Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage
Abstract
:1. Metal Care in Cultural Heritage: An Overview
2. Gels: A Reliable Delivery System
Cleaning Gels in Cultural Heritage
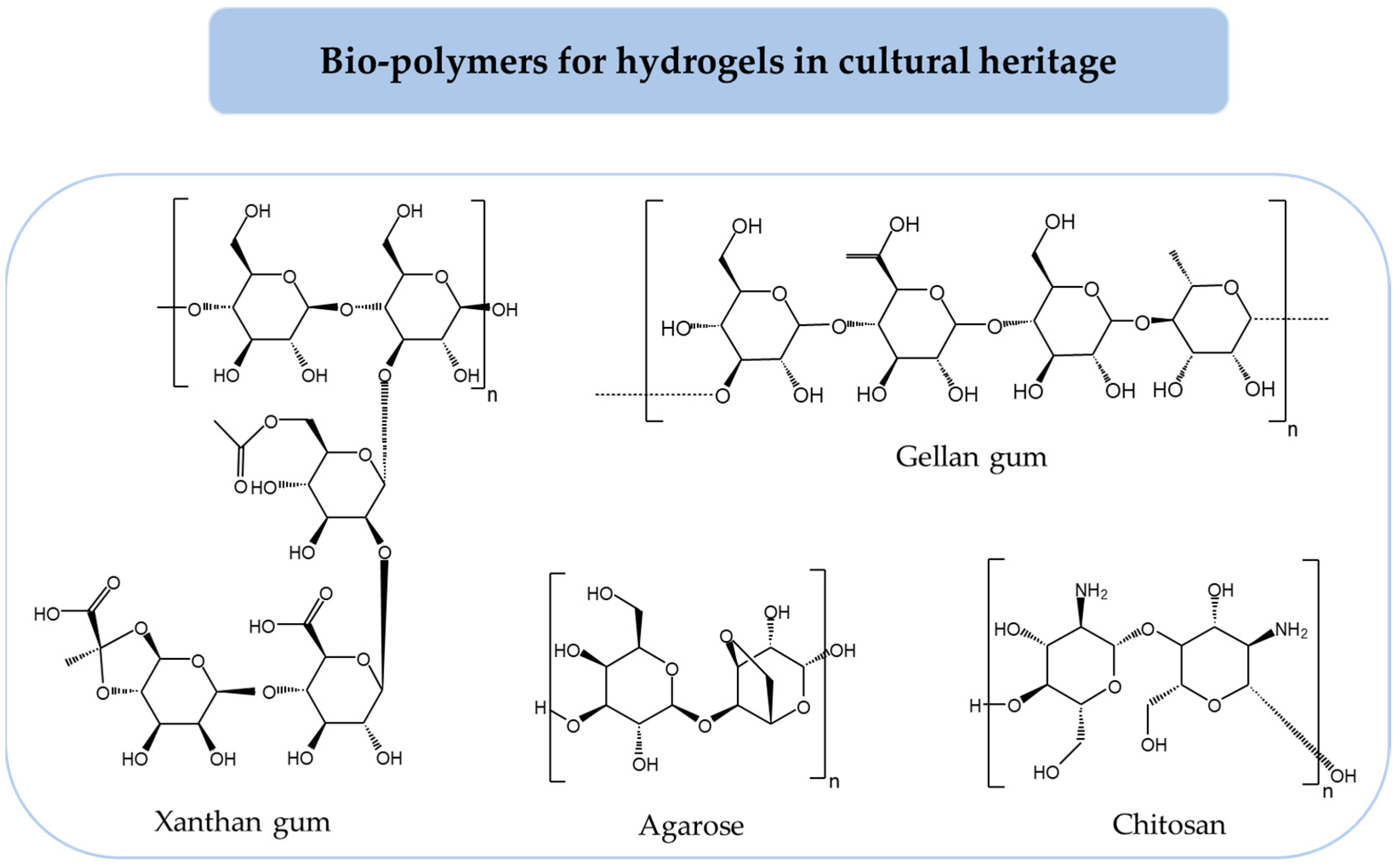
3. Bio-Based Hydrogels for Metal Preservation
3.1. Physical Hydrogels
3.1.1. Agar
3.1.2. Gellan Gum
3.1.3. Xanthan Gum
3.1.4. Chitosan
3.2. Chemical Hydrogels
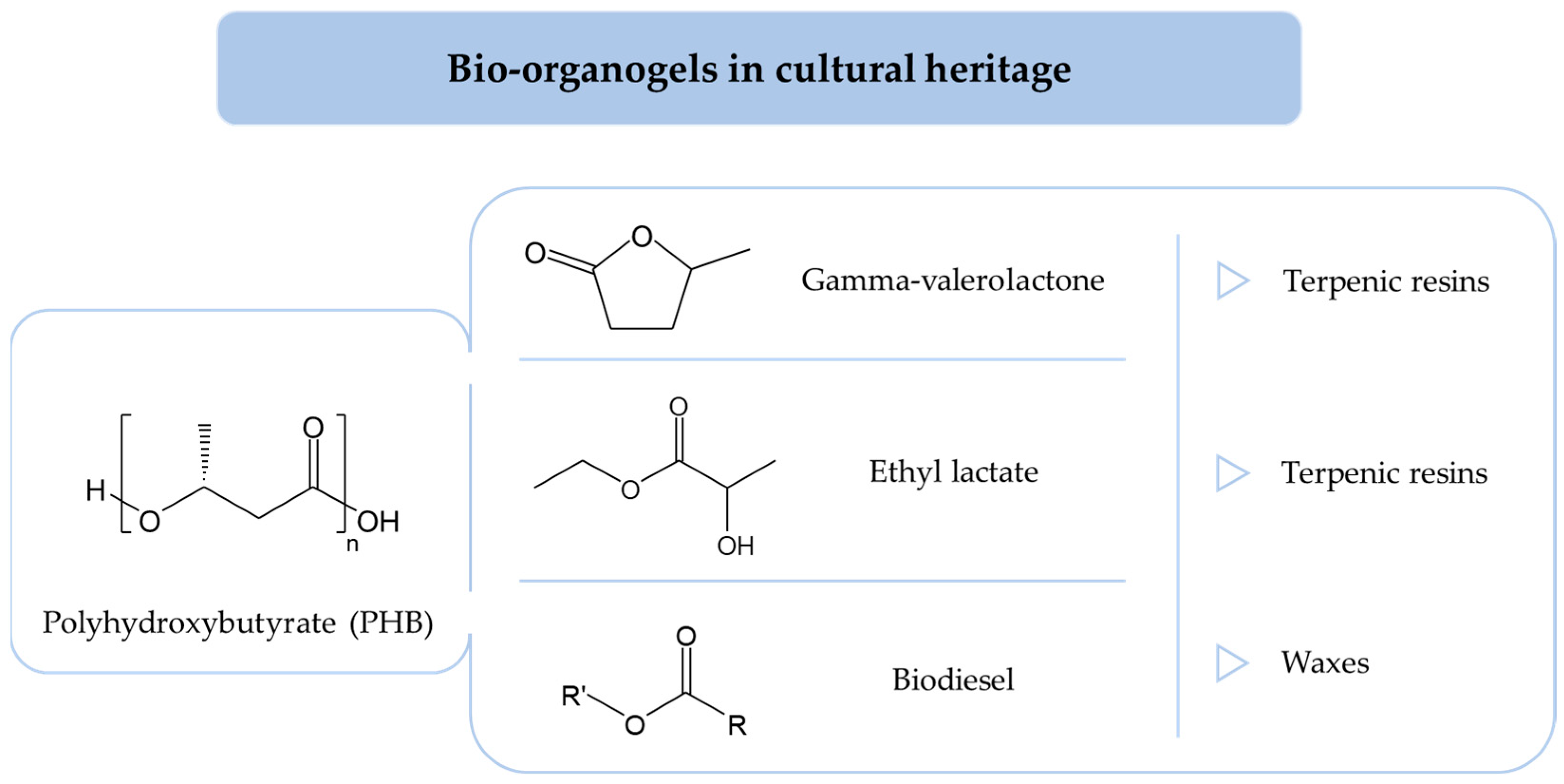
4. Organogels for Organic Coatings Removal
4.1. Solvent Greenness
4.2. Bio-Based Solvents
4.2.1. Bioethanol and Derivatives
4.2.2. n-Butyl Alcohol and Derivatives
4.2.3. Ethyl Lactate
4.2.4. Gamma-Valerolactone
4.2.5. Biodiesel and Derivatives
4.3. Bio-Based Organogels for Cleaning
4.3.1. Biopolymers
4.3.2. Bio-Organogels in Art Conservation
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kristiansen, K.; Larsson, T.B. L’âge du Bronze, une période historique. Ann. Hist. Sci. Soc. 2005, 60, 975–1007. [Google Scholar] [CrossRef]
- Bertolotti, G.; Bersani, D.; Lottici, P.P.; Alesiani, M.; Malcherek, T.; Schlüter, J. Micro-Raman study of copper hydroxychlorides and other corrosion products of bronze samples mimicking archaeological coins. Anal. Bioanal. Chem. 2012, 402, 1451–1457. [Google Scholar] [CrossRef]
- Felix, V.S.; Pereira, M.O.; Freitas, R.P.; Aranha, P.J.M.; Heringer, P.C.S.; Anjos, M.J.; Lopes, R.T. Analysis of silver coins from colonial Brazil by hand held XRF and micro-XRF. Appl. Radiat. Isotopes 2020, 166, 109409. [Google Scholar] [CrossRef]
- Gharib, A.; Mohamed, H.; Abdel Ghany, N. Nondestructive techniques in the study of a gilded metallic sword from the Islamic Art Museum. Egypt. J. Archaeol. Restor. Stud. 2018, 8, 15–21. [Google Scholar]
- Giumlia-Mair, A.; Lucchini, E. Surface analyses on modern and ancient copper based fakes. Surf. Eng. 2005, 21, 406–410. [Google Scholar] [CrossRef]
- Chiavari, C.; Martini, C.; Prandstraller, D.; Niklasson, A.; Johansson, L.-G.; Svensson, J.-E.; Åslund, A.; Bergsten, C.J. Atmospheric corrosion of historical organ pipes: The influence of environment and materials. Corros. Sci. 2008, 50, 2444–2455. [Google Scholar] [CrossRef]
- Lehmann, E.H.; Hartmann, S.; Speidel, M.O. Investigation of the content of ancient tibetan metallic buddha statues by means of neutron imaging methods. Archaeometry 2009, 52, 416–428. [Google Scholar] [CrossRef]
- Chilton, J.P. The Corrosion of Metals. J. R. Soc. Arts 1971, 119, 614–629. [Google Scholar]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Neff, D.; Reguer, S.; Bellot-Gurlet, L.; Dillmann, P.; Bertholon, R. Structural characterization of corrosion products on archaeological iron: An integrated analytical approach to establish corrosion forms. J. Raman Spectrosc. 2004, 35, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and Materials Science for the Conservation of Cultural Heritage: Cleaning, Consolidation, and Deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Turner-Walker, G. A Practical Guide to the Care and Conservation of Metals; Headquarters Administration of Cultural Heritage, Council for Cultural Affairs: Taichung City, Taiwan, 2008; ISBN 9789860172980. [Google Scholar]
- Comensoli, L.; Maillard, J.; Albini, M.; Sandoz, F.; Junier, P.; Joseph, E. Use of Bacteria To Stabilize Archaeological Iron. Appl. Environ. Microbiol. 2017, 83, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Albini, M.; Letardi, P.; Mathys, L.; Brambilla, L.; Schröter, J.; Junier, P.; Joseph, E. Comparison of a bio-based corrosion inhibitor versus benzotriazole on corroded copper surfaces. Corros. Sci. 2018, 143, 84–92. [Google Scholar] [CrossRef]
- Guaragnone, T.; Casini, A.; Chelazzi, D.; Giorgi, R. PVA-based peelable films loaded with tetraethylenepentamine for the removal of corrosion products from bronze. Appl. Mater. Today 2020, 19, 100549. [Google Scholar] [CrossRef]
- Joseph, E.; Simon, A.; Mazzeo, R.; Job, D.; Wörle, M. Spectroscopic characterization of an innovative biological treatment for corroded metal artefacts. J. Raman Spectrosc. 2012, 43, 1612–1616. [Google Scholar] [CrossRef]
- Palomar, T.; Ramírez Barat, B.; Cano, E. Evaluation of cleaning treatments for tarnished silver: The conservator’s perspective. Int. J. Conserv. Sci. 2018, 9, 81–90. [Google Scholar] [CrossRef]
- Watkinson, D. Preservation of Metallic Cultural Heritage. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3307–3340. ISBN 9780444527882. [Google Scholar]
- Argyropoulos, V.; Giannoulaki, M.; Michalakakos, G.P.; Siatou, A. A Survey of the Type of Corrosion Inhibitors and Protective Coatings Used for the Conservation of Metal Objects from Museum Collections in the Mediterranean Basin. In Strategies for Saving our Cultural Heritage. Proceedings of the International Conference on Conservation Strategies for Saving Indoor Metallic Collections, Cairo (Egypt). TEI of Athens, Athens; Academic Press: Cambridge, MA, USA, 2007; pp. 166–170. [Google Scholar]
- Wolfe, J.; Grayburn, R. A review of the development and testing of Incralac lacquer. J. Am. Inst. Conserv. 2017, 56, 225–244. [Google Scholar] [CrossRef]
- Perera, D.Y. Physical ageing of organic coatings. Prog. Org. Coat. 2003, 47, 61–76. [Google Scholar] [CrossRef]
- Couture-Rigert, D.E.; Sirois, P.J.; Moffatt, E.A. An investigation into the cause of corrosion on indoor bronze sculpture. Stud. Conserv. 2012, 57, 142–163. [Google Scholar] [CrossRef]
- Jaeger, T. Short Communication Removal of Paraffin Wax in the Re-treatment of Archaeological Iron. J. Am. Inst. Conserv. 2008, 47, 217–223. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; Salzano de Luna, M.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-based coatings for corrosion protection of copper-based alloys: A promising more sustainable approach for cultural heritage applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Mari, Y. Il Restauro Della Grande Croce del Pollaiolo: Un Intervento All’interno del Cantiere Organizzato per l’Altare di san Giovanni. In E l’informe si fa forma...: studi intorno a Santa Maria del Fiore in ricordo di Patrizio Osticresi; Mandragora: Florence, Italy, 2012; pp. 281–286. ISBN 9788874615346. [Google Scholar]
- Cagnini, A.; Gennai, S.; Mazzoni, M.D. La Banderuola di Palazzo Vecchio: Storia, vicende conservative, restauro. OPD Restauro 2012, 24, 13–32. [Google Scholar]
- Phenix, A. Effects of Organic Solvents on Artists Oil Paint Films: Swelling. In New Insights into the Cleaning of Paintings: Proceedings from the Cleaning 2010 International Conference, Universidad Politecnica de Valencia and Museum Conservation Institute; Smithsonian Institution: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Varnai, V.M.; Macan, J.; Ljubicic Calusic, A.; Prester, L.; Kanceljak Macan, B. Upper respiratory impairment in restorers of cultural heritage. Occup. Med. 2011, 61, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Meng, F.; Wang, F.; Liu, Q. Environmental behavior and eco-toxicity of xylene in aquatic environments: A review. Ecotoxicol. Environ. Saf. 2017, 145, 324–332. [Google Scholar] [CrossRef]
- De Silva, M.; Henderson, J. Sustainability in conservation practice. J. Inst. Conserv. 2011, 34, 5–15. [Google Scholar] [CrossRef]
- Kumar, R.; Sagar, P. Preface on Green Conservation of the Museum Objects. In A treatise on Recent Trends and Sustainability in Crafts & Design; Gupta, T., Mistry, B., Gupta, B.S., Eds.; Excel India: Mumbai, India, 2017; pp. 118–124. ISBN 978-93-86724-21-2. [Google Scholar]
- Appelbaum, B. Criteria for Treatment: Reversibility. J. Am. Inst. Conserv. 1987, 26, 65–73. [Google Scholar] [CrossRef]
- Brandi, C. Teoria del Restauro; Ed. di storia e letteratura: Rome, Italy, 1963. [Google Scholar]
- Di Marino, D.; Shalaby, M.; Kriescher, S.; Wessling, M. Corrosion of metal electrodes in deep eutectic solvents. Electrochem. Commun. 2018, 90, 101–105. [Google Scholar] [CrossRef]
- Heitz, E. Corrosion of Metals in Organic Solvents. In Advances in Corrosion Science and Technology; Springer: Boston, MA, USA, 1974; pp. 149–243. ISBN 9781461590590. [Google Scholar]
- Brajer, I.; Fossé-Le Rouzic, M.; Shashoua, Y.; Taube, M.; Chelazzi, D.; Baglioni, M.; Baglioni, P. The removal of aged acrylic coatings from wall paintings using microemulsions. In ICOM-CC 17th Triennial Conference Preprints, Melbourne, Australia, 15–19 September 2014; Bridgland, J., Ed.; art. 1103; International Council of Museums: Paris, France, 2014; ISBN 9789290124108. [Google Scholar]
- Chelazzi, D.; Giorgi, R.; Baglioni, P. Microemulsions, Micelles, and Functional Gels: How Colloids and Soft Matter Preserve Works of Art. Angew. Chem. Int. Ed. 2018, 57, 7296–7303. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef]
- Mazzoni, M.; Alisi, C.; Tasso, F.; Cecchini, A.; Marconi, P.; Sprocati, A.R. Laponite micro-packs for the selective cleaning of multiple coherent deposits on wall paintings: The case study of Casina Farnese on the Palatine Hill (Rome-Italy). Int. Biodeterior. Biodegrad. 2014, 94, 1–11. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F.; AL-Thabaiti, S.A.; Khan, Z. Anti-corrosion ability of surfactants: A review. Int. J. Electrochem. Sci. 2011, 6, 1927–1948. [Google Scholar]
- Fratini, E.; Carretti, E. CHAPTER 10. Cleaning IV: Gels and Polymeric Dispersions. In Nanoscience for the Conservation of Works of Art; Royal Society of Chemistry: London, UK, 2013; pp. 252–279. ISBN 978-1-84973-566-7. [Google Scholar]
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Baglioni, P.; Baglioni, M.; Bonelli, N.; Chelazzi, D.; Giorgi, R. Smart Soft Nanomaterials for Cleaning. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–204. [Google Scholar] [CrossRef]
- Wolbers, R. Restoration’92: Conservation, training, materials and techniques: Latest developments. In Proceedings of the Preprints to the Conference Held at the RAI International Exhibition and Congress Centre, Amsterdam, The Netherlands, 20–22 October 1992; pp. 74–75, ISBN 9781871656183. [Google Scholar]
- Wolbers, R. Cleaning Painted Surfaces: Aqueous Methods; Archetype Publications: London, UK, 2000; ISBN 1873132360. [Google Scholar]
- Van Loon, A.; Hartman, L.E.; van den Burg, J.; Haswell, R.; Pottasch, C. The Development of an Aqueous Gel Testing Procedure for the Removal of Lead-Rich Salt Crusts on the Surface of Paintings by Giovanni Antonio Pellegrini (1675–1741) in the “Golden Room” of the Mauritshuis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 283–296. [Google Scholar] [CrossRef]
- Toreno, G.; Isola, D.; Meloni, P.; Carcangiu, G.; Selbmann, L.; Onofri, S.; Caneva, G.; Zucconi, L. Biological colonization on stone monuments: A new low impact cleaning method. J. Cult. Herit. 2018, 30, 100–109. [Google Scholar] [CrossRef]
- Cushman, M.; Wolbers, R. A new approach to cleaning iron stained marble surfaces. WAAC Newsl. 2007, 29, 23–28. [Google Scholar]
- Mazzuca, C.; Micheli, L.; Carbone, M.; Basoli, F.; Cervelli, E.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Gellan hydrogel as a powerful tool in paper cleaning process: A detailed study. J. Colloid Interface Sci. 2014, 416, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delattre, C.; Bouvet, S.; Le Bourg, E. Gellan gum and agar compared to aqueous immersion for cleaning paper. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 57–61. ISBN 9781909492509. [Google Scholar]
- Guilminot, E.; Leroux, M.; Raimon, A.; Chalvidal, C. Projet collaboratif sur l’utilisation des gels pour le traitement des métaux: Démarche et fonctionnement. In Proceedings of the Journées des Restaurateurs en Archéologie, Journée d’Étude, de Recherche et d’Innovation Lyon, France, 29–30 November 2018; p. 14. [Google Scholar]
- Baij, L.; Hermans, J.; Ormsby, B.; Noble, P.; Iedema, P.; Keune, K. A review of solvent action on oil paint. Herit. Sci. 2020, 8, 43. [Google Scholar] [CrossRef]
- Duncan, T.T.; Berrie, B.H.; Weiss, R.G. A Comparison between Gel and Swab Cleaning: Physical Changes to Delicate Surfaces. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 250–256. ISBN 9781909492509. [Google Scholar]
- Guilminot, E.; Gomez, A.; Raimon, A.; Leroux, M. Use of Gels for the treatment of Metals. In Proceedings of the Metal 2019 Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group; Chemello, C., Brambilla, L., Joseph, E., Eds.; International Councils of Museums—Committee for Conservation: Paris, France, 2019; p. 473. ISBN 9789290124573. [Google Scholar]
- Smith, S.S. Layer by layer: The removal of complex soiling on a collection of modern art bronzes using buffered pH-adjusted aquous gels. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 349–355. ISBN 9781909492509. [Google Scholar]
- Parisi, E.I.; Bonelli, N.; Carretti, E.; Giorgi, R.; Ingo, G.M.; Baglioni, P. Film forming PVA-based cleaning systems for the removal of corrosion products from historical bronzes. Pure Appl. Chem. 2018, 90, 507–522. [Google Scholar] [CrossRef]
- Feldman, D. Poly(Vinyl Alcohol) Recent Contributions to Engineering and Medicine. J. Compos. Sci. 2020, 4, 175. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Duplat, V.; Rouchon, V.; Desloges, I.; Papillon, M.C. Steel versus Paper: The Conservation of a Piece of Modern Art Consisting of a Rust Print on Paper. J. Pap. Conserv. 2009, 10, 26–34. [Google Scholar]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of freezing under electrostatic field on selected properties of an agar gel. Innov. Food Sci. Emerg. Technol. 2017, 42, 151–156. [Google Scholar] [CrossRef]
- Cremonesi, P. Rigid Gels and Enzyme Cleaning. Smithson. Contrib. Museum Conserv. 2012, 3, 179–183. [Google Scholar]
- Tamura, M.; Takagi, K. Towards the sustainable use of agar/agarose in conservation: A case study of the Izu peninsula, Japan. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 152–154. ISBN 9781909492509. [Google Scholar]
- Bertasa, M.; Chiantore, O.; Poli, T.; Riedo, C.; Di Tullio, V.; Canevali, C.; Sansonetti, A.; Scalarone, D. A study of commercial agar gels as cleaning materials. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 11–18. ISBN 9781909492509. [Google Scholar]
- Wolbers, R. Terminology and properties of selected gels. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 381–394. ISBN 9781909492509. [Google Scholar]
- Marchand, G.; Chevallier, R.; Guilminot, E.; Rossetti, L.; Lemoine, S. Study of the conservation treatment applied to the archaeological horn silver artifacts. In Proceedings of the Interim Meeting for the International Council of Museums Committee for Conservation Metal Working Group, Metal 2013; International Councils of Museums—Committee for Conseravtion and Historic Scotland: Edinburgh, Scotland, 2013; pp. 245–250. ISBN 9781849171427. [Google Scholar]
- Fays, M. «D’or, D’argent et de Pate Noire: Incrustations Révélées» Étude et Conservation-Restauration de Cinq Objets Islamiques en Alliage Cuivreux Incrustés; Institut National du Patrimoine: Paris, France, 2018. [Google Scholar]
- Wolbers, R.; Rivers, S.; Yamashita, Y. Corroded applied lead-based decoration (hyomon) on Japanese lacquer: Principles and case studies. Stud. Conserv. 2014, 59, S191–S194. [Google Scholar] [CrossRef]
- Létrange, A.; Hourdet, D.; Guerrier, J.; Pons, E. Comparison of three hydrogels for cleaning tarnished silver threads using electrochemical treatment. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 369–371. ISBN 9781909492509. [Google Scholar]
- São João, J.; Branco, L.C.; Leite Fragoso, S. Trials fo agar gels and task-specific salts for the electrochemical reduction of silver sulphide on silver leaf. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 287–291. ISBN 9781909492509. [Google Scholar]
- Domon Beuret, E.; Mathys, L.; Brambilla, L.; Albini, M.; Cevey, C.; Bertholon, R.; Junier, P.; Joseph, E. Biopatines: Des champignons au service des alliages cuivreux. In Proceedings of the Cahier n°22—XXVIIIe Journées des restaurateurs en archéologie, Arles, 16–17 October 2014; ARAAFU: Paris, France, October 2015. [Google Scholar]
- Barberà Giné, A.; Marín Ortega, S. The removal of Paraloid B-72 coatings with aqueous gelled systems: Roman frescoesfrom Empúries, Catalonia. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 363–365. ISBN 9781909492509. [Google Scholar]
- Nie, J.; Wang, Z.; Hu, Q. Difference between Chitosan Hydrogels via Alkaline and Acidic Solvent Systems. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog. Org. Coat. 2012, 75, 8–13. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Chitosan as a smart coating for controlled release of corrosion inhibitor 2-mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2. [Google Scholar] [CrossRef]
- Brunet, M.; Cochard, A.; Deshayes, C.; Brouca-Cabarrecq, C.; Robbiola, L.; Olivier, J.-M.; Sciau, P. Study of Post-World War II French Aeronautical Aluminium Alloy and Coatings: Historical and Materials Science Approach. Stud. Conserv. 2020, 65, 103–117. [Google Scholar] [CrossRef]
- Wang, Y.N.; Dong, C.F.; Zhang, D.W.; Ren, P.P.; Li, L.; Li, X.G. Preparation and characterization of a chitosan-based low-pH-sensitive intelligent corrosion inhibitor. Int. J. Miner. Metall. Mater. 2015, 22, 998–1004. [Google Scholar] [CrossRef]
- Lai, H.; Liu, S.; Yan, J.; Xing, F.; Xiao, P. Facile Fabrication of Biobased Hydrogel from Natural Resources: L-Cysteine, Itaconic Anhydride, and Chitosan. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Murakami, S.; Aoki, N.; Matsumura, S. Bio-based biodegradable hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with L-lysine in aqueous solution. Polym. J. 2011, 43, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in art conservation: A novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Baglioni, P.; Bonelli, N.; Chelazzi, D.; Chevalier, A.; Dei, L.; Domingues, J.; Fratini, E.; Giorgi, R.; Martin, M. Organogel formulations for the cleaning of easel paintings. Appl. Phys. A 2015, 121, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Pianorsi, M.D.; Raudino, M.; Bonelli, N.; Chelazzi, D.; Giorgi, R.; Fratini, E.; Baglioni, P. Organogels for the cleaning of artifacts. Pure Appl. Chem. 2017, 89, 3–17. [Google Scholar] [CrossRef]
- Yiming, J.; Sciutto, G.; Prati, S.; Catelli, E.; Galeotti, M.; Porcinai, S.; Mazzocchetti, L.; Samorì, C.; Galletti, P.; Giorgini, L.; et al. A new bio-based organogel for the removal of wax coating from indoor bronze surfaces. Herit. Sci. 2019, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Duncan, T.T.; Berrie, B.H.; Weiss, R.G. Soft, Peelable Organogels from Partially Hydrolyzed Poly(vinyl acetate) and Benzene-1,4-diboronic Acid: Applications to Clean Works of Art. ACS Appl. Mater. Interfaces 2017, 9, 28069–28078. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, D.; Fratini, E.; Giorgi, R.; Mastrangelo, R.; Rossi, M.; Baglioni, P. Gels for the Cleaning of Works of Art. In Gels and Other Soft Amorphous Solids; ACS Publications: Washington, DC, USA, 2018; pp. 291–314. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Tabone, M.D.; Cregg, J.J.; Beckman, E.J.; Landis, A.E. Sustainability Metrics: Life Cycle Assessment and Green Design in Polymers. Environ. Sci. Technol. 2010, 44, 8264–8269. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide?application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 2004, 7, 42–50. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927. [Google Scholar] [CrossRef]
- Lens, C.; Malet, G.; Cupferman, S. Antimicrobial activity of Butyl acetate, Ethyl acetate and Isopropyl alcohol on undesirable microorganisms in cosmetic products. Int. J. Cosmet. Sci. 2016, 38, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Gettens, R.J.; Stout, G.L. Painting Materials. A Short Encyclopaedia; Dover Publications, Inc.: New York, NY, USA, 1966; ISBN 9780486215976. [Google Scholar]
- La Nasa, J.; Orsini, S.; Degano, I.; Rava, A.; Modugno, F.; Colombini, M.P. A chemical study of organic materials in three murals by Keith Haring: A comparison of painting techniques. Microchem. J. 2016, 124, 940–948. [Google Scholar] [CrossRef]
- Chércoles Asensio, R.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Kerton, F.; Marriott, R. Alternative Solvents for Green Chemistry; Royal Society of Chemistry: London, UK, 2013; ISBN 9781849735957. [Google Scholar]
- Nikles, S.M.; Piao, M.; Lane, A.M.; Nikles, D.E. Ethyl lactate: A green solvent for magnetic tape coating. Green Chem. 2001, 3, 109–113. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Vinçotte, A.; Beauvoit, E.; Boyard, N.; Guilminot, E. Effect of solvent on PARALOID® B72 and B44 acrylic resins used as adhesives in conservation. Herit. Sci. 2019, 7, 42. [Google Scholar] [CrossRef]
- Esson, J.M.; Scott, R.; Hayes, C.J. Chemistry and Art: Removal of Graffiti Ink from Paints Grounded in a Real-Life Scenario. J. Chem. Educ. 2018, 95, 400–402. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Volpi, F.; Rehorn, C.; Vurro, R.; Blümich, B.; Mazzocchetti, L.; Giorgini, L.; Samorì, C.; Galletti, P.; et al. Cleaning oil paintings: NMR relaxometry and SPME to evaluate the effects of green solvents and innovative green gels. New J. Chem. 2019, 43, 8229–8238. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Jia, Y.; Sciutto, G.; Mazzeo, R.; Samorì, C.; Focarete, M.L.; Prati, S.; Gualandi, C. Organogel Coupled with Microstructured Electrospun Polymeric Nonwovens for the Effective Cleaning of Sensitive Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 39620–39629. [Google Scholar] [CrossRef]
- Kaplan, D.L. Introduction to Biopolymers from Renewable Resources. Biopolym. Renew. Resour. 1998, 1–29. [Google Scholar] [CrossRef]
- Knutson, C.M.; Schneiderman, D.K.; Yu, M.; Javner, C.H.; Distefano, M.D.; Wissinger, J.E. Polymeric Medical Sutures: An Exploration of Polymers and Green Chemistry. J. Chem. Educ. 2017, 94, 1761–1765. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, B.; Jia, S.; Ma, M.; Hao, J. Novel supramolecular organogel based on β-cyclodextrin as a green drug carrier for enhancing anticancer effects. J. Mol. Liq. 2018, 250, 19–25. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.S.; Grosso, M. Bioplastics and waste management. Waste Manag. 2018, 78, 800–801. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct Transformation of Edible Vegetable Waste into Bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Onen Cinar, S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Yee, L.-N.; Yee, P.L.; Ariffin, H.; Raha, A.R.; Shirai, Y.; Sudesh, K. Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy 2013, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Determination of poly-β-hydroxybutyrate (PHB) production by some Bacillus spp. World J. Microbiol. Biotechnol. 2005, 21, 565–566. [Google Scholar] [CrossRef]
- Scalioni, L.V.; Gutiérrez, M.C.; Felisberti, M.I. Green composites of poly(3-hydroxybutyrate) and curaua fibers: Morphology and physical, thermal, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Walsh-Korb, Z.; Ruiz-Fourcade, S.; Avérous, L. Responsive bio-based gels for the preservation and treatment of archaeological wooden objects. In Gels in the Conservation of Art; Angelova, L.V., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 294–296. ISBN 9781909492509. [Google Scholar]
- Samorì, C.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Prati, S.; Sciutto, G.; Volpi, F.; Tagliavini, E. The Green Attitude in Art Conservation: Polyhydroxybutyrate–based Gels for the Cleaning of Oil Paintings. ChemistrySelect 2016, 1, 4502–4508. [Google Scholar] [CrossRef]
- Fawell, J. Risk assessment case study—Chloroform and related substances. Food Chem. Toxicol. 2000, 38, S91–S95. [Google Scholar] [CrossRef]
- Baij, L.; Buijs, J.; Hermans, J.J.; Raven, L.; Iedema, P.D.; Keune, K.; Sprakel, J. Quantifying solvent action in oil paint using portable laser speckle imaging. Sci. Rep. 2020, 10, 10574. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passaretti, A.; Cuvillier, L.; Sciutto, G.; Guilminot, E.; Joseph, E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Appl. Sci. 2021, 11, 3405. https://doi.org/10.3390/app11083405
Passaretti A, Cuvillier L, Sciutto G, Guilminot E, Joseph E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Applied Sciences. 2021; 11(8):3405. https://doi.org/10.3390/app11083405
Chicago/Turabian StylePassaretti, Arianna, Luana Cuvillier, Giorgia Sciutto, Elodie Guilminot, and Edith Joseph. 2021. "Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage" Applied Sciences 11, no. 8: 3405. https://doi.org/10.3390/app11083405





