meerbeek, B. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur. J. Oral Sci. 2010, 118, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc reduces collagen degradation in demineralized human dentin explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
- Boelen, G.J.; Boute, L.; D’Hoop, J.; EzEldeen, M.; Lambrichts, I.; Opdenakker, G. Matrix metalloproteinases and inhibitors in dentistry. Clin. Oral Investig. 2019, 23, 2823–2835. [Google Scholar] [CrossRef]
- Sulkala, M.; Wahlgren, J.; Larmas, M.; Sorsa, T.; Teronen, O.; Salo, T.; Tjäderhane, L. The Effects of MMP Inhibitors on Human Salivary MMP Activity and Caries Progression in Rats. J. Dent. Res. 2001, 80, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.O.; Ceballos, G.; Villarreal, F.J. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011, 63, 102–107. [Google Scholar] [CrossRef] [PubMed]
- De Castilho, A.R.F.; Duque, C.; Negrini, T.D.C.; Sacono, N.T.; De Paula, A.B.; Sacramento, P.A.; De Souza Costa, C.A.; Spolidorio, D.M.P.; Puppin-Rontani, R.M. Mechanical and biological characterization of resin-modified glass-ionomer cement containing doxycycline hyclate. Arch. Oral Biol. 2012, 57, 131–138. [Google Scholar] [CrossRef]
- De Castilho, A.R.F.; Duque, C.; Kreling, P.F.; Pereira, J.A.; de Aula, A.B.; Sinhoreti, M.A.C.; Puppin-Rontani, R.M. Doxycycline-containing glass ionomer cement for arresting residual caries: An in vitro study and a pilot trial. J. Appl. Oral Sci. 2018, 26, e20170116. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic acid antagonists: Antimicrobial and immunomodulating mechanisms and applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef]
- Semyari, H.; Salehi, M.; Taleghani, F.; Ehterami, A.; Bastami, F.; Jalayer, T.; Semyari, H.; Hamed Nabavi, M.; Semyari, H. Fabrication and characterization of collagen–hydroxyapatite-based composite scaffolds containing doxycycline via freeze-casting method for bone tissue engineering. J. Biomater. Appl. 2018, 33, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015. ID 720654. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Deb, S. Polymer Therapeutics in Relation to Dentistry. Front. Oral Biol. 2015, 17, 13–21. [Google Scholar]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing polymeric microparticles for biomedical and industrial applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Kurniawan, Y.S.; Syah, Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef]
- dos Santos, A.; André, C.B.; Martim, G.C.; Schuquel, I.T.A.; Pfeifer, C.S.; Ferracane, J.L.; Tominaga, T.T.; Khalil, N.M.; Radovanovic, E.; Girotto, E.M. Methacrylate saccharide-based monomers for dental adhesive systems. Int. J. Adhes. Adhes. 2018, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Huang, Q.; Liu, F.; Lin, Z.; He, J. Synthesis of antibacterial methacrylate monomer derived from thiazole and its application in dental resin. J. Mech. Behav. Biomed. Mater. 2015, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, ester-free monomers: Polymerization kinetics, mechanical properties, biocompatibility and anti-biofilm activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Y.; Weir, M.D.; Gao, J.; Imazato, S.; Oates, T.W.; Lei, L.; Wang, S.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020, 36, 296–309. [Google Scholar] [CrossRef]
- Kaplan, A.E.; Picca, M.; Gonzalez, M.I.; Macchi, R.L.; Molgatini, S.L. Antimicrobial effect of six endodontic sealers: An in vitro evaluation. Dent. Traumatol. 1999, 15, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Jendresen, M.D.; Phillips, R.W.; Swartz, M.L.; Norman, R.D. A comparative study of four zinc oxide and eugenol formulations as restorative materials. Part I. J. Prosthet. Dent. 1969, 21, 176–183. [Google Scholar] [CrossRef]
- Jendresen, M.D.; Phillips, R.W. A comparative study of four zinc oxide and eugenol formulations as restorative materials. Part II. J. Prosthet. Dent. 1969, 21, 300–309. [Google Scholar] [CrossRef]
- Millstein, P.L.; Nathanson, D. Effect of eugenol and eugenol cements on cured composite resin. J. Prosthet. Dent. 1983, 50, 211–215. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Action of eugenol as a retarder against polymerization of methyl methacrylate by benzoyl peroxide. Biomaterials 1997, 18, 701–703. [Google Scholar] [CrossRef]
- Rojo, L.; Vazquez, B.; Parra, J.; Bravo, A.L.; Deb, S.; San Roman, J. From natural products to polymeric derivatives of “Eugenol”: A new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; San Román, J. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.J.; De Bont, J.A.M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta Rev. Biomembr. 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Markowitz, K.; Moynihan, M.; Liu, M.; Kim, S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 729–737. [Google Scholar] [CrossRef]
- Manabe, A.; Nakayama, S.; Sakamoto, K. Effects of Essential Oils on Erythrocytes and Hepatocytes from Rats and Dipalmitoyl Phosphatidylcholine-Liposomes. Jpn. J. Pharmacol. 1987, 44, 77–84. [Google Scholar] [CrossRef]
- Rojo, L.; Borzacchiello, A.; Parra, J.; Deb, S.; Vázquez, B.; San Román, J. The preparation of high conversion polymeric systems containing eugenol residues and their rheological characterization. J. Mater. Sci. Mater. Med. 2008, 19, 1467–1477. [Google Scholar] [CrossRef]
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Al-Taee, L.; Deb, S.; Banerjee, A. An in vitro assessment of the physical properties of manually-mixed and encapsulated glass-ionomer cements. BDJ Open 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Bielas, R.; Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. Choline supported poly(ionic liquid) graft copolymers as novel delivery systems of anionic pharmaceuticals for anti-inflammatory and anti-coagulant therapy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Effect of butyl lactate methacrylate content on the properties of acrylic acid copolymers. Polym. Sci. Ser. A 2016, 58, 368–378. [Google Scholar] [CrossRef]
- Rojo, L.; Vázquez, B.; Román, J.S.; Deb, S. Eugenol functionalized poly(acrylic acid) derivatives in the formation of glass-ionomer cements. Dent. Mater. 2008, 24, 1709–1716. [Google Scholar] [CrossRef]
- Nicholson, J.W. Maturation processes in glass-ionomer dental cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Ruse, N.D. Acidity of glass ionomer cements during setting and its relation to pulp sensitivity. J. Am. Dent. Assoc. 1986, 112, 654–657. [Google Scholar] [CrossRef]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Almaroof, A.; Rojo, L.; Mannocci, F.; Deb, S. A resin composite material containing an eugenol derivative for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 149–160. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Influence of a polymerizable eugenol derivative on the antibacterial activity and wettability of a resin composite for intracanal post cementation and core build-up restoration. Dent. Mater. 2016, 32, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Finger, W.J.; Inoue, M.; Asmussen, E. Effect of wettability of adhesive resins on bonding to dentin. Am. J. Dent. 1994, 7, 35–38. [Google Scholar]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Moreira Da Silva, E.; Dos Santos, G.O.; Guimarães, J.G.A.; Barcellos, A.D.A.L.; Sampaio, E.M. The influence of C-factor, flexural modulus and viscous flow on gap formation in resin composite restorations. Oper. Dent. 2007, 32, 356–362. [Google Scholar] [CrossRef]
- Swift, E.J.; Triolo, P.T.; Barkmeier, W.W.; Bird, J.L.; Bounds, S.J. Effect of low-viscosity resins on the performance of dental adhesives. Am. J. Dent. 1996, 9, 100–104. [Google Scholar]
- Rojo, L.; Vázquez, B.; Deb, S.; Román, J.S. Eugenol derivatives immobilized in auto-polymerizing formulations as an approach to avoid inhibition interferences and improve biofunctionality in dental and orthopedic cements. Acta Biomater. 2009, 5, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, e239–e254. [Google Scholar] [CrossRef]
- Kurita, K. Chemistry and application of chitin and chitosan. Polym. Degrad. Stab. 1998, 59, 117–120. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Zamora, L.S.I.; Murillo, S.J.M.; Zapata, M.E.V.; Hernandez, J.H.M.; Valencia, C.H.; Rojo, L.; Grande Tovar, C.D. Influence of the chitosan morphology on the properties of acrylic cements and their biocompatibility. RSC Adv. 2020, 10, 31156–31164. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Osseointegration of antimicrobial acrylic bone cements modified with graphene oxide and chitosan. Appl. Sci. 2020, 10, 6528. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Lussi, A. Combined effect of a fluoride-stannous-and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion-abrasion. J. Dent. 2014, 42, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surf. B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef]
- Diolosà, M.; Donati, I.; Turco, G.; Cadenaro, M.; Di Lenarda, R.; Breschi, L.; Paoletti, S. Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules 2014, 15, 4606–4613. [Google Scholar] [CrossRef]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef]
- Javed, R.; Rais, F.; Kaleem, M.; Jamil, B.; Ahmad, M.A.; Yu, T.; Qureshi, S.W.; Ao, Q. Chitosan capping of CuO nanoparticles: Facile chemical preparation, biological analysis, and applications in dentistry. Int. J. Biol. Macromol. 2021, 167, 1452–1467. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Takashiba, S.; Nishina, Y. Graphene oxide: A new direction in dentistry. Appl. Mater. Today 2020, 19, 100576. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Escobar, J.A.D.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Novel bioactive and antibacterial acrylic bone cement nanocomposites modified with graphene oxide and chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Kundu, R. Cationic Amphiphilic Peptides: Synthetic Antimicrobial Agents Inspired by Nature. Chem. Med. Chem. 2020, 15, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Fang, Z.H.; Li, Q.L.; Cao, C.Y. A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Targeting the oral plaque microbiome with immobilized anti-biofilm peptides at tooth-restoration interfaces. PLoS ONE 2020, 15, e0235283. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.P.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).