Abstract
In order to develop new oleanolic acid (OA) derivatives endowed with improved antitumor activities, for the first time, a number of new hybrid compounds were reported by combining OA or 3-oxooleanolic acid with appropriate H2S-donor moiety, coupled via a suitable linker. The anti-tumor evaluation indicated that they exhibited excellent anti-cancer activities against the tested cancer cell lines. Moreover, 18d with 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione moiety as H2S donor and β-alanine as the linker, showed more potent cytotoxicity against the tested cancer cell lines than OA and 3-oxooleanolic acid, especially for A549 cells. Furthermore, the preferred compound, 18d, preferentially accumulates in cancer cells (13.6 μM) over the matched normal cells LO2 (>100 μM) in vitro. The improved antitumor activity of this hybrid was probably due to its H2S-releasing capability.
1. Introduction
One of the major causes of death in patients is cancer, and a major factor to explore in cancer treatment is novel chemotherapy. Approximately 90% of patient deaths are due to cancer or metastatic cancer. Current anticancer agents cannot cure primary tumor and metastatic cells. Thus, the design of novel antitumor therapeutic agents, including oleanolic acid (OA) activities, represents an area in need of urgent attention.
The optimization of compounds derived from natural origins represents one of the promising strategies which is widely used in seeking and developing anticancer drug molecules [1]. Oleanolic acid (OA), a well-known natural pentacyclic triterpenoid, has anti-inflammatory and antitumor activities [2,3]. A number of studies have demonstrated that OA can act at various stages of tumor development to inhibit tumor initiation and promotion, as well as to induce tumor cell differentiation and apoptosis [4]. However, the antitumor activity of OA is relatively weak, and the aqueous solubility of OA is also very poor, which greatly hinders its clinical application [5]. Thus, on the basis of the structure of OA, there have been multiple studies concerning the structural modification and improvement of their antitumor activities and aqueous solubility [6,7]. More importantly, many hybrid OA compounds have been synthesized, including amino acid/dipeptide prodrugs of OA [4,8,9] and nitric oxide (NO) donor hybrid OA compounds [4,10,11,12,13], which showed significantly improved antitumor activity and pharmacokinetic properties. However, to the best of our knowledge, there were no reports focused on hydrogen sulfide (H2S) donor hybrid OA compounds.
Similar to the other two gasotransmitters, NO and carbon monoxide (CO), hydrogen sulfide (H2S) is a signaling molecule and plays an important part in various physiological processes [14,15,16,17]. Recently, H2S donor hybrid compounds have focused on the treatment of tumors; H2S is a signaling molecule involved in the apoptosis of tumor cells, and H2S donors have reported antiangiogenic effects [18,19,20,21]. In previous reports, several H2S donor hybrid compounds have been synthesized and biologically evaluated, which were identified to display increased effectiveness and reduced toxicity when compared with their parent drugs due to synergic effects [22,23,24,25,26,27,28,29,30]. However, the linkage between drugs and hydrogen sulfide donors was relatively simple, most of which was connected by ester bonds, not a heterozygous modification [26,27,28,29,30]. Additionally, compared with the other reported slow-releasing hydrogen sulfide (H2S) donors, only a slow-releasing hydrogen sulfide (H2S) donor in aqueous media over a period of hours to days reportedly compared with sulfide salts such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) in its evaluation of biological effects in cells, tissues and animals, but there are few examples of connecting it to other parent anticancer drugs [26,27,28,29,30]. In recent years, it has been found that the efficiency can be improved by coupling different types of specific or sensitive linkages. Among them, the coupling of amino acids has captured the greatest attention in pro-drug design. The coupling of amino acids can be selectively identified and taken up by peptide transporter 1 (PepT1) to improve the oral absorption of parent drugs with undesirable biopharmaceutical characteristics [4,9]. PepT1 appears to be an attractive target with its high capacity, broad substrate specificity, and high level of expression in the intestinal epithelium. Many PepT1 targeted prodrugs have been designed and synthesized with the aim of improving oral bioavailability of parent drugs, in which valacyclovir and valganciclovir are two excellent examples (they are L-Val ester prodrugs of acyclovir and ganciclovir, respectively) [8]. Furthermore, the redox sensitiveness disulfide structure is a promising linkage because the redox process is widely present in physiological environments. Especially, there is a significant redox gradient for tumors between the intracellular components and the extracellular environments. Many studies have reported that polymer micelles with disulfide structures have a good ability to transfer antitumor drug to cancer cells and effectively perform intracellular drug release [31,32].
Regarding the abovementioned studies, it may be interesting to study the hybrid OA compounds containing H2S-donating species through different linkers. Herein, oleanolic acid and 3-oxooleanolic acid (3-oxo-OA) were coupled with a number of classic H2S-releasing moieties via different linkers, including ester bonds, amino acids, reduction-sensitive disulfide bonds, and the chemically and metabolically stable ether bonds. Their anticancer, H2S-releasing ability and the effects of the type of linker were biologically evaluated.
2. Materials and Methods
2.1. General Procedure for the Synthesis of Targeted Compounds
We purchased all reagents from Shanghai Chemical Reagent Company. The silica gel of the Column chromatography was 200–300 mesh and the Column chromatography was examined by thin layer chromatography performed on GF/UV 254 plates and was visualized with UV light at 365 and 254 nm. Results of the 1H NMR spectra: BrukerAVANCE Ⅲ equipment at 300 or 400 MHz, in CDCl3 unless otherwise specified; δ in ppm relative to Me4Si, J in Hz. The 13C NMR spectra: BrukerAVANCE Ⅲ equipment at 75 or 100 MHz, in CDCl3 unless otherwise specified; δ in ppm relative to Me4Si. High-resolution mass spectra (HRMS; m/z) were taken using a Thermo QE spectrometer.
The starting materials OA and 4-OH-TBZ were purchased directly. The compound 3-oxo-OA was synthesized using the reported method [33]. The H2S-releasing groups ADT-OH and N-benzyl-4-hydroxybenzothioamide were prepared according to the reported methods [34,35].
2.1.1. General Procedure for the Preparation of 2a–c
Oleanolic acid, white needles (CH3OH): 1H NMR (CDCl3, 400 MHz) δ ppm 5.27 (t, 1H, J = 3.2 Hz, 12-H), 3.22 (dd, 1H, J = 11.2, 4.0 Hz, 3-H), 2.81 (dd, 1H, J = 14.4, 4.0 Hz, 18-H), 1.13–2.20 (m, 22H, methylene and methine protons), 1.12 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.92 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.77 (s, 3H, 27-H), 0.74 (s, 3H, 24-H), OH and COOH show no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 183.1, 143.6, 122.6, 79.1, 55.2, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.8, 38.4, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7, 28.1, 27.7, 27.2, 25.9, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3.
EDCI (2.4 mmol), DMAP (0.1 mmol) were added to OA (1.0 mmol) in anhydrous CH2Cl2 (20 mL) solution. The mixture was stirred for 20 min under room temperature [36,37]. Then, ADT-OH or 4-OH-TBZ or N-benzyl-4-hydroxybenzothioamide (2.0 mmol, dissolved in 10 mL dry CH2Cl2) was added dropwise to the mixture and the reaction solution was whisked until the end of the reaction. Then, we washed the whole solution using water (20 mL × 3), saturated NaHCO3 aqueous solution (20 mL × 3) and saturated brine (20 mL × 3), dried and filtered it with anhydrous Na2SO4, inspissated it under reduced pressure to obtain a remainder, and purified it by flash column chromatography to acquire pure product.
Oleanolic acid 28-(4-thiocarbamoyl) benzoate (2a). Yield: 41%. White solid, m.p.: 200.3–202.0 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.65 (d, 2H, J = 8.2 Hz, Ar-H), 7.13 (d, 2H, J = 8.2 Hz, Ar-H), 5.34 (brs, 1H, 12-H), 3.20 (m, 1H, 3-H), 2.93 (br d, 1H, J = 11.3 Hz, 18-H), 1.18–2.20 (m, 22H, methylene and methine protons), 1.17 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.95 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.80 (s, 3H, 27-H), 0.76 (s, 3H, 24-H), OH and NH2 show no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 175.5, 154.5, 143.0, 133.6, 122.8, 123.1, 109.4, 78.9, 55.2, 47.5, 47.4, 45.7, 41.8, 41.4, 39.5, 38.8, 38.5, 37.0, 33.8, 33.1, 32.8, 32.3, 30.7, 28.1, 27.8, 27.2, 25.8, 23.6, 23.4, 23.0, 18.3, 17.4, 15.6, 15.4; HRMS (ESI) Calcd for C37H54NO3S [M + H]+ 592.3824, found: 592.3813.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl)]benzoate (2b). Yield: 57%. Yellow solid, m.p.: 154.5–156.1 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.64 (d, 2H, J = 8.2 Hz, Ar-H), 7.39 (s, 1H, 8′-H), 7.15 (d, 2H, J = 8.2 Hz, Ar-H), 5.35 (br s, 1H, 12-H), 3.21 (m, 1H, 3-H), 2.80 (br d, 1H, J = 11.7 Hz, 18-H), 1.19–2.20 (m, 22H, methylene and methine protons), 1.18 (s, 3H, 29-H), 0.96 (s, 3H, 30-H), 0.96 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.82 (s, 3H, 27-H), 0.77 (s, 3H, 24-H), OH shows no no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 215.3, 175.7, 172.0, 147.1, 143.1, 128.9, 128.2, 124.0, 123.1, 122.8, 79.0, 55.2, 47.6, 47.4, 45.7, 41.8, 41.5, 39.5, 38.8, 38.5, 37.0, 33.8, 33.1, 32.8, 32.4, 30.7, 28.1, 27.8, 27.1, 25.9, 23.6, 23.5, 23.1, 18.3, 17.5, 15.7, 15.4; HRMS (ESI) Calcd for C39H53O3S3 [M + H]+ 665.3157, found: 665.3153.
Oleanolic acid 28-[4-(N-benzyl)-thiocarbamoyl] benzoate (2c). Yield: 40%. Yellow solid, m.p.: 171.8–173.4 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.76 (d, 2H, J = 8.2 Hz, Ar-H), 7.37–7.33 (m, 5H, Ar-H), 7.03 (d, 2H, J = 8.2 Hz, Ar-H), 5.34 (br s, 1H, 12-H), 4.98 (d, 2H, J = 5.2 Hz, 1′′-H), 3.20 (m, 1H, 3-H), 2.90 (br d, 1H, J = 11.2 Hz, 18-H), 1.13–2.20 (m, 22H, methylene and methine protons), 1.18 (s, 3H, 29-H), 0.99 (s, 3H, 30-H), 0.97 (s, 3H, 26-H), 0.94 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.80 (s, 3H, 27-H), 0.78 (s, 3H, 24-H), OH and -CSNH- show no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 175.5, 154.5, 143.0, 134.8, 133.6, 128.5, 128.5, 126.9, 126.9, 126.7, 123.1, 122.8, 109.4, 78.9, 55.2, 50.3, 47.5, 47.4, 45.7, 41.8, 41.4, 39.5, 38.8, 38.5, 37.0, 33.8, 33.1, 32.8, 32.3, 30.7, 28.1, 27.8, 27.2, 25.8, 23.6, 23.4, 23.0, 18.3, 17.4, 15.6, 15.4; HRMS (ESI) Calcd for C44H60NO3S [M + H]+ 682.4294, found: 682.4296.
2.1.2. General Procedure for the Preparation of 4a–d
The corresponding dibromoalkane (3.0 mmol), KOH (1.0 mmol) and KI (0.1 mmol) were added to OA (1.0 mmol) which was dissolved in anhydrous tetrahydrofuran (20 mL) solution. The reaction solution was heated to reflux and whisked until the end of the reaction. The solvent was removed under vacuum. The remainder was diluted with water and extracted with EtOAc. The organic layer was washed with brine and then dried with sodium sulfate, filtered, and evaporated in vacuo to give corresponding middle product, 3a or 3b, respectively, which was used in the next step without further purification. ADT-OH or 4-OH-TBZ (2.0 mmol), KOH (2.0 mmol) and KI (0.1 mmol) were added to compounds 3a or 3b (1.0 mmol), which were dissolved in anhydrous tetrahydrofuran (20 mL) solution. The reaction mixture was heated to reflux and whisked until the end of the reaction. Then, the solvent was removed under vacuum, and the remainder was diluted with water and extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. The resulting crude product was purified by flash chromatography on silica gel column to provide the target compound 4a–d, respectively.
Oleanolic acid 28-[4-(4-carbamothioylphenoxy)] butyrate (4a). Yield: 24%. Oil: 1H NMR (CDCl3, 300 MHz) δ ppm 7.65 (d, 2H, J = 8.5 Hz, Ar-H), 6.93 (d, 2H, J = 8.5 Hz, Ar-H), 5.27 (brs, 1H, 12-H), 4.09 (t, 2H, J = 6.8 Hz, 1′-H), 4.03 (t, 2H, J = 6.8 Hz, 4′-H), 3.20 (m, 1H, 3-H), 2.87 (br d, 1H, J = 11.8 Hz, 18-H), 1.18–2.20 (m, 26H, methylene and methine protons), 1.13 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.92 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.77 (s, 3H, 27-H), 0.72 (s, 3H, 24-H), OH and NH2 show no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 177.8, 162.2, 143.8, 134.0, 122.4, 115.2, 79.0, 67.7, 63.6, 55.2, 47.6, 46.8, 45.8, 41.7, 41.3, 39.3, 38.7, 38.4, 37.0, 33.8, 33.1, 32.5, 32.4, 31.4, 30.7, 30.1, 28.1, 27.6, 27.1, 25.8, 23.6, 23.4, 23.0, 18.3, 17.0, 15.6, 15.3; HRMS (ESI) Calcd for C41H62NO4S [M + H]+ 664.4394, found: 664.4387.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenoxy] butyrate (4b). Yield: 57%. Brown yellow solid, m.p.: 109.1–111.0 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.61 (d, 2H, J = 8.4 Hz, Ar-H), 7.40 (s, 1H, 8′′-H), 6.96 (d, 2H, J = 8.4 Hz, Ar-H), 5.28 (brs, 1H, 12-H), 4.10 (t, 2H, J = 6.8 Hz, 1′-H), 4.05 (t, 2H, J = 6.8 Hz, 4′-H), 3.20 (m, 1H, 3-H), 2.87 (br d, 1H, J = 12.4 Hz, 18-H), 1.13–2.80 (m, 26H, methylene and methine protons), 1.13 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.92 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.77 (s, 3H, 27-H), 0.72 (s, 3H, 24-H), OH shows no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 215.1, 177.7, 162.3, 147.1, 143.8, 134.6, 128.6, 124.0, 122.4, 115.4, 79.0, 67.8, 63.6, 55.2, 47.6, 46.8, 45.8, 41.7, 41.4, 39.3, 38.8, 38.4, 37.0, 33.9, 33.1, 32.7, 32.5, 31.5, 30.7, 30.2, 28.1, 27.7, 27.2, 25.9, 23.7, 23.4, 23.0, 18.3, 17.1, 15.6, 15.3; HRMS (ESI) Calcd for C43H61O4S3 [M + H]+ 737.3726, found: 737.3723.
Oleanolic acid 28-[4-(4-carbamothioylphenoxy)] valerate (4c). Yield: 21%. Oil: 1H NMR (CDCl3, 400 MHz) δ ppm 7.66 (d, 2H, J = 8.5 Hz, Ar-H), 6.93 (d, 2H, J = 8.5 Hz, Ar-H), 5.30 (brs, 1H, 12-H),4.05 (t, 2H, J = 6.8 Hz, 1′-H), 4.03 (t, 2H, J = 6.8 Hz, 5′-H),3.20 (m, 1H, 3-H), 2.87 (brd, 1H, J = 11.8 Hz, 18-H), 1.12–2.20 (m, 28H, methylene and methine protons), 1.11 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.95 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.80 (s, 3H, 27-H), 0.78 (s, 3H, 24-H), OH and NH2 show no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 177.8, 162.2, 143.8, 134.0, 122.3, 115.2, 109.4, 79.0, 68.2, 63.9, 55.2, 47.6, 46.8, 45.9, 41.7, 41.3, 39.3, 38.7, 38.4, 37.0, 33.8, 33.1, 32.5, 31.6, 30.7, 28.7, 28.1, 27.5, 27.2, 26.9, 25.9, 23.6, 23.4, 22.7, 18.3, 17.1, 15.6, 15.3; HRMS (ESI) Calcd for C42H64NO4S [M + H]+ 678.4551, found: 678.4545.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenoxy] valerate (4d). Yield: 26%. Reddish brown solid, m.p.: 164.9–165.9 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.61 (d, 2H, J = 8.4 Hz, Ar-H), 7.39 (s, 1H, 8′′-H), 6.95 (d, 2H, J = 8.4 Hz, Ar-H), 5.27 (brs, 1H, 12-H), 4.05 (t, 2H, J = 6.8 Hz, 1′-H), 4.03 (t, 2H, J = 6.8 Hz, 5′-H), 3.19 (m, 1H, 3-H), 2.86 (brd, 1H, J = 10.9 Hz, 18-H), 1.13–2.20 (m, 28H, methylene and methine protons), 1.12 (s, 3H, 29-H), 0.97 (s, 3H, 30-H), 0.92 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.76 (s, 3H, 27-H), 0.72 (s, 3H, 24-H), OH shows no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 215.3, 177.8, 162.4, 143.8, 134.6, 128.6, 122.3, 115.4, 79.0, 68.2, 63.9, 55.2, 47.6, 46.7, 45.9, 41.7, 41.3, 39.3, 38.7, 38.4, 37.0, 33.8, 33.1, 32.6, 32.5, 31.6, 30.7, 28.7, 28.1, 27.5, 27.2, 26.9, 25.9, 23.6, 23.4, 22.7, 18.3, 17.1, 15.6, 15.3; HRMS (ESI) Calcd for C44H63O4S3 [M + H]+ 751.3883, found: 751.3886.
2.1.3. General Procedure for the Preparation of 6a, 6b and 8a–c
OA (1.0 mmol) was dissolved in 20 mL anhydrous dichloromethane solvent, to which 1,5,6-oxadithionane-2,9-dione (4.0 mmol) or succinic anhydride (4.0 mmol) and a certain amount of catalyst DMAP were added. This reaction mixture was heated to reflux while continuously stirring to ensure full reaction. The resulting reaction mixture was first diluted with anhydrous dichloromethane solvent (10 mL). The diluted solution was washed with water (20 mL × 3), saturated aqueous sodium bicarbonate solution (20 mL × 3), and saturated brine (20 mL × 3) three times. The organic layer was collected, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain intermediate product 5 or 7. The intermediate product 5 or 7 (1.0 mmol) was dissolved in 20 mL of anhydrous dichloromethane solvent, dehydrating agent EDCI (2.4 mmol) was added, and a certain amount of dimethylaminopyridine catalyst was present in the flask. This was stirred for 20 min at room temperature. Then, N-benzyl-4-hydroxybenzothioamide or 4-OH-TBZ or ADT-OH 2.0 mmol was dissolved in 10 mL of anhydrous dichloromethane solvent, dropwise added to the flask, and continued to react until the reaction was completed. Then, the whole solution was washed 3 times with water (20 mL × 3), saturated aqueous sodium bicarbonate solution (20 mL × 3), and saturated brine (20 mL × 3), in that order. The organic layer was collected, dried over anhydrous sodium sulfate, filtered, evaporated in vacuo, and passed through the column; the residues obtained after separation and purification were the corresponding products 6a, 6b and 8a–c.
Oleanolic acid 3-[4-(4-carbamothioylphenyl)] succinate (6a). Yield: 29%. Yellow solid, m.p.: 140.4–142.0 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.14 (d, 2H, J = 7.6 Hz, Ar-H), 7.06 (d, 2H, J = 7.6 Hz, Ar-H), 5.35 (brs, 1H, 12-H), 4.55 (t, 1H, J = 7.2 Hz, 3-H), 2.95 (dd, 1H, J = 13.6, 2.4 Hz, 18-H), 2.90 (t, 2H, J = 6.0 Hz, 3′-H), 2.75 (t, 2H, J = 6.0 Hz, 2′-H), 1.19–2.20 (m, 22H, methylene and methine protons), 1.18 (s, 3H, 29-H), 0.97 (s, 3H, 30-H), 0.94 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.87 (s, 3H, 27-H), 0.81 (s, 3H, 24-H), COOH and NH2 show no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 182.5, 173.8, 172.8, 154.5, 143.6, 133.6, 122.8, 122.6, 109.4, 80.5, 55.2, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.4, 38.0, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7, 30.1, 29.8, 28.1, 27.7, 25.9, 24.7, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3; HRMS (ESI) Calcd for C41H58NO6S [M + H]+ 692.3979, found: 692.3973.
Oleanolic acid 3-[4-(5-thioxo-5H-1,2-dithiol-3-yl)phenyl] succinate (6b). Yield: 30%. Red brown solid, m.p.: 126.6–127.6 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.40 (s, 1H, 8′′-H), 7.25 (d, 2H, J = 8.8 Hz, Ar-H),7.17 (d, 2H, J = 8.8 Hz, Ar-H), 5.36 (t, 1H, J = 3.2 Hz, 12-H), 4.57 (t, 1H, J = 7.6 Hz, 3-H), 2.97 (dd, 1H, J = 10.2, 2.4 Hz, 18-H), 2.91 (t, 2H, J = 6.4 Hz, 2′-H), 2.76 (t, 2H, J = 6.4 Hz, 3′-H), 1.27–2.20 (m, 22H, methylene and methine protons), 1.26 (s, 6H, 29-H and 30-H), 1.19 (s, 3H, 26-H), 0.98 (s, 3H, 23-H), 0.95 (s, 3H, 25-H), 0.88 (s, 3H, 27-H), 0.83 (s, 3H, 24-H), COOH shows no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 215.3, 182.5, 173.8, 172.8,172.0, 147.1, 143.6, 128.9, 128.2, 124.0, 123.1, 122.6, 80.5, 55.2, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.4, 38.0, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7, 30.1, 29.8, 28.1, 27.7, 25.9, 24.7, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3; HRMS (ESI) Calcd for C43H57O6S3 [M + H]+ 765.3312, found: 765.3317.
Oleanolic acid 3-[((3-(4-carbamothioylphenoxy)-3-oxopropyl)disulfanyl)propanoyl)oxy] carboxylic acid (8a). Yield: 43%. Brown yellow solid, m.p.: 127.4–128.9 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.90 (d, 2H, J = 8.2 Hz, Ar-H), 7.15 (d, 2H, J = 8.2 Hz, Ar-H), 5.30 (t, 1H, J = 3.6 Hz, 12-H), 4.54 (t, 1H, J = 7.6 Hz, 3-H), 3.03 (m, 4H, 1′′-H and 3′-H), 2.97 (t, 2H, J = 6.8 Hz, 2′′-H), 2.82 (dd, 1H, J = 13.6, 3.2 Hz, 18-H), 2.75 (t, 2H, J = 6.8 Hz, 2′-H), 1.14–2.20 (m, 22H, methylene and methine protons), 1.13 (s, 3H, 29-H), 0.93 (s, 3H, 30-H), 0.93 (s, 3H, 26-H), 0.91 (s, 3H, 23-H), 0.88 (s, 3H, 25-H), 0.86 (s, 3H, 27-H), 0.76 (s, 3H, 24-H), COOH and NH2 show no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 182.5,173.8, 172.8, 154.5, 143.6, 133.6, 122.8, 122.6, 109.4, 80.5, 55.2, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.4, 38.0, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7,30.3, 30.2, 30.1, 30.0, 28.1, 27.7, 25.9, 24.7, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3; HRMS (ESI) Calcd for C43H62NO6S3 [M + H]+ 784.3734, found: 784.3738.
Oleanolic acid 3-[((3-oxo-3-(4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)propyl) disulfanyl)propanoyl)oxy)] carboxylic acid (8b). Yield: 40%. Red brown solid, m.p.: 130.8–140.8 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.68 (d, 2H, J = 8.2 Hz, Ar-H), 7.40 (s, 1H, 8′′′-H), 7.26 (d, 2H, J = 8.2 Hz, Ar-H), 5.27 (t, 1H, J = 3.6 Hz, 12-H), 4.54 (t, 1H, J = 8.0 Hz, 3-H), 3.04 (m, 4H, 1′′-H and 3′-H), 2.98 (t, 2H, J = 6.8 Hz, 2′′-H), 2.82 (dd, 1H, J = 13.6, 3.2 Hz, 18-H), 2.75 (t, 2H, J = 6.8 Hz, 2′-H), 1.11–2.20 (m, 22H, methylene and methine protons), 1.13 (s, 3H, 29-H), 0.93 (s, 3H, 30-H), 0.93 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.85 (s, 3H, 27-H), 0.75 (s, 3H, 24-H), COOH shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 215.3, 182.5, 173.8, 172.8, 172.0, 147.1, 143.6, 128.9, 128.2, 124.0, 123.1, 122.6, 80.5, 55.2, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.4, 38.0, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7, 30.3, 30.2, 30.1, 30.0, 28.1, 27.7, 25.9, 24.7, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3; HRMS (ESI) Calcd for C45H61O6S5 [M + H]+ 857.3066, found: 857.3061.
Oleanolic acid 3-[((3-(4-(benzylcarbamothioyl)phenoxy)-3-oxopropyl)disulfanyl) propanoyl)oxy)] carboxylic acid (8c). Yield: 39%. Bright yellow solid, m.p.: 139.5–140.8 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.77 (d, 2H, J = 8.8 Hz, Ar-H), 7.35–7.40 (m, 5H, Ar-H), 7.11 (d, 2H, J = 8.8 Hz, Ar-H), 5.27 (t, 1H, J = 3.6 Hz, 12-H), 4.98 (d, 2H, J = 5.2 Hz, 1′′′′-H), 4.53 (t, 1H, J = 8.0 Hz, 3-H), 3.01 (t, 4H, J = 3.6 Hz, 1′′-H and 3′-H), 2.96 (t, 2H, J = 6.8 Hz, 2′′-H), 2.82 (dd, 1H, J = 13.6, 3.2 Hz, 18-H), 2.74 (t, 2H, J = 6.8 Hz, 2′-H), 1.14–2.20 (m, 22H, methylene and methine protons), 1.13 (s, 3H, 29-H), 0.93 (s, 3H, 30-H), 0.93 (s, 3H, 26-H), 0.91 (s, 3H, 23-H), 0.87 (s, 3H, 25-H), 0.85 (s, 3H, 27-H), 0.75 (s, 3H, 24-H), COOH shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 182.5, 173.8, 172.8, 154.5, 143.6, 134.8, 133.6, 128.6, 128.5, 126.9, 126.7, 122.8 122.6 109.4 80.5, 55.2, 50.4, 47.6, 46.5, 45.9, 41.6, 41.0, 39.3, 38.4, 38.0, 37.1, 33.8, 33.1, 32.6, 32.4, 30.7, 30.3, 30.2, 30.1, 28.1, 27.7, 25.9, 24.7, 23.6, 23.4, 22.9, 18.3, 17.1, 15.5, 15.3; HRMS (ESI) Calcd for C50H68NO6S3 [M + H]+ 874.4203, found: 874.4214.
2.1.4. General Procedure for the Preparation of 12a, 12b
A mixture of OA (1.0 mmol) and Et3N (1.0 mmol) in anhydrous CH2Cl2 (25 mL) was stirred at room temperature for 10 min. The reaction mixture was cooled to 0 °C, oxalyl chloride (5.0 mmol) in anhydrous CH2Cl2 (2 mL) was added dropwise, and the mixture was allowed to stir at 0 °C for an additional 30 min. Then, the solvent was removed in vacuo to give the middle product 9, which was used in the next step without further purification. To a solution of different ethyl-amino acid (5.0 mmol) and TEA (10.0 mmol) in dry CH2Cl2 (25 mL), the middle product 9 (1.0 mmol) in anhydrous CH2Cl2 (2 mL) was added dropwise at 0 °C, and the mixture was stirred at room temperature for about 5 h. Then, the solvent was removed in vacuo, and the residue was diluted with water and extracted with EtOAc. The organic layer was washed with saturated NH4Cl aqueous solution, brine, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure to obtain the middle products 10a or 10b, respectively. The middle product, 10a or 10b (1.0 mmol), was dissolved in dry THF (25 mL), and 4 N NaOH (20.0 mmol) was added into the flask. The mixture was stirred at room temperature until the starting material was totally consumed, as indicated by TLC. The reaction mixture was diluted with H2O (20 mL), neutralized with 1 N HCl, and extracted three times with CHCl3. The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under vacuum to afford the middle products 11a or 11b. The reaction mixture with 20 mL of water was diluted and neutralized with 1 N HCl for acid–base, the neutralized solution was extracted three times with chloroform, and the organic layer was collected. The brine was used to wash the composite organic layer, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain intermediate product 11a or 11b. A solution of 20 mL anhydrous dichloromethane was added to 11a or 11b (1.0 mmol) to dissolve it, and certain amounts of dimethyl-aminopyridine and dehydrating agent EDCI (490 mg, 2.4 mmol) were added. This mixture was stirred for 20 min at room temperature. ADT-OH 2.0 mmol was dissolved in 10 mL anhydrous dichloromethane solution and added to the reaction solution, and the reaction continued at room temperature until the end of the reaction. Then, the reaction solution was washed with water (20 mL × 3), saturated aqueous sodium bicarbonate solution (20 mL × 3), and saturated brine (20 mL × 3) successively, dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off under reduced pressure; after separation and purification by column chromatography, the pure products 12a or 12b could be obtained.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenyl] glycinate (12a). Yield: 20%. Orange solid, m.p.: 222.9–224.5 °C: 1H NMR (CDCl3, 300 MHz) δ ppm 7.66 (d, 2H, J = 8.8 Hz, Ar-H), 7.39 (s, 1H, 8′′-H), 7.16 (d, 2H, J = 8.8 Hz, Ar-H), 6.29 (t, 1H, J = 5.6 Hz, NH), 5.36 (t, 1H, J = 3.2 Hz, 12-H), 4.36 (d, 1H, J = 7.2 Hz, 1′-Ha), 4.32 (d, 1H, J = 7.2 Hz, 1′-Hb), 3.24 (m, 1H, 3-H), 2.97 (dd, 1H, J = 10.2, 3.0 Hz, 18-H), 1.13–2.20 (m, 22H, methylene and methine protons), 1.12 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.95 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.80 (s, 3H, 27-H), 0.76 (s, 3H, 24-H), OH shows no signal; 13C NMR (CDCl3, 75 MHz) δ ppm 215.5, 178.5, 171.6, 171.2, 153.5, 143.0, 136.0, 129.2, 128.2, 123.1, 122.9, 78.9, 55.2, 47.5, 47.4, 46.9, 45.7, 41.8, 41.4, 39.5, 38.8, 38.5, 37.0, 33.8, 33.1, 32.8, 32.3, 30.7, 28.1, 27.8, 27.2, 25.8, 23.6, 23.4, 23.0, 18.3, 17.4, 15.6, 15.4; HRMS (ESI) Calcd for C41H56NO4S3 [M + H]+ 722.3366, found: 722.3359.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenyl] carboxamido propanoate (12b). Yield: 24%. Brick red solid, m.p.: 129.4–131.2 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.56 (d, 2H, J = 8.4 Hz, Ar-H), 7.13 (s, 1H, 8′′-H), 7.11 (d, 2H, J = 8.4 Hz, Ar-H), 6.28 (t, 1H, J = 5.6 Hz, NH), 5.32 (brs, 1H, 12-H), 3.62 (m, 1H, 1′-Ha), 3.31 (m, 1H, 1′-Hb), 3.20 (m, 1H, 3-H), 2.74 (m, 1H, 18-H), 2.70 (m, 2H, 2′-H), 1.13–2.20 (m, 22H, methylene and methine protons), 1.12 (s, 3H, 29-H), 0.98 (s, 3H, 30-H), 0.95 (s, 3H, 26-H), 0.93 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.80 (s, 3H, 27-H), 0.76 (s, 3H, 24-H), OH shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 215.5, 178.5, 171.6, 171.2, 153.5, 143.0, 136.0, 129.2, 128.2, 123.1, 122.9, 78.9, 55.2, 47.5, 47.4, 46.6, 45.7, 41.8, 41.4, 39.5, 38.8, 38.5, 37.0, 33.8, 33.1, 32.8, 32.3, 30.7, 28.1, 27.8, 27.2, 25.8, 24.9, 23.6, 23.4, 23.0, 18.3, 17.4, 15.6, 15.4; HRMS (ESI) Calcd for C42H58NO4S3 [M + H]+ 736.3522, found: 736.3519.
2.1.5. General Procedure for the Preparation of 14a–c
Compound 1 was treated in acetone at 0 °C with Jones reagent to form the C-3 oxidized derivative 13 in an almost quantitative yield. Then, using compound 13 as a starting material, the target products 14a–c were obtained according to the method of preparing 2a–c described above.
Compound 3-oxo-oleanolic acid 28-(4-thiocarbamoyl) benzoate (14a). Yield: 45%. Yellow solid, m.p.: 144.5–145.0 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.66 (d, 2H, J = 8.2 Hz, Ar-H), 7.13 (d, 2H, J = 8.2 Hz, Ar-H), 5.37 (t, 1H, J = 3.2 Hz, 12-H), 2.95 (dd, 1H, J = 13.6, 2.4 Hz, 18-H), 2.53 (m, 1H, 2-Ha), 2.35 (m, 1H, 2-Hb), 1.20–2.20 (m, 20H, methylene and methine protons), 1.19 (s, 3H, 29-H), 1.07 (s, 3H, 30-H), 1.03 (s, 3H, 26-H), 1.03 (s, 3H, 23-H), 0.97 (s, 3H, 25-H), 0.93 (s, 3H, 27-H), 0.83 (s, 3H, 24-H), NH2 shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 175.5, 154.5, 145.0, 133.6, 122.8, 122.5, 109.4, 55.2, 47.4, 46.7, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C37H52NO3S [M + H]+ 590.3662, found: 590.3667.
Compound 3-oxo-oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl)] benzoate (14b). Yield: 58%. Orange solid, m.p.: 197.4–198.7 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.63 (d, 2H, J = 8.2 Hz, Ar-H), 7.38 (s, 1H, 8′-H), 7.13 (d, 2H, J = 8.2 Hz, Ar-H), 5.33 (t, 1H, J = 3.2 Hz, 12-H), 2.95 (dd, 1H, J = 13.6, 2.4 Hz, 18-H), 2.53 (m, 1H, 2-Ha), 2.35 (m, 1H, 2-Hb), 1.20–2.20 (m, 20H, methylene and methine protons), 1.19 (s, 3H, 29-H), 1.08 (s, 3H, 30-H), 1.03 (s, 3H, 26-H), 1.03 (s, 3H, 23-H), 0.99 (s, 3H, 25-H), 0.93 (s, 3H, 27-H), 0.81 (s, 3H, 24-H); 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 215.3, 175.5, 172.0, 147.1, 145.0, 128.9, 128.2, 124.0, 123.1, 122.5, 55.2, 47.4, 46.7, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C39H51O3S3 [M + H]+ 663.2995, found: 663.2990.
Compound 3-oxo-oleanolic acid 28-[4-(N-benzyl)-thiocarbamoyl] benzoate (14c). Yield: 55%. Bright yellow solid, m.p.: 127.2–128.4 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.77 (d, 2H, J = 8.2 Hz, Ar-H), 7.39–7.33 (m, 5H, Ar-H), 7.03 (d, 2H, J = 8.2 Hz, Ar-H), 5.38 (t, 1H, J = 3.2 Hz, 12-H), 4.98 (d, 2H, J = 5.2 Hz, 1′′-H), 2.96 (dd, 1H, J = 14.0, 4.0 Hz, 18-H), 2.53 (m, 1H, 2-Ha), 2.35 (m, 1H, 2-Hb), 1.20–2.20 (m, 20H, methylene and methine protons), 1.19 (s, 3H, 29-H), 1.08 (s, 3H, 30-H), 1.04 (s, 3H, 26-H), 1.04 (s, 3H, 23-H), 0.97 (s, 3H, 25-H), 0.94 (s, 3H, 27-H), 0.85 (s, 3H, 24-H), -CSNH- shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 175.5, 154.5, 145.0, 134.8, 133.6, 128.5, 128.5, 126.9, 126.7, 122.8, 122.5, 109.4, 55.2, 50.3, 47.4, 46.7, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C44H58NO3S [M + H]+ 680.4132, found: 680.4133.
2.1.6. General Procedure for the Preparation of 18a–f
Using compound 13 instead of compound 1 as raw material, the target products 18a–f were obtained according to the method of preparing 12a and 12b described above.
Compound 3-oxo-oleanolic acid 28-(4-thiocarbamoyl) glycinate (18a). Yield: 19%. Bright yellow solid, m.p.: 139.0–140.3 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.91 (d, 2H, J = 8.8 Hz, Ar-H), 7.15 (d, 2H, J = 8.8 Hz, Ar-H), 6.55 (t, 1H, J = 4.8 Hz, NH), 5.45 (t, 1H, J = 3.2 Hz, 12-H), 4.38 (dd, 1H, J = 18.4, 5.2 Hz, 1′-H), 4.08 (dd, 1H, J = 18.4, 4.0 Hz, 1′-H), 2.65 (dd, 1H, J = 12.8, 3.2 Hz, 18-H), 2.54 (m, 1H, 2-Ha), 2.36 (m, 1H, 2-Hb), 1.19–2.20 (m, 20H, methylene and methine protons), 1.18 (s, 3H, 29-H), 1.08 (s, 3H, 30-H), 1.04 (s, 3H, 26-H), 1.03 (s, 3H, 23-H), 0.91 (s, 3H, 25-H), 0.91 (s, 3H, 27-H), 0.80 (s, 3H, 24-H), NH2 shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 171.6, 171.2, 154.5, 145.0, 133.6, 122.8, 122.5, 109.4, 55.2, 47.4, 46.8, 46.7, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C39H55N2O4S3 [M + H]+ 647.3877, found: 647.3872.
Compound 3-oxo-oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenyl] glycinate (18b). Yield: 28%. Brick red solid, m.p.: 107.9–109.1 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.57 (d, 2H, J = 8.8 Hz, Ar-H), 7.14 (d, 2H, J = 8.8 Hz, Ar-H), 7.13 (s, 1H, 8′′-H), 6.41 (t, 1H, J = 4.8 Hz, NH), 5.33 (t, 1H, J = 3.2 Hz, 12-H), 4.28 (dd, 1H, J = 18.4, 5.6 Hz, 1′-H), 3.96 (dd, 1H, J = 18.4, 4.0 Hz, 1′-H), 2.52 (dd, 1H, J = 12.8, 3.2 Hz, 18-H), 2.42 (m, 1H, 2-Ha), 2.23 (m, 1H, 2-Hb), 1.06–2.20 (m, 20H, methylene and methine protons), 1.05 (s, 3H, 29-H), 0.95 (s, 3H, 30-H), 0.91 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.78 (s, 3H, 25-H), 0.78 (s, 3H, 27-H), 0.68 (s, 3H, 24-H); 13C NMR (CDCl3, 100 MHz) δ ppm 217.5, 215.5, 178.6, 171.3, 168.3, 153.0, 144.5, 136.1, 129.6, 128.3, 123.0, 122.6, 55.2, 47.4, 46.8, 46.6, 46.4, 42.2, 42.1, 41.8, 39.3, 39.1, 36.7, 34.1, 34.0, 32.9, 32.4, 31.8, 30.7, 27.3, 26.4, 25.6, 23.9, 23.6, 23.5, 19.5, 16.5, 15.0; HRMS (ESI) Calcd for C41H54NO4S3 [M + H]+ 720.3209, found: 720.3215.
Compound 3-oxo-oleanolic acid 28-(4-carbamothioylphenyl) carboxamido propanoate (18c). Yield: 15%. Yellow solid, m.p.: 161.6–162.9 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.90 (d, 2H, J = 8.8 Hz, Ar-H), 7.12 (d, 2H, J = 8.8 Hz, Ar-H), 6.06 (t, 1H, J = 5.2 Hz, NH), 5.34 (t, 1H, J = 3.2 Hz, 12-H), 3.50 (m, 1H, 1′-H), 3.14 (m, 1H, 1′-H), 2.80 (m, 1H, 18-H), 2.66 (m, 1H, 2-Ha), 2.40 (m, 2H, 2′-H), 2.22 (m, 1H, 2-Hb), 1.18–2.20 (m, 20H, methylene and methine protons), 1.17 (s, 3H, 29-H), 1.08 (s, 3H, 30-H), 1.03 (s, 3H, 26-H), 1.01 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.88 (s, 3H, 27-H), 0.83 (s, 3H, 24-H), NH2 shows no signal; 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 171.6, 171.2, 154.5, 145.0, 133.6, 122.8, 122.5, 109.4, 55.2, 47.4, 46.7, 46.6, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 31.8, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C40H57N2O4S [M + H]+ 661.4034, found: 661.4036.
Compound 3-oxo-oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenyl] carboxamido propanoate (18d). Yield: 23%. Brick red solid, m.p.: 113.5–114.7 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.55 (d, 2H, J = 8.4 Hz, Ar-H), 7.12 (s, 1H, 8′′-H), 7.11 (d, 2H, J = 8.4 Hz, Ar-H), 6.28 (t, 1H, J = 5.6 Hz, NH), 5.24 (t, 1H, J = 3.2 Hz, 12-H), 3.62 (m, 1H, 1′-H), 3.31 (m, 1H, 1′-H), 2.73 (m, 1H, 18-H), 2.66 (m, 1H, 2-Ha), 2.40 (m, 2H, 2′-H), 2.22 (m, 1H, 2-Hb), 1.04–2.20 (m, 20H, methylene and methine protons), 1.03 (s, 3H, 29-H), 0.94 (s, 3H, 30-H), 0.90 (s, 3H, 26-H), 0.86 (s, 3H, 23-H), 0.76 (s, 3H, 25-H), 0.75 (s, 3H, 27-H), 0.69 (s, 3H, 24-H); 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 215.5, 178.4, 171.4, 170.6, 153.3, 144.4, 136.0, 129.3, 128.2, 122.7, 122.7, 55.2, 47.4, 46.7, 46.5, 46.4, 42.1, 42.1, 39.3, 39.1, 36.7, 34.7, 34.4, 34.1, 32.9, 32.7, 31.9, 30.7, 27.3, 26.4, 25.6, 23.7, 23.6, 23.5, 21.5, 19.5, 16.9, 15.0; HRMS (ESI) Calcd for C42H56NO4S3 [M + H]+ 734.3366, found: 734.3361.
Compound 3-oxo-oleanolic acid 28-(4-carbamothioylphenyl) carboxamido butanoate (18e). Yield: 15%. Bright yellow solid, m.p.: 124.0–126.0 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.91 (d, 2H, J = 8.8 Hz, Ar-H), 7.13 (d, 2H, J = 8.8 Hz, Ar-H), 6.08 (t, 1H, J = 5.2 Hz, NH), 5.40 (t, 1H, J = 3.2 Hz, 12-H), 3.49 (m, 1H, 1′-H), 3.11 (m, 1H, 1′-H), 2.61(t, 2H, J = 7.2 Hz, 3′-H), 2.55 (m, 1H, 18-H), 2.52 (m, 1H, 2-Ha), 2.36 (m, 1H, 2-Hb), 1.18–2.20 (m, 22H, methylene and methine protons), 1.17 (s, 3H, 29-H), 1.08 (s, 3H, 30-H), 1.03 (s, 3H, 26-H), 1.03 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.88 (s, 3H, 27-H), 0.81 (s, 3H, 24-H); 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 171.6, 171.2, 154.5, 145.0, 133.6, 122.8, 122.5, 109.4, 55.2, 47.4, 46.7, 46.6, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 24.6, 23.7, 23.6, 23.5, 21.4, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C41H59N2O4S [M + H]+ 675.4190, found: 675.4189.
Oleanolic acid 28-[4-(5-thioxo-5H-1,2-dithiol-3-yl) phenyl] carboxamido butanoate (18f). Yield: 25%. Red brown solid, m.p.: 139.8–141.4 °C: 1H NMR (CDCl3, 400 MHz) δ ppm 7.54 (d, 2H, J = 8.8 Hz, Ar-H), 7.13 (s, 1H, 8′′-H), 7.11 (d, 2H, J = 8.8 Hz, Ar-H), 5.93 (t, 1H, J = 5.2 Hz, NH), 5.27 (t, 1H, J = 3.2 Hz, 12-H), 3.39 (m, 1H, 1′-H), 3.00 (m, 1H, 1′-H), 2.51(t, 2H, J = 7.2 Hz, 3′-H), 2.43 (m, 1H, 18-H), 2.38 (m, 1H, 2-Ha), 2.23 (m, 1H, 2-Hb), 1.18–2.20 (m, 22H, methylene and methine protons), 1.17 (s, 3H, 29-H), 1.04 (s, 3H, 30-H), 0.95 (s, 3H, 26-H), 0.90 (s, 3H, 23-H), 0.90 (s, 3H, 25-H), 0.77 (s, 3H, 27-H), 0.69 (s, 3H, 24-H); 13C NMR (CDCl3, 100 MHz) δ ppm 217.4, 215.5, 178.5, 171.6, 171.2, 153.5, 145.0, 136.0, 129.2, 128.2, 122.9, 122.5, 55.2, 47.4, 46.7, 46.6, 46.4, 42.2, 42.1, 39.3, 39.1, 38.6, 36.7, 34.1, 32.9, 32.7, 31.8, 30.7, 27.3, 26.4, 25.6, 24.6, 23.7, 23.6, 23.5, 21.4, 19.5, 16.8, 15.0; HRMS (ESI) Calcd for C43H58NO4S3 [M + H]+ 748.3522, found: 748.3527.
2.2. Cell Lines
Human hepatocellular carcinoma cell line (HepG2), breast cancer cell line (MCF-7) and lung adenocarcinoma cell line (A549) were cultured in a humidified 5% CO2 atmosphere at 37 °C and incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).
2.3. MTT Assay
HepG2, A549, or MCF-7 cell lines were grown on 96-well plates at a cell density of 5000 cells/well in RPMI-1640 medium with 10% FBS. The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2/95% air overnight. The cells were then exposed to different concentrations of selected compounds and cisplatin for another 48 h in an atmosphere of 95% air with 5% CO2 at 37 °C. The cells were stained with MTT in the incubator for 4 h at 37 °C, and the absorbance was read at 490 nm on a microplate reader (Thermo Fisher Scientific). Experiments were carried out in triplicates and independently repeated three times.
2.4. Evaluation of Hydrogen Sulfide Release Activity
DNS-Az by itself is nonfluorescent. Nevertheless, the DNS-Az solution with an addition of hydrogen sulfide formed dansyl amide, which revealed strongly enhanced fluorescence. Without specimen pre-processing, the rapid response by DNS-Az to sulfide can be used to detect the instantaneous changes of hydrogen sulfide levels. The initial H2S–OA concentration of 200 μM was added to 60 μL of dansylazide solution (10 mM in ethanol) and 40 μL of H2S–OA stock solution (10 mM in DMSO) in preheated 1900 µL of culture medium (DMEM) at 37 °C. All trials were carried out at least three times. The Waters e2695-2998 chromatograph system which performed the HPLC analyses was equipped with a 2475 fluorescence detector integrated in the Waters e2695 system. Data analysis was implemented using an Empower system (Waters Technologies). The sample was eluted on Symmetry C18 (250 × 4.6 mm, 5 μm; Waters); the amount of injection was 20 μL. The mobile phase was composed of 0.1% aqueous HCOOH/ CH3CN (18:82, v/v); elution-adopted isasteric mode and flow rate was 1.0 mL/min. Using excitation and emission wavelengths of 340 and 535 nm, respectively, the fluorescence signals were obtained (gain factor = 10). Data operation was implemented by Empower 3. The values acquired from integration of the peak of dansyl amide were interpolated in NaHS as a standard calibration line; therefore, the concentration of dansyl amide in each specimen was an indicatrix of H2S amount [24]. The detection limit was about 1 μM with a signal:noise ratio (S/N) of 3:1; the amounts of H2S could be tested and quantified at 0.5 mM combined concentrations. A linear relationship between the fluorescence intensity and in DMEM against hydrogen sulfide was shown, and the assessment of the linear range of the H2S release was [3 μM, 250 μM]. The measured concentrations were within the range of the standard curve.
3. Results and Discussion
3.1. Chemistry
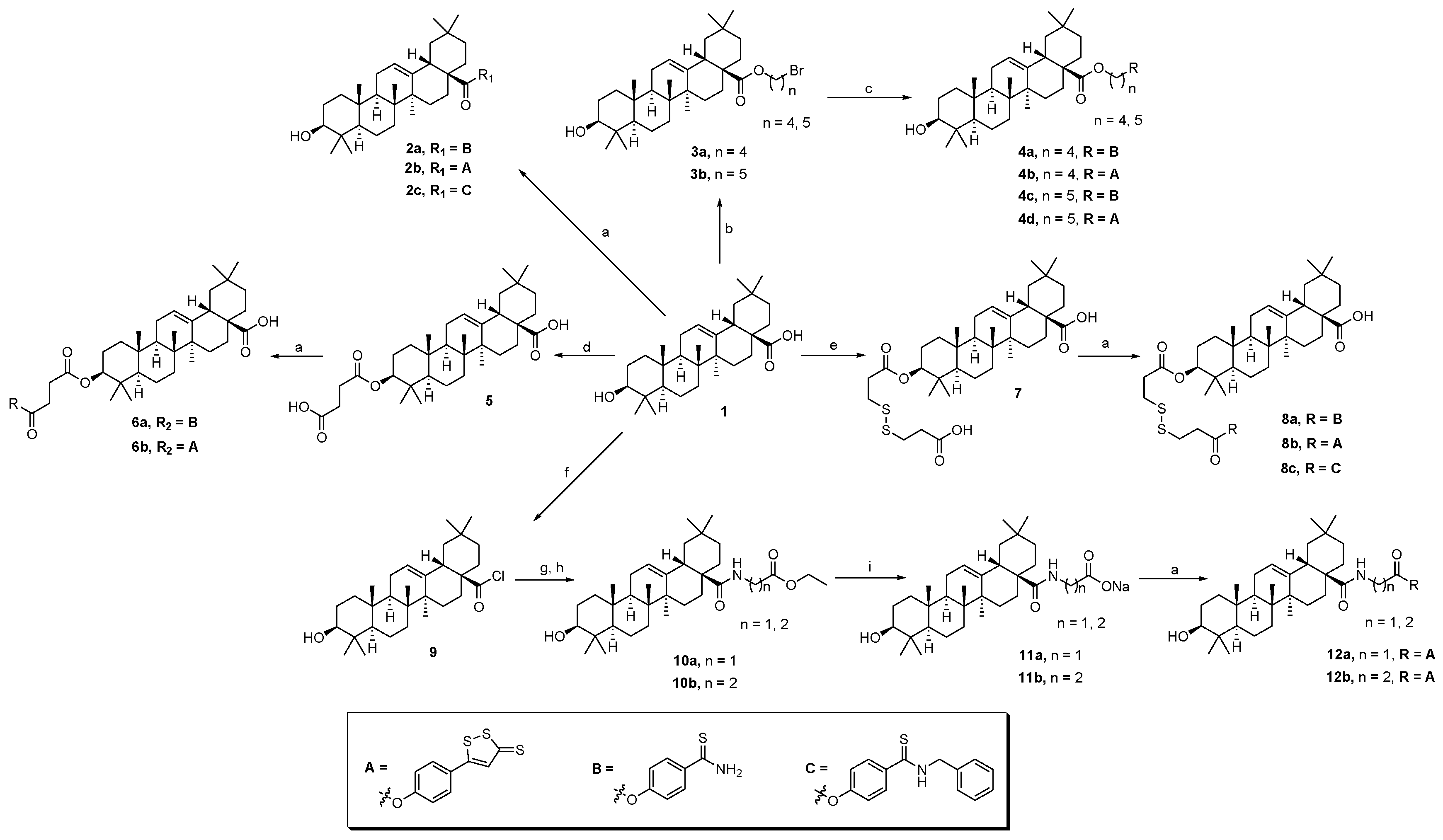
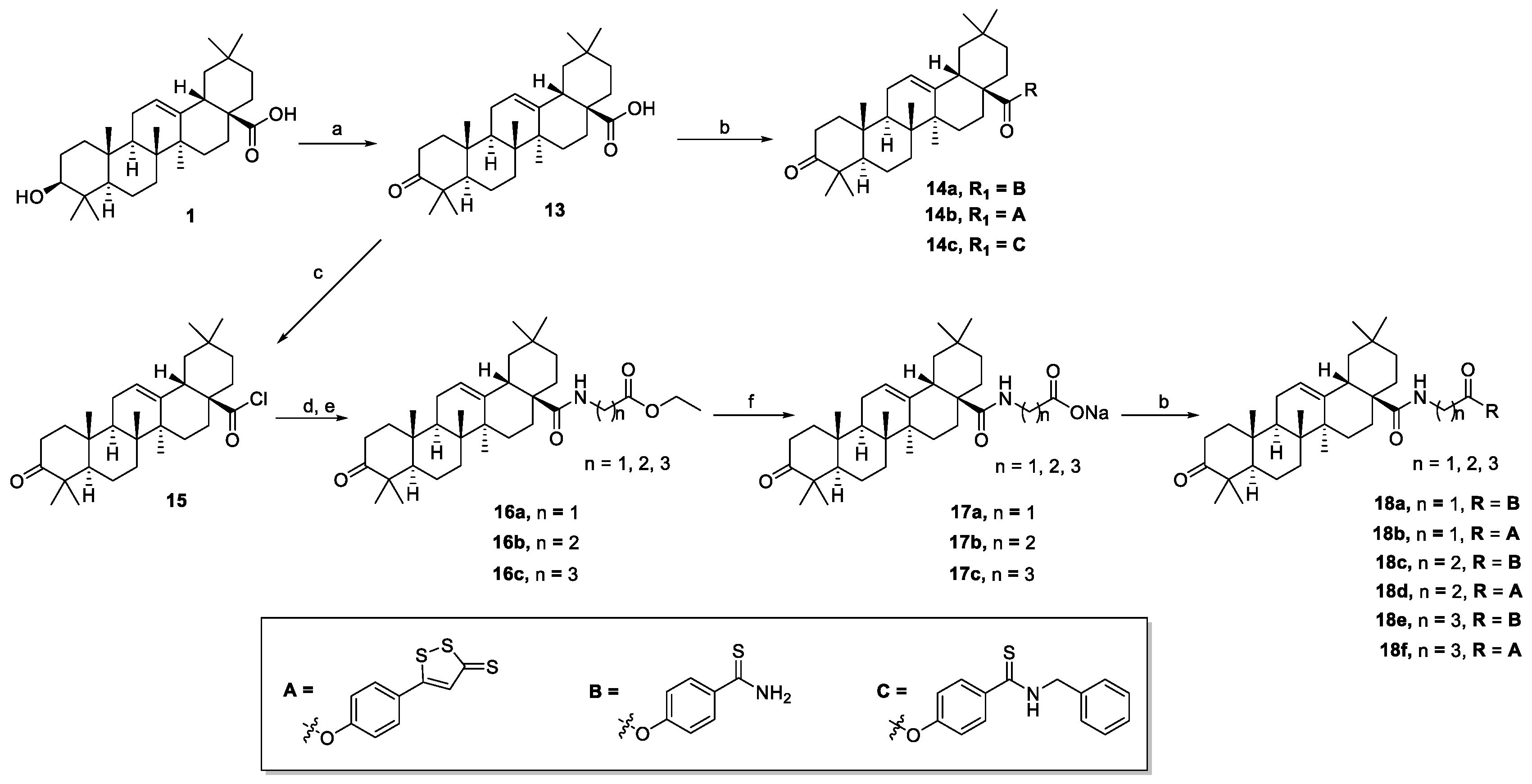
The synthesis of oleanolic acid–hydrogen sulfide donor hybrids and 3-oxooleanolic acid–hydrogen sulfide donor hybrids are outlined in Scheme 1 and Scheme 2, respectively. In this study, we selected 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH), 4-hydroxythiobenzamide (4-OH-TBZ), and N-benzyl-4-hydroxybenzothioamide (N-Bn-4-OH-TBZ) as H2S-releasing moieties. OA or 3-oxo-OA was directly modified with different H2S donors in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and 4-dimethylaminopyridine (DMAP) to produce the corresponding C-28 ester derivatives (2a–c and 14a–c). In order to enrich the types of linking bonds, we tried to connect the dibromoalkane to 3-OH of OA to obtain C-3 ether derivatives. Reaction of OA with dibromoalkane in the presence of potassium hydroxide and 0.1 mmol of potassium iodide in dry tetrahydrofuran afforded intermediates 3a,b. Unexpectedly, the NMR spectra of the intermediates 3a,b showed that they were C-28 ester derivatives. These results indicated that the reactivity of 3-OH was too low to participate in the reaction under these conditions. Thereafter, treating the intermediates 3a,b with H2S donors under basic conditions afforded the hybrids 4a–d. Furthermore, the preparation of C-3 ester derivatives (6a,b and 8a–c) was carried out in two steps. OA was reacted with succinic anhydride or 1,5,6-oxadithionane-2,9-dione in the presence of DMAP to form 3-O-acyl derivatives (5 and 7), which were further esterified with corresponding H2S donors in the presence of EDCI/DMAP to generate target compounds. Then, we tried to couple the amino acid moiety to the 28-carboxyl group. In order to improve the reactivity and avoid the generation the C-28 ester by-products, the reaction was carried out in multiple steps. Generally, OA or 3-oxo-OA first reacted with oxalyl chloride to give intermediates (9 or 15), and then a different ethyl-amino acid in the presence of triethylamine (TEA) was added to yield intermediates (10a,b or 16a–c). Thereafter, the ethyl group was successfully removed by treating the above intermediates with sodium hydroxide, resulting in 11a,b or 17a–c, respectively. In the presence of EDCI/DMAP, the OA- or 3-oxo-OA-amino acid-H2S donor trihybrids (12a,b or 18a–f) were successfully obtained by further coupling with different H2S donors. Their structures were characterized by 1H NMR, 13C NMR, and HRMS.
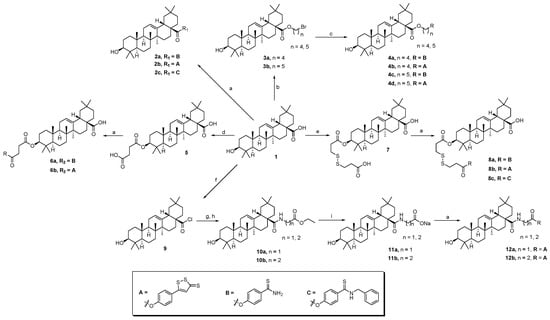
Scheme 1.
Reactions and conditions: (a) H2S donor, EDCI, DMAP, dry CH2Cl2, reflux; (b) dibromoalkane, KOH, KI, dry THF, reflux; (c) H2S donor, KOH, KI, dry THF, reflux; (d) succinic anhydride, DMAP, dry CH2Cl2, reflux; (e) 1,5,6-oxadithionane-2,9-dione, DMAP, dry CH2Cl2, reflux; (f) (COCl)2, dry CH2Cl2, 0 °C; (g) amino acid, EtOH, H2SO4; (h) ethyl-amino acid, TEA, dry CH2Cl2, 0 °C—rt; (i) NaOH, TFH.
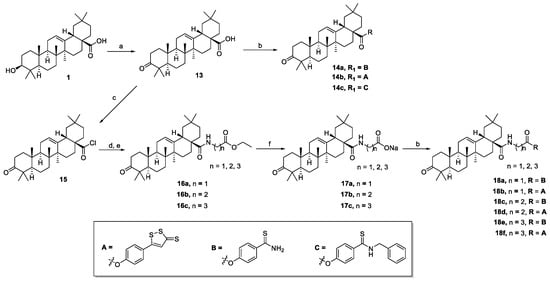
Scheme 2.
Reactions and conditions: (a) CrO3, H2SO4, acetone, 0 °C; (b) H2S donor, EDCI, DMAP, dry CH2Cl2, reflux; (c) (COCl)2, dry CH2Cl2, 0 °C; (d) amino acid, EtOH, H2SO4; (e) ethyl-amino acid, TEA, dry CH2Cl2, 0 °C—rt; (f) NaOH, TFH.
3.2. The Antiproliferative Activities of Compounds in Different Cancer Cell Lines
The target complexes 2a–c, 4a–d, 6a,b, 8a–c, 12a,b, 14a–c, and 18a–f, in parallel with OA and 3-oxo-OA, were initially evaluated by the MTT assays for their cytotoxicities on human hepatocellular carcinoma cell line (HepG2), human lung adenocarcinoma cell line (A549), and human breast cancer cell line (MCF-7), using general chemotherapeutic drug oxaliplatin as positive control. As the results show in Table 1, compared with their lead compounds OA or 3-oxo-OA, the synthesized prodrugs showed significantly improved cytotoxicities against all of the tested tumor cell lines. In contrast, the precursor compounds (3a, 3b, 5, 7, 11a, and 11b) without the H2S-donor moiety, showed only a weak activity, indicating that the H2S-donor moiety exerts an important influence on the cytotoxic activities of the prodrugs (data not shown). Furthermore, 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH), 4-hydroxythiobenzamide (4-OH-TBZ), and N-benzyl-4-hydroxybenzothioamide (N-Bn-4-OH-TBZ), three kinds of hydrogen sulfide donors (Figure 1), were initially evaluated by the MTT assays for their cytotoxicities on human hepatocellular carcinoma cell line (HepG2), human lung adenocarcinoma cell line (A549), and human breast cancer cell line (MCF-7) as well. (Table 2).

Table 1.
The antiproliferative activities of targeted compounds in HepG2, A549 and MCF-7.
Figure 1.
The structures of three kinds of H2S donors.

Table 2.
The antiproliferative activities of hydrogen sulfide donors in HepG2, A549 and MCF-7.
These results indicated that the enhanced cytotoxicity of the H2S donor derivatives may be due to their H2S-releasing ability, which can achieve the synergic action of cytotoxic H2S and the parent compounds OA or 3-oxo-OA. In addition, because the cytotoxic activity of 3-oxo-OA was higher than that of OA, the cytotoxic activities of H2S-donating 3-oxo-OA derivatives (14a–c, 18b, 18d) were also superior to those of the corresponding H2S-donating OA derivatives (2a–c, 12a,b).
The above results revealed that the type of the H2S-donor moiety was crucial for the cytotoxicity of H2S-donating OA or 3-oxo-OA derivatives. Under the same conditions of the connecting arm and coupling site, the order of potencies was ADT-OH > 4-OH-TBZ > N-Bn-4-OH-TBZ (Table 1), which was almost the same (2b > 2a > 2c, 8b > 8a > 8c, and 14b > 14a > 14c) against the tested tumor cell lines.
In addition, the linkers which linked the H2S donor to OA or 3-oxo-OA had a great influence on the cytotoxic potency of the derivatives. Firstly, the compounds that directly coupled H2S donor to the 28-COOH of OA (2a–c) or 3-oxo-OA (14a–c) exhibited lower cytotoxicities. Secondly, the 3-OH ester derivatives of OA (6a,b, and 8a–c) showed moderate cytotoxicities. Unexpectedly, the 3-OH ester derivatives with redox-sensitized disulfide bonds did not significantly promote cytotoxicities. IC50 values of the compounds with redox-sensitized disulfide bond (8a, 8b) were slightly lower than those without redox-sensitized disulfide bond derivatives (6a, 6b). In contrast, compared with their lead compounds OA or 3-oxo-OA, the amino acid prodrugs 12a,b and 18a–f showed significantly improved cytotoxicity against the three tested cell lines. Among them, compounds 18c and 18d with β-alanine as a linker showed potent cytotoxicity against HepG2 and A549 cell lines. Especially, the cytotoxicities of 18c and 18d against A549 were higher than those of other cell lines, which may be due to the expression of PepT1 in A549, but not in other cells.
On the basis of the above results, the most promising compounds, 18c and 18d, were evaluated for possible cytotoxicity towards normal human liver cells (LO2) to investigate whether the prodrugs have a tumor-cell-selecting activity. Interestingly, the growth of LO2 cell lines was not significantly affected by 18c and 18d, indicating that 18c and 18d selectively inhibit the growth of tumor cells (Table 3).

Table 3.
The antiproliferative activities of compounds in human normal liver cell lines by the MTT assay.
3.3. H2S-Releasing Ability
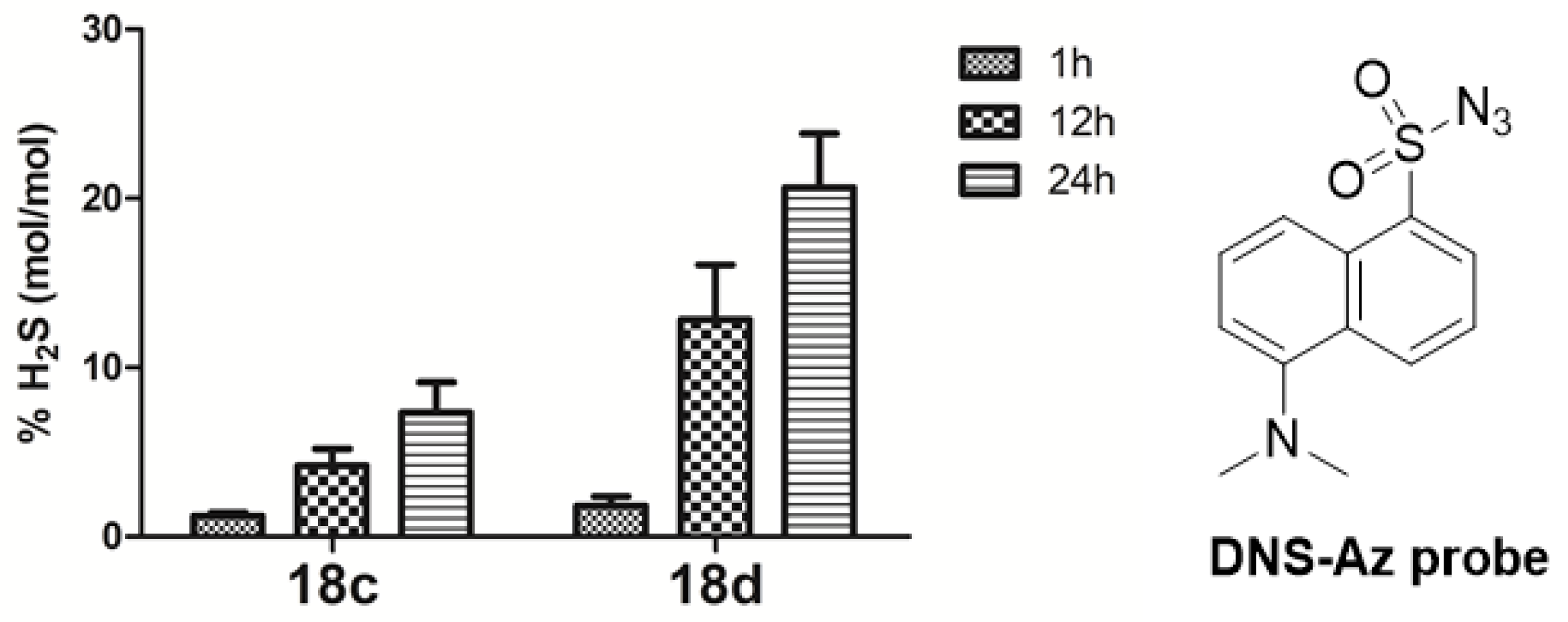
To investigate whether the H2S-donating prodrugs possessed the ability to release H2S, 18c and 18d were selected to perform a fluorometric assay. In brief, the confirmation of H2S release was based on the reaction of the H2S donor compound with the H2S fluorescence probe to produce the fluorescent related amide detected by HPLC [24,25]. H2S released in DMEM is shown in Figure 2. As expected, compounds 18c and 18d did have the ability to release H2S. The percentages of H2S released from compound 18c in DMEM were 1.2%, 4.2%, and 7.3% at 1 h, 12 h, and 24 h, respectively, which was indicative of a slow and steady H2S release. The trend of compound 18d was very similar to that of 18c. Interestingly, at all points, the % H2S released from 18d were higher than that of 18c, which were consistent with the order of the cytotoxicity results. This finding further confirmed that the H2S-releasing abilities were quite well associated with their cytotoxic activities.
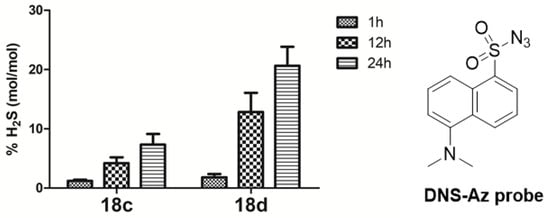
Figure 2.
H2S release assessment from the compounds 18c and 18d with incubation time 1, 12, and 24 h in DMEM. Data are presented as the mean ± SEM (SEM ≤ 3; number of determinations, 3–5).
4. Conclusions
In conclusion, with the hydrogen sulfide donor, 23 new hybrid compounds derived from the combination of OA as well as 3-oxo-OA via various chemical linkers were prepared to improve their pharmaceutical profiles. In this study, the synthesized compounds were screened for anticancer activities against three human cancer cell lines using the MTT assay. The H2S donors or materials were controllable or slow-releasing. We can deduce that the specific H2S-releasing moiety and linker strongly influenced the anticancer activities. Additionally, all of the synthesized pro-drugs showed moderate to potent cytotoxicity against the three tested cell lines. In particular, the cytotoxicity of 18d (IC50 = 11.8 μM) against PepT1 expressed in A549 cell lines was basically equal to that of oxaliplatin. The higher activity of 18d may be attributable to the introduction of the H2S donor and amino acid moieties. In addition, our tests revealed that 18d could release H2S in vitro and has little toxicity against normal human liver cells. Overall, for the first time, H2S-releasing derivatives as anticancer drugs have been reported and indicate a promising strategy for the further development of anticancer drugs.
Author Contributions
Investigation, F.Z., J.L., K.Y., B.S., E.S., X.C., J.M. and L.Y.; Project administration, J.M. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (U1704185 and 21807025,) and the Key Scientific, Technological Project of Henan Province (202102310147) and Project of Innovation and Entrepreneurship Support Program for College Students of Henan University (Grant No. 2020102006 and 2020102002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Li, B.; Zhang, Z.; Wei, Y.; Xu, Z.; Qin, S.; Liu, N.; Zhao, R.; Peng, J.; Yang, G.; et al. Synthesis and Discovery Novel Anti-Cancer Stem Cells Compounds Derived from the Natural Triterpenoic Acids. J. Med. Chem. 2018, 61, 10814–10833. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New Synthetic Triterpenoids: Potent Agents for Prevention and Treatment of Tissue Injury Caused by Inflammatory and Oxidative Stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Fang, L.; Wang, M.; Gou, S.; Liu, X.; Zhang, H.; Cao, F. Combination of Amino Acid/Dipeptide with Nitric Oxide Donating Oleanolic Acid Derivatives as PepT1 Targeting Antitumor Prodrugs. J. Med. Chem. 2014, 57, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.-K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Jia, J.; Yin, Z.; Gao, Y.; Sha, L.; Lai, Y.; Ping, Q.; Zhang, Y. Ethylene Glycol-Linked Amino Acid Diester Prodrugs of Oleanolic Acid for PepT1-Mediated Transport: Synthesis, Intestinal Permeability and Pharmacokinetics. Mol. Pharm. 2012, 9, 2127–2135. [Google Scholar] [CrossRef]
- Cao, F.; Gao, Y.; Wang, M.; Fang, L.; Ping, Q. Propylene Glycol-Linked Amino Acid/Dipeptide Diester Prodrugs of Oleanolic Acid for PepT1-Mediated Transport: Synthesis, Intestinal Permeability, and Pharmacokinetics. Mol. Pharm. 2013, 10, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Kong, X.; Lan, E.; Huang, Z.; Peng, S.; Kaufman, D.L.; Tian, J. Design, Synthesis, and Antihepatocellular Carcinoma Activity of Nitric Oxide Releasing Derivatives of Oleanolic Acid. J. Med. Chem. 2008, 51, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Kang, F.; Huang, Z.; Xue, X.; Lai, Y.; Peng, S.; Tian, J.; Zhangjian, H. Synthesis of CDDO–Amino Acid–Nitric Oxide Donor Trihybrids as Potential Antitumor Agents against Both Drug-Sensitive and Drug-Resistant Colon Cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Feng, M.; Chen, F.; Liu, X.; Shen, H.; Zhao, J.; Gou, S. Oleanolic acid-NO donor-platinum(II) trihybrid molecules: Targeting cytotoxicity on hepatoma cells with combined action mode and good safety. Bioorg. Med. Chem. 2016, 24, 4611–4619. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, L.; Huang, Z.; Lai, Y.; Ji, H.; Peng, S.; Tian, J.; Zhang, Y. Hybrid molecule from O2-(2,4-dinitrophenyl)diazeniumdiolate and oleanolic acid: A glutathione S-transferase pi-activated nitric oxide prodrug with selective anti-human hepatocellular carcinoma activity and improved stability. J. Med. Chem. 2013, 56, 4641–4655. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Wong, P.T.-H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Berenyiova, A.; Kristek, F. The Role of Hydrogen Sulphide in Blood Pressure Regulation. Physiol. Res. 2016, 65, S273–S289. [Google Scholar] [CrossRef]
- Feng, W.; Teo, X.Y.; Novera, W.; Ramanujulu, P.M.; Liang, D.; Huang, D.; Moore, P.K.; Deng, L.W.; Dymock, B.W. Discovery of New H2S Releasing Phosphordithioates and 2,3-Dihydro-2-phenyl-2-sulfanylenebenzo[d][1,3,2]oxazaphospholes with Improved Antiproliferative Activity. J. Med. Chem. 2015, 58, 6456–6480. [Google Scholar] [CrossRef]
- Ewelina, Z.; Lenka, T.; Dominik, K.; Ryszard, O.; Marcin, U. Hydrogen Sulfide in Pharmacotherapy, Beyond the Hydrogen Sulfide-Donors. Biomolecules 2020, 10, 323. [Google Scholar]
- Chun, T.; Li, C.; Shi, X.; Jacob, J.D.; Xiang, L.; Ming, X. Recent Development of Hydrogen Sulfide Releasing/Stimulating Reagents and Their Potential Applications in Cancer and Glycometabolic Disorders. Front. Pharmacol. 2017, 8, 664. [Google Scholar]
- Chadwick, R.P.; Kearsley, M.D.; John, B.M. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar]
- Ji, J.; Xiang, P.; Li, T.; Lan, L.; Xu, X.; Lu, G.; Ji, H.; Zhang, Y.; Li, Y. NOSH-NBP, a Novel Nitric Oxide and Hydrogen Sulfide- Releasing Hybrid, Attenuates Ischemic Stroke-Induced Neuroinflammatory Injury by Modulating Microglia Polarization. Front. Cell. Neurosci. 2017, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Lougiakis, N.; Papapetropoulos, A.; Gikas, E.; Toumpas, S.; Efentakis, P.; Wedmann, R.; Zoga, A.; Zhou, Z.; Iliodromitis, E.K.; Skaltsounis, A.-L.; et al. Synthesis and Pharmacological Evaluation of Novel Adenine–Hydrogen Sulfide Slow Release Hybrids Designed as Multitarget Cardioprotective Agents. J. Med. Chem. 2016, 59, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Rolando, B.; Cortese, D.; Gazzano, E.; Buondonno, I.; Lazzarato, L.; Fanelli, M.; Hattinger, C.M.; Serra, M.; Riganti, C.; et al. H2S-Donating Doxorubicins May Overcome Cardiotoxicity and Multidrug Resistance. J. Med. Chem. 2016, 59, 4881–4889. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Nie, C.; Song, B.; Yan, L.; Qian, H. Design, synthesis and biological evaluation of novel hydrogen sulfide releasing capsaicin derivatives. Bioorg. Med. Chem. 2018, 26, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.-Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.-S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Maria, P.S. The Slow-Releasing Hydrogen Sulfide Donor, GYY4137, Exhibits Novel Anti-Cancer Effects In Vitro and In Vivo. PLoS ONE 2011, 6, e21077. [Google Scholar]
- Li-xin, S.; Jia-yan, H.; Chun-mei, L.; Ju-zheng, Z.; Ke-guang, C. Synthesis of oleanolic acid/ursolic acid/glycyrrhetinic acid-hydrogen sulfide donor hybrids and their antitumor activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar]
- Uppuluri, V.M.; Anita, M.; Banita, P.; Nagireddy, V.; Nitasha, S.; Ajit, K.S. Synthesis and anti-cancer activity of some novel C-17 analogs of ursolic and oleanolic acids. Med. Chem. Res. 2013, 22, 1263–1269. [Google Scholar]
- Matthew, W.; Alexis, P.; Zongmin, Z.; Mariarosaria, B.; Andreas, P.; Guiseppe, C.; Mark, E.W. Phosphinodithioate and Phosphoramidodithioate Hydrogen Sulfide Donors. Handb. Exp. Pharmacol. 2015, 230, 337–363. [Google Scholar]
- Zhu, Y.; Lin, W.; Wang, X.; Zhang, W.; Chen, L.; Xie, Z. Constructing reduction-sensitive PEGylated NIRF mesoporous silica nanoparticles via a one-pot Passerini reaction for photothermal/chemo-therapy. Chem. Commun. 2018, 54, 11921–11924. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Z.; Li, Y.; Cao, J.; Chen, Y.; Luo, X. Bioinspired mimics: Self-assembly of redox-activated phosphorylcholine–based biodegradable copolymers for enhancing antitumor efficiency. Mater. Sci. Eng. C 2018, 89, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Konoike, T.; Takahashi, K.; Araki, Y.; Horibe, I. Practical Partial Synthesis of Myriceric Acid A, an Endothelin Receptor Antagonist, from Oleanolic Acid. J. Org. Chem. 1997, 62, 960–966. [Google Scholar] [CrossRef]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Nesi, G.; Citi, V.; Piragine, E.; Piano, I.; Taliani, S.; Da Settimo, F.; Rapposelli, S.; Testai, L.; Breschi, M.C.; et al. Iminothioethers as Hydrogen Sulfide Donors: From the Gasotransmitter Release to the Vascular Effects. J. Med. Chem. 2017, 60, 7512–7523. [Google Scholar] [CrossRef]
- Chen, M.; Li, K.; Li, H.; Song, C.P.; Miao, Y. The glutathione peroxidase gene family in gossypium hirsutum: Genome-wide identification, classification, gene expression and functional analysis. Sci. Rep. 2017, 7, 44743. [Google Scholar] [CrossRef]
- Dai, Y.J.; Hui, K.M.; Zhang, Y.H.; Liu, Y.; Wang, Y.Q.; Zhao, L.J.; Lin, L.; Chai, L.Q.; Wei, S.; Lan, J.F. Three STATs are involved in the regulation of the expression of antimicrobial peptides in the triangle sail mussel, Hyriopsis cumingii. Fish Shellfish Immunol. 2017, 63, 181–188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).