Tensile Test of Human Lumbar Ligamentum Flavum: Age-Related Changes of Stiffness
Abstract
:1. Introduction
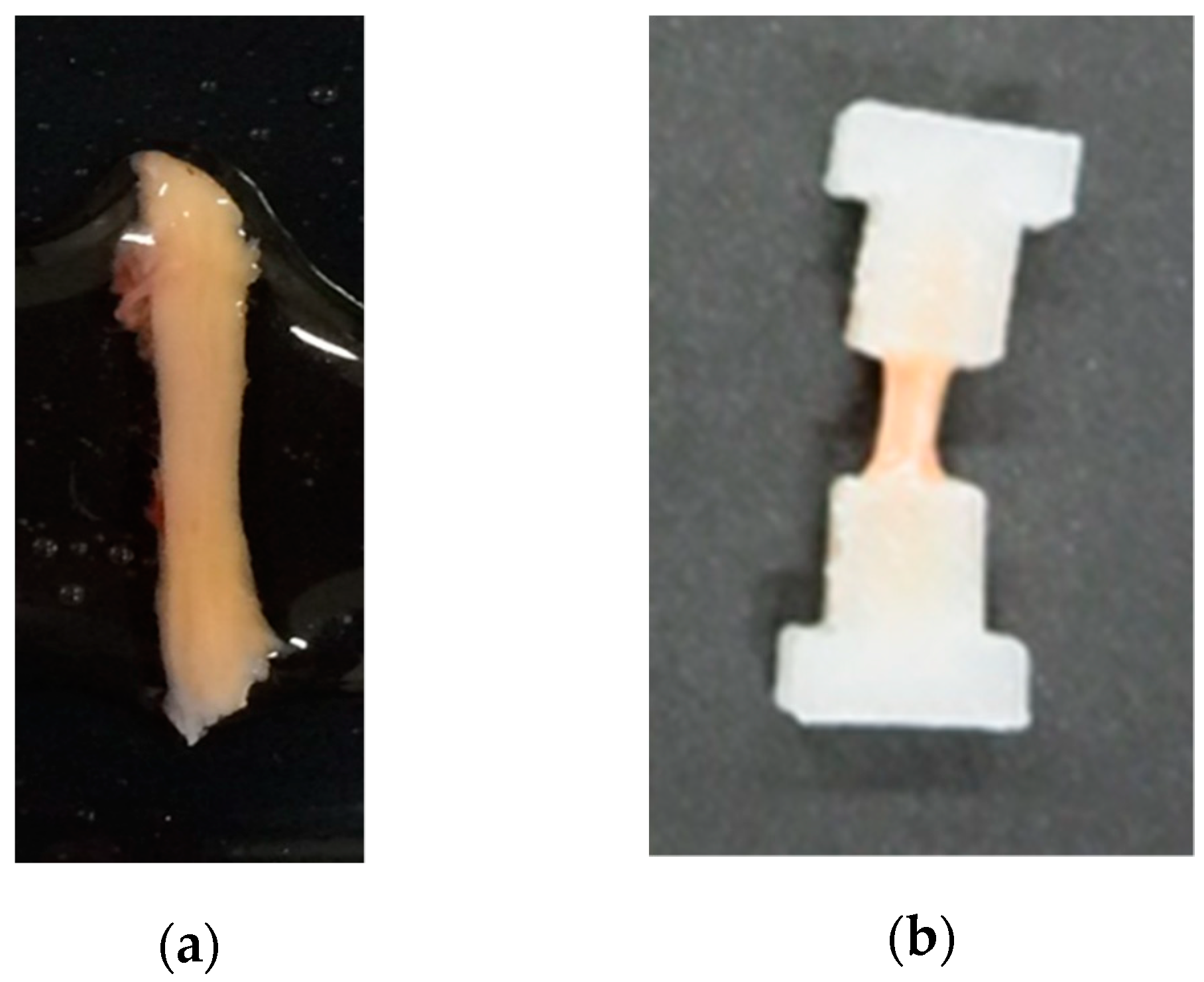
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nachemson, A.L.; Evans, J.H. Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J. Biomech. 1968, 1, 211–220. [Google Scholar] [CrossRef]
- Nachemson, A.; Lewin, T.; Maroudas, A.; Freeman, M.A. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop. Scand. 1970, 41, 589–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trigg, S.D.; Devilbiss, Z. Spine Conditions: Lumbar Spinal Stenosis. FP Essent. 2017, 461, 21–25. [Google Scholar] [PubMed]
- Lee, J.Y.; Whang, P.G.; Lee, J.Y.; Phillips, F.M.; Patel, A.A. Lumbar spinal stenosis. Instr. Course Lect. 2013, 62, 383–396. [Google Scholar]
- Yabe, Y.; Hagiwara, Y.; Ando, A.; Tsuchiya, M.; Minowa, T.; Takemura, T.; Honda, M.; Hatori, K.; Sonofuchi, K.; Kanazawa, K.; et al. Chondrogenic and fibrotic process in the ligamentum flavum of patients with lumbar spinal canal stenosis. Spine 2015, 40, 429–435. [Google Scholar] [CrossRef]
- Poletti, C.E. Central lumbar stenosis caused by ligamentum flavum: Unilateral laminotomy for bilateral ligamentectomy: Preliminary report of two cases. Neurosurgery 1995, 37, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, H.; Wang, X.; Liu, X. Ligamentum flavum fibrosis and hypertrophy: Molecular pathways, cellular mechanisms, and future directions. FASEB J. 2020, 34, 9854–9868. [Google Scholar] [CrossRef]
- Yong-Hing, K.; Reilly, J.; Kirkaldy-Willis, W.H. The ligamentum flavum. Spine 1976, 1, 226–234. [Google Scholar] [CrossRef]
- Yayama, T.; Baba, H.; Furusawa, N.; Kobayashi, S.; Uchida, K.; Kokubo, Y.; Noriki, S.; Imamura, Y.; Fukuda, M. Pathogenesis of calcium crystal deposition in the ligamentum flavum correlates with lumbar spinal canal stenosis. Clin. Exp. Rheumatol. 2005, 23, 637–643. [Google Scholar] [PubMed]
- Schräder, P.K.; Grob, D.; Rahn, B.A.; Cordey, J.; Dvorak, J. Histology of the ligamentum flavum in patients with degenerative lumbar spinal stenosis. Eur. Spine J. 1999, 8, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Shima, K.; Taniguchi, Y.; Tamaki, T.; Tanaka, T. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Pathogenesis and morphologic and immunohistochemical observation. Spine 1992, 17, 1353–1360. [Google Scholar] [CrossRef]
- Zhong, Z.M.; Zha, D.S.; Xiao, W.D.; Wu, S.H.; Wu, Q.; Zhang, Y.; Liu, F.Q.; Chen, J.T. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J. Orthop. Res. 2011, 29, 1592–1597. [Google Scholar] [CrossRef]
- Okuda, T.; Baba, I.; Fujimoto, Y.; Tanaka, N.; Sumida, T.; Manabe, H.; Hayashi, Y.; Ochi, M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine 2004, 29, 1689–1697. [Google Scholar] [CrossRef]
- Hur, J.W.; Kim, B.J.; Park, J.H.; Kim, J.H.; Park, Y.K.; Kwon, T.H.; Moon, H.J. The mechanism of ligamentum flavum hypertrophy: Introducing angiogenesis as a critical link that couples mechanical stress and hypertrophy. Neurosurgery 2015, 77, 274–282. [Google Scholar] [CrossRef] [PubMed]
- L€ohr, M.; Hampl, A.J.; Lee, J.Y.; Ernestus, R.I.; Deckert, M.; Stenzel, W. Hypertrophy of the lumbar ligamentum flavum is associated with inflamation-related TGF-beta expression. Acta Neurochir. 2011, 153, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, H. Tensile properties of human lumbar longitudinal ligaments. Acta Orthop. Scand. 1968, 39 (Suppl. 115), 1–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jezek, J.; Sepitka, J.; Daniel, M.; Kujal, P.; Blankova, A.; Waldauf, P.; Krbec, M.; Dousa, P.; Skala-Rosenbaum, J.; Samal, F.; et al. The role of vascularization on changes in ligamentum flavum mechanical properties and development of hypertrophy in patients with lumbar spinal stenosis. Spine J. 2020, 20, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Tsai, K.L.; Tsai, K.J.; Tu, T.Y.; Shyong, Y.J.; Jou, I.M.; Hsu, C.C.; Shih, S.S.; Liu, Y.F.; Lin, C.L. Oxidative stress mediates age-related hypertrophy of ligamentum flavum by inducing inflammation, fibrosis, and apoptosis through activating Akt and MAPK pathways. Aging 2020, 12, 24168–24183. [Google Scholar] [CrossRef]
- Abbas, J.; Hamoud, K.; Masharawi, Y.M.; May, H.; Hay, O.; Medlej, B.; Peled, N.; Hershkovitz, I. Ligamentum flavum thickness in normal and stenotic lumbar spines. Spine 2010, 35, 1225–1230. [Google Scholar] [CrossRef]
- Safak, A.A.; Is, M.; Sevinc, O.; Barut, C.; Eryoruk, N.; Erdogmus, B.; Dosoglu, M. The thickness of the ligamentum flavum in relation to age and gender. Clin. Anat. 2010, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Park, J.Y.; Kim, D.H.; Karm, M.H.; Lee, J.Y.; Yoo, J.I.; Chon, S.W.; Suh, J.H. The Role of the Ligamentum Flavum Area as a Morphological Parameter of Lumbar Central Spinal Stenosis. Pain Physician 2017, 20, E419–E424. [Google Scholar]
- Sairyo, K.; Biyani, A.; Goel, V.; Leaman, D.; Booth, R., Jr.; Thomas, J.; Gehling, D.; Vishnubhotla, L.; Long, R.; Ebraheim, N. Pathomechanism of ligamentum flavum hypertrophy: A multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine 2005, 30, 2649–2656. [Google Scholar] [CrossRef]
- Myklebust, J.B.; Pintar, F.; Yoganandan, N.; Cusick, J.F.; Maiman, D.; Myers, T.J.; Sances, A., Jr. Tensile strength of spinal ligaments. Spine 1988, 13, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Pintar, F.A.; Yoganandan, N.; Myers, T.; Elhagediab, A.; Sances, A., Jr. Biomechanical properties of human lumbar spine ligaments. J. Biomech. 1992, 25, 1351–1356. [Google Scholar] [CrossRef]
- Chazal, J.; Tanguy, A.; Bourges, M.; Gaurel, G.; Escande, G.; Guillot, M.; Vanneuville, G. Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J. Biomech. 1985, 18, 167–176. [Google Scholar] [CrossRef]
- Dumas, G.A.; Beaudoin, L.; Drouin, G. In situ mechanical behavior of posterior spinal ligaments in the lumbar region. An in vitro study. J. Biomech. 1987, 20, 301–310. [Google Scholar] [CrossRef]
- Nishida, N.; Kanchiku, T.; Kato, Y.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Ohgi, J.; Chen, X.; Taguchi, T. Cervical ossification of the posterior longitudinal ligament: Factors affecting the effect of posterior decompression. J. Spinal Cord Med. 2017, 40, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, T.; Kanchiku, T.; Nishida, N.; Ichihara, K.; Sakuramoto, I.; Ohgi, J.; Funaba, M.; Imajo, Y.; Suzuki, H.; Chen, X.; et al. Age-related changes of the spinal cord: A biomechanical study. Exp. Ther. Med. 2018, 15, 2824–2829. [Google Scholar] [CrossRef]


| Age | Gender 1 | Disease 2 | Stiffness Mean ± STD (MPa) |
|---|---|---|---|
| 33 | F | Schwannoma | 2.951 ± 0 |
| 36 | F | LDH | 2.545 ± 0 |
| 37 | M | LDH | 2.470 ± 0.917 |
| 41 | M | LDH | 2.656 ± 0 |
| 42 | M | LSS | 2.803 ± 0.582 |
| 51 | M | LDH | 3.198 ± 0.471 |
| 51 | F | LDH | 3.501 ± 0.865 |
| 53 | M | LSS | 3.518 ± 0 |
| 56 | F | LSS | 4.310 ± 0.403 |
| 60 | M | LDH | 2.826 ± 0.172 |
| 60 | M | LDH | 3.894 ± 0.429 |
| 60 | F | LSS | 3.341 ± 1.150 |
| 61 | M | LSS | 3.177 ± 0 |
| 62 | M | LSS | 3.769 ± 0.762 |
| 65 | M | LSS | 2.982 ± 0.440 |
| 65 | M | LSS | 3.557 ± 0 |
| 65 | F | LSS | 4.831 ± 0.533 |
| 66 | M | LDH | 2.832 ± 0.947 |
| 67 | M | LSS | 6.592 ± 0.543 |
| 68 | F | LDH | 3.475 ± 0.549 |
| 68 | F | LSS | 4.081 ± 0 |
| 69 | F | LDH | 2.662 ± 0.746 |
| 70 | F | LSS | 4.632 ± 0.984 |
| 71 | M | LSS | 6.849 ± 0 |
| 73 | F | LDH | 4.047 ± 0.993 |
| 73 | F | LSS | 7.538 ± 0.572 |
| 74 | F | LDH | 9.892 ± 2.527 |
| 74 | M | LSS | 4.309 ± 0.523 |
| 74 | F | LSS | 4.510 ± 0.386 |
| 75 | F | Schwannoma | 5.490 ± 0.661 |
| 76 | F | LSS | 4.656 ± 1.211 |
| 76 | F | LSS | 5.141 ± 0.025 |
| 77 | F | LSS | 4.889 ± 1.032 |
| 77 | M | LSS | 4.150 ± 0.776 |
| 77 | F | LSS | 2.314 ± 0.669 |
| 78 | M | LSS | 4.470 ± 1.940 |
| 78 | M | LSS | 7.063 ± 0 |
| 79 | F | LSS | 3.260 ± 0.670 |
| 79 | F | LSS | 5.581 ± 0.852 |
| 79 | M | LSS | 3.427 ± 0.754 |
| 81 | M | LSS | 6.749 ± 0 |
| 82 | M | LSS | 6.773 ± 0 |
| 83 | M | LSS | 4.832 ± 1.020 |
| 85 | M | LSS | 5.223 ± 0.975 |
| Variable | stdβ | p-Value |
|---|---|---|
| Age | 0.5731 | 0.0004 |
| Gender | −0.0374 | n.s. |
| LSS 1 | 0.1108 | n.s. |
| Non-LSS 1 | 0.1203 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihara, A.; Nishida, N.; Jiang, F.; Ohgi, J.; Imajo, Y.; Suzuki, H.; Funaba, M.; Yamagata, H.; Chen, X.; Sakai, T. Tensile Test of Human Lumbar Ligamentum Flavum: Age-Related Changes of Stiffness. Appl. Sci. 2021, 11, 3337. https://doi.org/10.3390/app11083337
Mihara A, Nishida N, Jiang F, Ohgi J, Imajo Y, Suzuki H, Funaba M, Yamagata H, Chen X, Sakai T. Tensile Test of Human Lumbar Ligamentum Flavum: Age-Related Changes of Stiffness. Applied Sciences. 2021; 11(8):3337. https://doi.org/10.3390/app11083337
Chicago/Turabian StyleMihara, Atsushi, Norihiro Nishida, Fei Jiang, Junji Ohgi, Yasuaki Imajo, Hidenori Suzuki, Masahiro Funaba, Hiroki Yamagata, Xian Chen, and Takashi Sakai. 2021. "Tensile Test of Human Lumbar Ligamentum Flavum: Age-Related Changes of Stiffness" Applied Sciences 11, no. 8: 3337. https://doi.org/10.3390/app11083337






