Environmentally Friendly Anticorrosive Polymeric Coatings
Abstract
1. Introduction
Corrosion Cost and Environmental Impact
2. Anticorrosive Coating Market and Its Challenges
3. Protective Mechanism of Anticorrosive Coatings
- Barrier coatings or impermeable coatings: They act by blocking the transport of aggressive species into the surface such as water, gases (i.e., CO2, SO2 in industrial atmosphere), ions (Cl- in marine atmosphere), or electrons. This can be obtained by a chemical conversion layer, or by addition of pigments to the coating. This type of coating may be used as primer, intermediate, or topcoat, and are often applied on immersed structures.
- Inhibitive coatings: In contrast with coatings based on impermeability, inhibitive coatings avoid corrosion by reacting with the environment to provide a protective film or barrier on the metallic surface. The inhibitive pigments are generally inorganic salts, which are slightly water soluble. This type of coating is primarily applied as primer because they are solely effective if dissolved constituents can react with the metal. They are mainly applied in industrial environment when the risk of atmospheric corrosion is high and are generally not recommended for immersion in water or burial in soil.
- Sacrificial coatings: They rely on the principle of galvanic corrosion for the protection of metals against corrosion. The substrate is coated by a metal or an alloy that is electrochemically more active than the substrate itself. Coatings formulated with metallic zinc powder are extensively employed for corrosion protection of steel structures. This type of coatings is only applied as primers because they need an electrical contact between the substrate and the sacrificial metal to be effective.
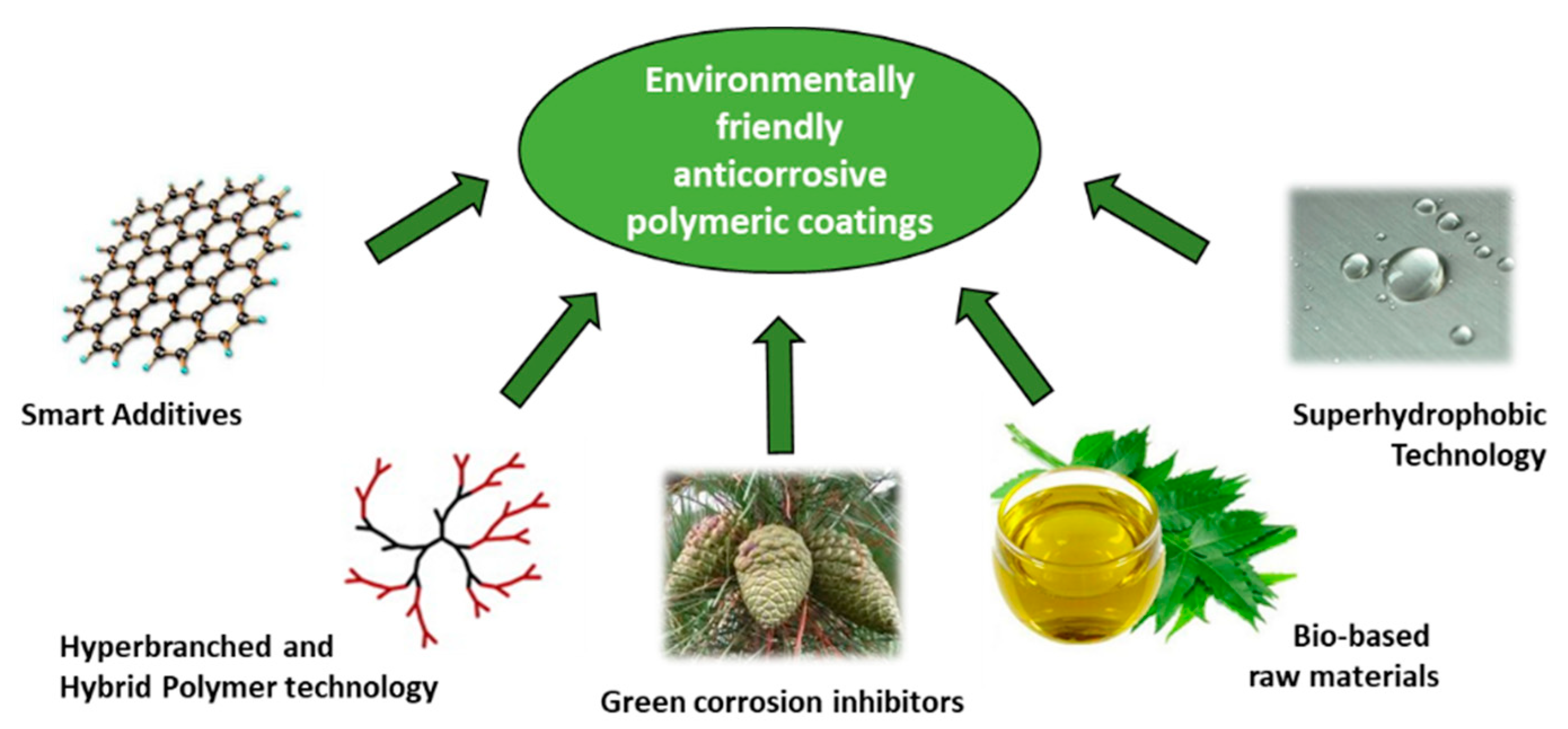
4. Developments in Environmentally Friendly Polymeric Coatings
4.1. Epoxy
4.2. Polyester
4.3. Polyurethane
4.4. Acrylic
5. New Trends
6. Applications
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillon Forms of Corrosion-Recognition and Prevention: NACE Handbook 1, Volume 1. Available online: https://store.nace.org/forms-of-corrosion-recognition-and-prevention-nace (accessed on 4 June 2020).
- Roberge, P.R. Corrosion Engineering: Principles and Practice; McGraw Hill Professional: New York, NY, USA, 2008; ISBN 978-0-07-164087-9. [Google Scholar]
- Chiavari, C.; Bernardi, E.; Martini, C.; Passarini, F.; Motori, A.; Bignozzi, M. Atmospheric corrosion of Cor-Ten steel with different surface finish: Accelerated ageing and metal release. Mater. Chem. Phys. 2012, 136, 477–486. [Google Scholar] [CrossRef]
- Christiansen, B.C.; Balic-Zunic, T.; Dideriksen, K.; Stipp, S. Identification of Green Rust in Groundwater. Environ. Sci. Technol. 2009, 43, 3436–3441. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Avci, R.; Fox, R.V.; Deliorman, M.; Suo, Z.; Kellerman, L. Characterization of Arsenic Contamination on Rust from Ton Containers. Ind. Eng. Chem. Res. 2013, 52, 1396–1404. [Google Scholar] [CrossRef]
- Lindström, D.; Wallinder, I.O. Long-term use of galvanized steel in external applications. Aspects of patina formation, zinc runoff, barrier properties of surface treatments, and coatings and environmental fate. Environ. Monit. Assess. 2010, 173, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Van Der Lugt, W.; Euser, S.M.; Bruin, J.P.; Boer, J.W.D.; Walker, J.T.; Crespi, S. Growth of Legionella anisa in a model drinking water system to evaluate different shower outlets and the impact of cast iron rust. Int. J. Hyg. Environ. Heal. 2017, 220, 1295–1308. [Google Scholar] [CrossRef]
- Kirchgeorg, T.; Weinberg, I.; Hörnig, M.; Baier, R.; Schmid, M.; Brockmeyer, B. Emissions from corrosion protection systems of offshore wind farms: Evaluation of the potential impact on the marine environment. Mar. Pollut. Bull. 2018, 136, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Si, R.; Dai, Q.; You, Z.; Ma, Y.; Wang, J. A critical review of corrosion development and rust removal techniques on the structural/environmental performance of corroded steel bridges. J. Clean. Prod. 2019, 233, 126–146. [Google Scholar] [CrossRef]
- Koch, G.H.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. Nace International Report. International Measures of Prevention Application and Economics of Corrosion Technologies Study. Available online: http://impact.nace.org/executive-summary.aspx (accessed on 19 March 2021).
- Sud, A. Anticorrosive Coatings. European Coatings Journal, 10 May 2015; 8–9. [Google Scholar]
- Gagro, D. Protective Coatings. European Coatings Journal, 8 April 2020; 10–11. [Google Scholar]
- Paints and Coatings Global Market Report 2021: COVID 19 Impact and Recovery to 2030. Available online: https://www.reportlinker.com/p06018803/Paints-And-Coatings-Global-Market-Report-COVID-19-Impact-and-Recovery-to.html?utm_source=GNW (accessed on 19 March 2021).
- Interview: The Covid-19 Pandemic Has Put Everything Upside Down. Available online: https://www.european-coatings.com/articles/2021/03/interview-the-covid-19-pandemic-has-put-everything-upside-down (accessed on 19 March 2021).
- Paints and Coatings Market | Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/paints-and-coatings-market (accessed on 19 March 2021).
- Lyon, S.; Bingham, R.; Mills, D. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Tsn, S.N. Surface Pretreatment by Phosphate Conversion Coatings—A Review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Osborne, J.H. Observations on chromate conversion coatings from a sol–gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Parashar, G.; Bajpayee, M.; Kamani, P. Water-borne non-toxic high-performance inorganic silicate coatings. Surf. Coat. Int. Part B Coat. Trans. 2003, 86, 209–216. [Google Scholar] [CrossRef]
- Verma, A.; Van Ooij, W. High-temperature batch hot-dip galvanizing. Part 2. Comparison of coatings formed in the temperature range 520–555 °C. Surf. Coat. Technol. 1997, 89, 143–150. [Google Scholar] [CrossRef]
- Jakobson, S.; Crotty, D.; Griffin, R.; Phipps, D.; Rubin, E. Zinc anodizing. Met. Finish. 1998, 96, 114–118. [Google Scholar] [CrossRef]
- Ping, Z.; He, Y.; Gu, C.; Zhang, T.-Y. Mechanically assisted electroplating of Ni–P coatings on carbon steel. Surf. Coat. Technol. 2008, 202, 6023–6028. [Google Scholar] [CrossRef]
- Gharbi, O.; Thomas, S.; Smith, C.; Birbilis, N. Chromate replacement: What does the future hold? NPJ Mater. Degrad. 2018, 2, 12. [Google Scholar] [CrossRef]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Adhikari, S.; Unocic, K.; Zhai, Y.; Frankel, G.; Zimmerman, J.; Fristad, W. Hexafluorozirconic acid based surface pretreatments: Characterization and performance assessment. Electrochimica Acta 2011, 56, 1912–1924. [Google Scholar] [CrossRef]
- Rudd, A.L.; Breslin, C.B.; Mansfeld, F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium. Corros. Sci. 2000, 42, 275–288. [Google Scholar] [CrossRef]
- Walker, D.E.; Wilcox, G.D. Molybdate based conversion coatings for zinc and zinc alloy surfaces: A review. Trans. IMF 2008, 86, 251–259. [Google Scholar] [CrossRef]
- Hodge, J.; Mirabile, D. Pearson Most appropriate treatments to control the environmental impact of effluents in the iron and steel industry. Available online: http://op.europa.eu/es/publication-detail/-/publication/bc305c8d-1a4d-462c-8f4e-483da0aa4b74 (accessed on 30 November 2020).
- Cunningham, M.F.; Campbell, J.D.; Fu, Z.; Bohling, J.; Leroux, J.G.; Mabee, W.; Robert, T. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 2019, 21, 4919–4926. [Google Scholar] [CrossRef]
- Salata, R.R.; Pellegrene, B.; Soucek, M.D. Synthesis and properties of a high solids triethoxysilane-modified alkyd coatings. Prog. Org. Coat. 2019, 133, 340–349. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.; Udayabhanu, G.; Narayan, R. Water-based & eco-friendly epoxy-silane hybrid coating for enhanced corrosion protection & adhesion on galvanized steel. Prog. Org. Coat. 2016, 101, 24–44. [Google Scholar] [CrossRef]
- Ramlan, S.N.A.; Basirun, W.J.; Phang, S.-W.; Ang, D.T.-C. Electrically conductive palm oil-based coating with UV curing ability. Prog. Org. Coat. 2017, 112, 9–17. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Asemani, H.; Skuza, J.; Mannari, V. Synthesis of non-isocyanate polyurethanes and their application in radiation-curable aerospace coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Derksen, J.T.; Cuperus, F.; Kolster, P. Paints and coatings from renewable resources. Ind. Crop. Prod. 1995, 3, 225–236. [Google Scholar] [CrossRef]
- Sherwood, J.; De Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2018, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Macmillan: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control. Available online: https://www.elsevier.com/books/principles-of-corrosion-engineering-and-corrosion-control/ahmad/978-0-7506-5924-6 (accessed on 4 June 2020).
- Cramer, S.D.; Covino, B.S. ASM Handbook Volume 13B, Corrosion: Materials—ASM International. Available online: https://www.asminternational.org/search/-/journal_content/56/10192/06508G/PUBLICATION (accessed on 4 June 2020).
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Weiss, K.D. Paint and coatings: A mature industry in transition. Prog. Polym. Sci. 1997, 22, 203–245. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully Biobased Epoxy Resins from Fatty Acids and Lignin. Mol. 2020, 25, 1158. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Elmore, J.D.; Kincaid, D.S.; Komar, P.C.; Nielsen, J.E. Waterborne epoxy protective coatings for metal. J. Coat. Technol. 2002, 74, 63–72. [Google Scholar] [CrossRef]
- Galgoci, E.C.; Komar, P.C.; Elmore, J.D. High performance waterborne coatings based on dispersions of a solid epoxy resin and an amine-functional curing agent. J. Coat. Technol. 1999, 71, 45–52. [Google Scholar] [CrossRef]
- Mišković-Stanković, V.B. The mechanism of cathodic electrodeposition of epoxy coatings and the corrosion behaviour of the electrodeposited coatings. J. Serbian Chem. Soc. 2002, 67, 305–324. [Google Scholar] [CrossRef]
- Hunakoshi, F.; Ishii, T. Cathodic Electrodeposition Paint. US6054033A, 25 April 2000. [Google Scholar]
- Wapner, K.; GASPAR, F.; Hammer, C. Aqueous Dip-Coating Composition for Electroconductive Substrates, Comprising Dissolved Bismuth. US9920205B2, 20 March 2018. [Google Scholar]
- Almeida, E.; Santos, D.; Fragata, F.; de la Fuente, D.; Morcillo, M. Anticorrosive painting for a wide spectrum of marine atmospheres: Environmental-friendly versus traditional paint systems. Prog. Org. Coat. 2006, 57, 11–22. [Google Scholar] [CrossRef]
- Waterborne Epoxy Zinc-Rich Primers: There Are Viable Options. Available online: https://www.pcimag.com/articles/96850-waterborne-epoxy-zinc-rich-primers--there-are-viable-options-?v=preview (accessed on 12 March 2021).
- Wang, J.; Qi, Y.; Zhao, X.; Zhang, Z. Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution. Coatings 2020, 10, 444. [Google Scholar] [CrossRef]
- Cooper, C.; Galick, P.; Harris, S.; Pourreau, D.; Rodriguez, C. Tert-butyl acetate: Non-HAP solvent for high-solids epoxy formulations. J. Coat. Technol. 2001, 73, 19–24. [Google Scholar] [CrossRef]
- Chen, X.; Wen, S.; Feng, T.; Yuan, X. High solids organic-inorganic hybrid coatings based on silicone-epoxy-silica coating with improved anticorrosion performance for AA2024 protection. Prog. Org. Coat. 2020, 139, 105374. [Google Scholar] [CrossRef]
- Foix, D.; Francos, X.F.; Ramis, X.; Serra, A.; Sangermano, M. New pegylated hyperbranched polyester as chemical modifier of epoxy resins in UV cationic photocuring. React. Funct. Polym. 2011, 71, 417–424. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef]
- Ni, L.; Chemtob, A.; Croutxé-Barghorn, C.; Moreau, N.; Bouder, T.; Chanfreau, S.; Pébère, N. Direct-to-metal UV-cured hybrid coating for the corrosion protection of aircraft aluminium alloy. Corros. Sci. 2014, 89, 242–249. [Google Scholar] [CrossRef]
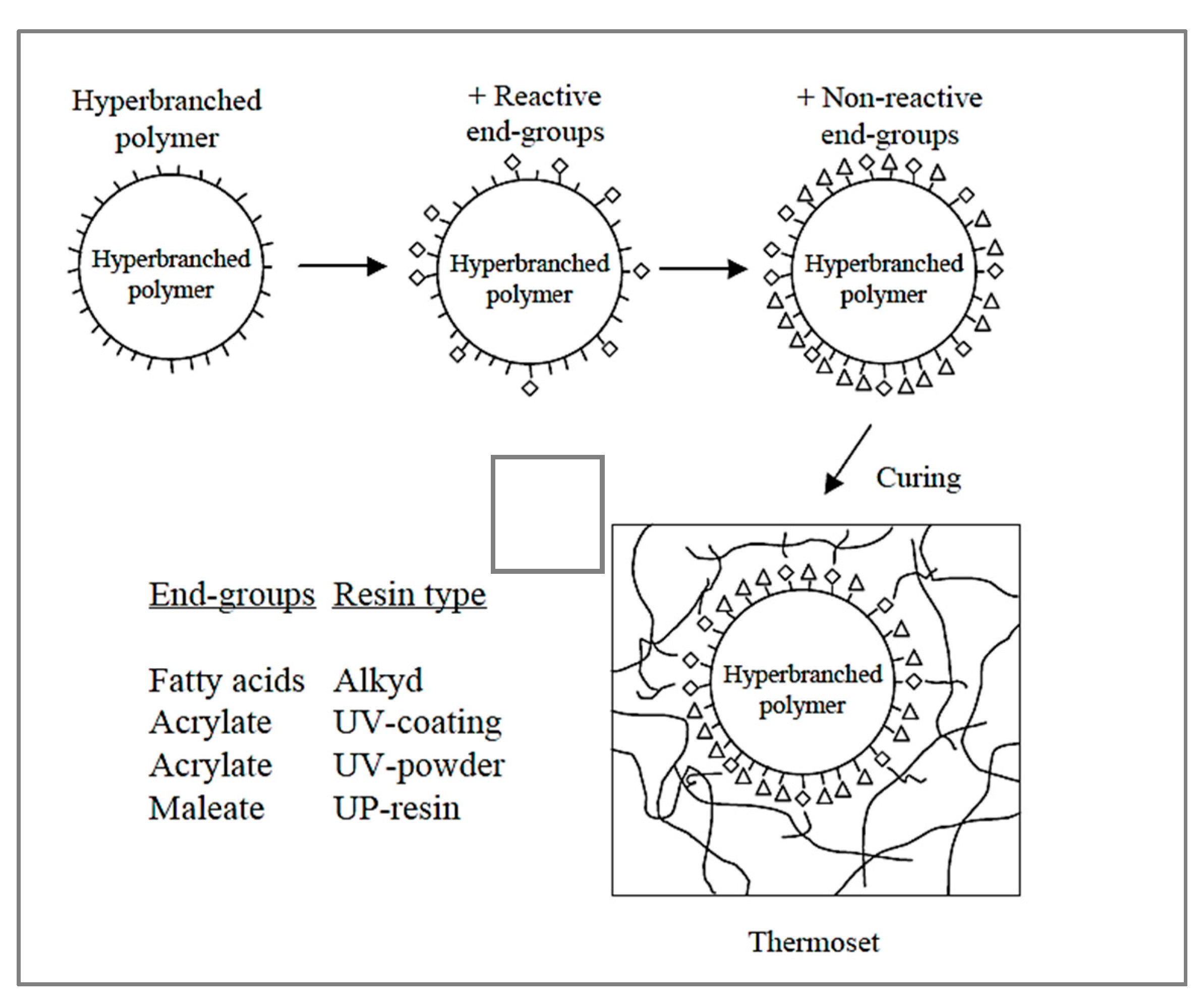
- Singh, A.P.; Suryanarayana, C.; Naik, R.B.; Gunasekaran, G. Development of hyperbranched polyester polyol-based waterborne anticorrosive coating. J. Coat. Technol. Res. 2016, 13, 41–51. [Google Scholar] [CrossRef]
- Unnisa, C.B.N.; Devi, G.N.; Hemapriya, V.; Chitra, S.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Linear polyesters as effective corrosion inhibitors for steel rebars in chloride induced alkaline médium—An electrochemical approach. Constr. Build. Mater. 2018, 165, 866–876. [Google Scholar] [CrossRef]
- Johansson, M.; Glauser, T.; Jansson, A.; Hult, A.; Malmström, E.; Claesson, H. Design of coating resins by changing the macromolecular architecture: Solid and liquid coating systems. Prog. Org. Coat. 2003, 48, 194–200. [Google Scholar] [CrossRef]
- Hadzich, A.; Gross, G.A.; Leimbach, M.; Ispas, A.; Bund, A.; Flores, S. Effect of polyalcohols on the anticorrosive behaviour of alkyd coatings prepared with drying oils. Prog. Org. Coat. 2020, 145, 105671. [Google Scholar] [CrossRef]
- Ikladious, N.E.; Asaad, J.N.; Emira, H.S.; Mansour, S.H. Alkyd resins based on hyperbranched polyesters and PET waste for coating applications. Prog. Org. Coat. 2017, 102, 217–224. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Abdelghaffar, F.; Haroun, A.A. UV-curable hyperbranched polyester acrylate encapsulation of phthalocyanine pigments for high performance synthetic fabrics printing. Dye. Pigment. 2020, 177, 108307. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Roussak, O.; Gesser, H.D. Applied Chemistry; Metzler, J.B., Ed.; Springer US: Boston, MA, USA, 2013. [Google Scholar]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Cashew nut shell liquid terminated self-healable polyurethane as an effective anticorrosive coating with biodegradable attribute. Prog. Org. Coat. 2020, 139, 105472. [Google Scholar] [CrossRef]
- Marathe, R.; Tatiya, P.; Chaudhari, A.; Lee, J.; Mahulikar, P.; Sohn, D.; Gite, V. Neem acetylated polyester polyol—Renewable source based smart PU coatings containing quinoline (corrosion inhibitor) encapsulated polyurea microcapsules for enhance anticorrosive property. Ind. Crop. Prod. 2015, 77, 239–250. [Google Scholar] [CrossRef]
- Gite, V.V.; Tatiya, P.D.; Marathe, R.J.; Mahulikar, P.P.; Hundiwale, D.G. Microencapsulation of quinoline as a corrosion inhibitor in polyurea microcapsules for application in anticorrosive PU coatings. Prog. Org. Coat. 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Jang, J.H.; Patil, C.; Jirimali, H.; Gite, V.; Hong, S.C. Functional soybean oil-based polyols as sustainable feedstocks for polyurethane coatings. Ind. Crop. Prod. 2018, 113, 249–258. [Google Scholar] [CrossRef]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Fei, G.; Wang, C.; Shen, Y.; Zhu, K.; Sun, L.; Rang, N.; Guo, D.; Wallace, G.G. Functionalizing graphene with titanate coupling agents as reinforcement for one-component waterborne poly(urethane-acrylate) anticorrosion coatings. Chem. Eng. J. 2019, 359, 331–343. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, L.; Feng, L.; Lu, H.; Xu, Y.; Wang, J.; Xiang, B.; Zou, X. Polydopamine functionalized graphene oxide nanocomposites reinforced the corrosion protection and adhesion properties of waterborne polyurethane coatings. Eur. Polym. J. 2019, 120, 109249. [Google Scholar] [CrossRef]
- Siyanbola, T.; Neelambaram, P.; Mohanty, S.; Somisetti, V.; Basak, P.; Narayan, R.; Kothapalli, R.V. The effects of carbonized Eucalyptus globulus leaves on castor seed oil based urethane coating system. Prog. Org. Coat. 2019, 131, 42–48. [Google Scholar] [CrossRef]
- Hou, L.; Zhou, M.; Wang, S. Synthesis, thermal and anticorrosion performance of WPU nanocomposites with low carbon-black content by adding amine-modified multiwall carbon nanotube. Diam. Relat. Mater. 2018, 90, 166–171. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; Hasan, A.M.; Keshawy, M.; El Saeed, A.M.; Aboelenien, O.M. Nanocrystalline cellulose as an eco-friendly reinforcing additive to polyurethane coating for augmented anticorrosive behavior. Carbohydr. Polym. 2018, 183, 311–318. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; Waghoo, G. Effect of incorporation of surface treated zinc oxide on non-isocyanate polyurethane based nano-composite coatings. Prog. Org. Coat. 2013, 76, 1215–1229. [Google Scholar] [CrossRef]
- Gao, F.; Du, A.; Ma, R.; Lv, C.; Yang, H.; Fan, Y.; Zhao, X.; Wu, J.; Cao, X. Improved corrosion resistance of acrylic coatings prepared with modified MoS2 nanosheets. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 587, 124318. [Google Scholar] [CrossRef]
- Song, D.; Yin, Z.; Liu, F.; Wan, H.; Gao, J.; Zhang, D.; Li, X. Effect of carbon nanotubes on the corrosion resistance of water-borne acrylic coatings. Prog. Org. Coat. 2017, 110, 182–186. [Google Scholar] [CrossRef]
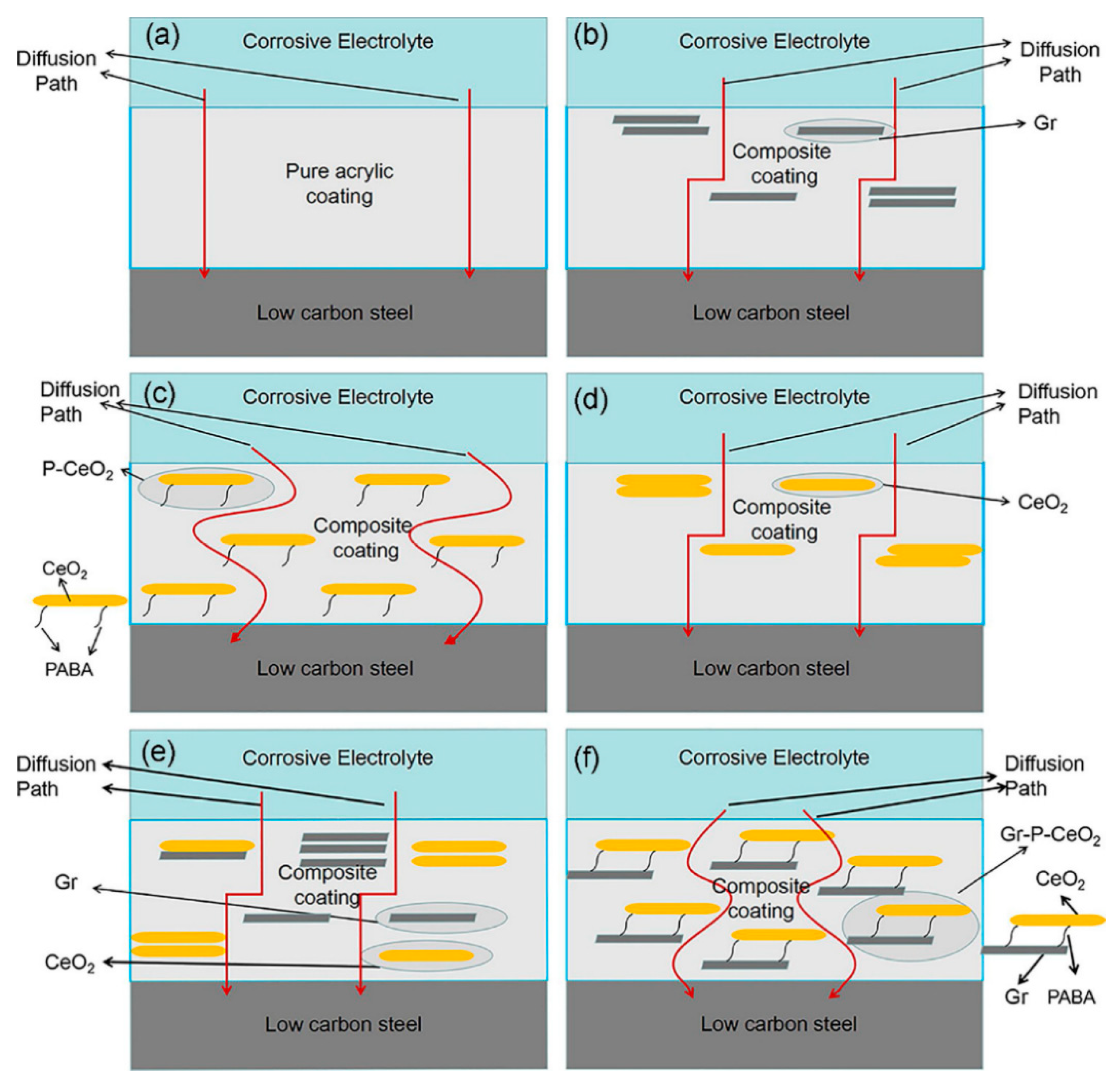
- Li, H.; Wang, J.; Yang, J.; Zhang, J.; Ding, H. Large CeO2 nanoflakes modified by graphene as barriers in waterborne acrylic coatings and the improved anticorrosion performance. Prog. Org. Coat. 2020, 143, 105607. [Google Scholar] [CrossRef]
- Rajkumar, R.; Vedhi, C. A study of corrosion protection efficiency of silica nanoparticles acrylic coated on mild steel electrode. Vacuum 2019, 161, 1–4. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; De Souza, T.A.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem. Eng. J. 2020, 383, 123219. [Google Scholar] [CrossRef]
- Zulkifli, F.; Ali, N.; Yusof, M.S.M.; Isa, M.; Yabuki, A.; Nik, W.W. Henna leaves extract as a corrosion inhibitor in acrylic resin coating. Prog. Org. Coat. 2017, 105, 310–319. [Google Scholar] [CrossRef]
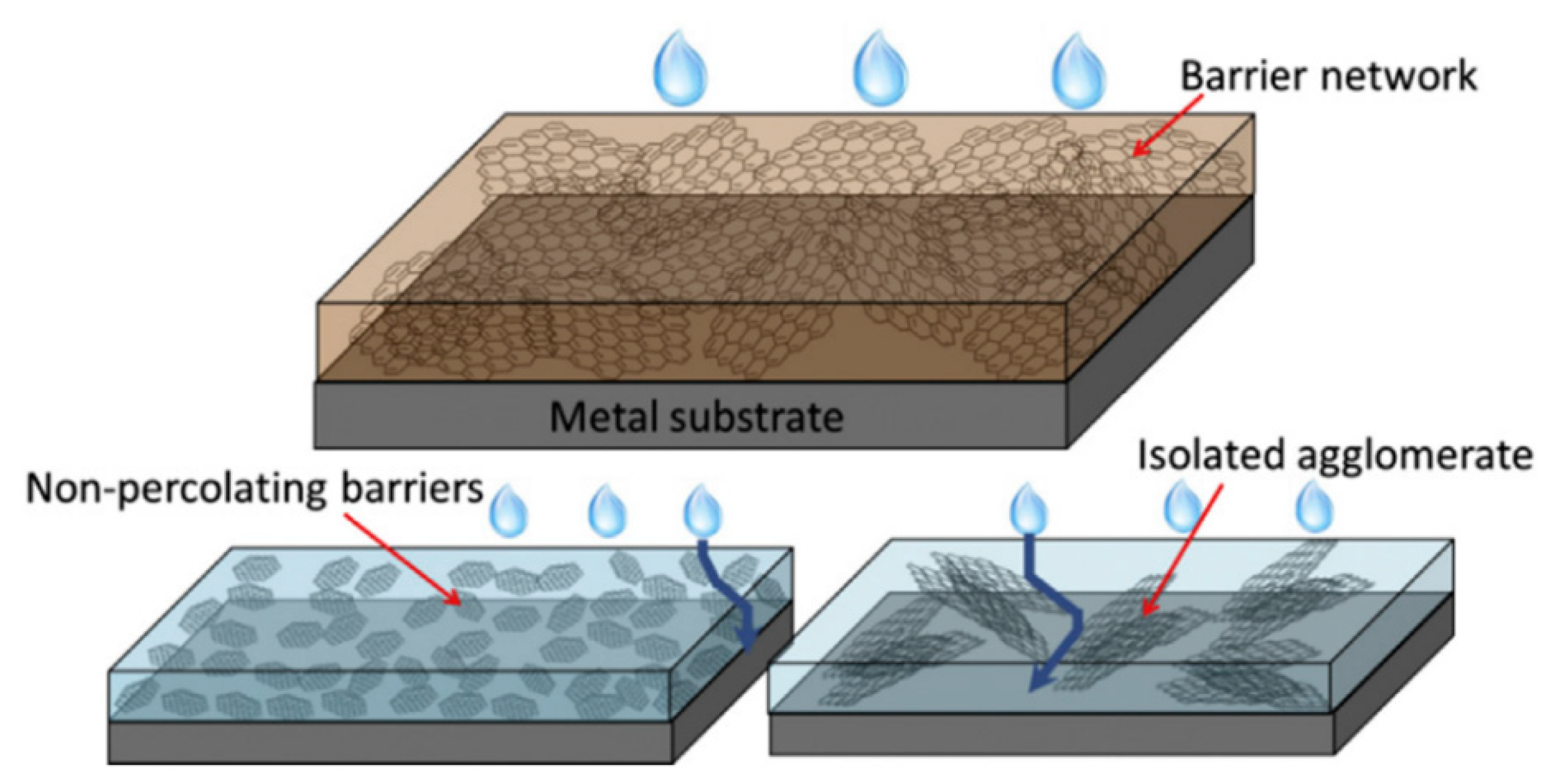
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Teng, S.; Gao, Y.; Cao, F.; Kong, D.; Zheng, X.; Ma, X.; Zhi, L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018, 123, 185–189. [Google Scholar] [CrossRef]
- Odarczenko, M.; Thakare, D.; Li, W.; Yang, K.; Tang, S.; Venkateswaran, S.P.; Sottos, N.R.; White, S.R. Self-Protecting Epoxy Coatings with Anticorrosion Microcapsules. ACS Omega 2018, 3, 14157–14164. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Proverbio, E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings 2019, 9, 409. [Google Scholar] [CrossRef]
- Wang, G.; Wen, S.; Qian, S.; Wang, J.; Wang, C.; Chen, Y. Synthesis of novel nano hyperbranched polymer resin and its corrosion resistance in coatings. Prog. Org. Coat. 2020, 140, 105496. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Peres, R.S.; Freiberger, K.; Ferreira, C.A.; Tamborim, S.M.M.; Azambuja, D.S. Zinc tannate and magnesium tannate as anticorrosion pigments in epoxy paint formulations. Prog. Org. Coat. 2018, 121, 23–29. [Google Scholar] [CrossRef]
- Peres, R.S.; Zmozinski, A.V.; Brust, F.R.; Macedo, A.J.; Armelin, E.; Alemán, C.; Ferreira, C.A. Multifunctional coatings based on silicone matrix and propolis extract. Prog. Org. Coat. 2018, 123, 223–231. [Google Scholar] [CrossRef]
- Veedu, K.K.; Kalarikkal, T.P.; Jayakumar, N.; Gopalan, N.K. Anticorrosive Performance of Mangifera indica L. Leaf Extract-Based Hybrid Coating on Steel. ACS Omega 2019, 4, 10176–10184. [Google Scholar] [CrossRef]
- Montoya, L.; Contreras, D.; Jaramillo, A.; Carrasco, C.; Fernández, K.; Schwederski, B.; Rojas, D.; Melendrez, M. Study of anticorrosive coatings based on high and low molecular weight polyphenols extracted from the Pine radiata bark. Prog. Org. Coat. 2019, 127, 100–109. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; He, Y. Preparation of environmental friendly coatings based on natural shellac modified by diamine and its applications for copper protection. Prog. Org. Coat. 2008, 62, 307–312. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Montemor, M. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Fitridge, I.; Dempster, T.; Guenther, J.; De Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Self-healable and thiol–ene UV-curable waterborne polyurethane for anticorrosion coating. J. Appl. Polym. Sci. 2019, 136, 47700. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, D.; Zhou, M.; Xia, Y.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Bio-based omniphobic polyurethane coating providing anti-smudge and anti-corrosion protection. Prog. Org. Coat. 2020, 148, 105844. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- VCI In Singapore | Preservemetals. Available online: https://www.preservemetals.com/environment-friendly-anti-corrosion-solution (accessed on 17 December 2020).
- Bizet, B.; Grau, E.; Cramail, H.; Asua, J.M. Water-based non-isocyanate polyurethane-ureas (NIPUUs). Polym. Chem. 2020, 11, 3786–3799. [Google Scholar] [CrossRef]
- SYLOMASK Anti-Corrosion Pigment | Fuji Silysia Chemical. Available online: https://www.fujisilysia.com/products/sylomask/ (accessed on 12 March 2021).
- Pigmentan | Environmentally Friendly Anti Corrosive Protection. Available online: https://www.pigmentan.com/ (accessed on 12 March 2021).
- El-Hamid, D.; Blustein, G.; Deyá, M.; Del Amo, B.; Romagnoli, R. The anticorrosive performance of zinc-free non-toxic pigment for paints. Mater. Chem. Phys. 2011, 127, 353–357. [Google Scholar] [CrossRef]
- Langer, E.; Zubielewicz, M.; Kuczyńska, H.; Królikowska, A.; Komorowski, L. Anticorrosive effectiveness of coatings with reduced content of Zn pigments in comparison with zinc-rich primers. Corros. Eng. Sci. Technol. 2019, 54, 627–635. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Momber, A.W.; Marquardt, T. Protective coatings for offshore wind energy devices (OWEAs): A review. J. Coat. Technol. Res. 2017, 15, 13–40. [Google Scholar] [CrossRef]
- Li, Y.; Ning, C. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures—A technical review. J. Loss Prev. Process. Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Buskens, P.; Wouters, M.; Rentrop, C.; Vroon, Z. A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J. Coat. Technol. Res. 2012, 10, 29–36. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control Through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Almeida, E.; Santos, D.; Fragata, F.; Rincon, O.; Morcillo, M. Alternative Environmentally Friendly Coatings for Mild Steel and Electrogalvanized Steel to Be Exposed to Atmospheres. Mater. Corros. 2001, 52, 904–919. [Google Scholar] [CrossRef]
- Anticorrosion Coating Industry Transitioning to Sustainable Development. Available online: https://www.pcimag.com/articles/103192-anticorrosion-coating-industry-transitioning-to-sustainable-development (accessed on 12 March 2021).
- NanoPrime Water Based Primer, No VOCs—Nanorustrx. Available online: https://www.nanorustx.com/ (accessed on 17 December 2020).
- Hemucryl—Hempel. Available online: https://www.hempel.com/products/brand/hemucryl/explore (accessed on 17 December 2020).
- Eco-Friendly Corrosion Protection Systems—Evonik Industries. Available online: https://corporate.evonik.com/en/eco-friendly-corrosion-protection-systems-109077.html (accessed on 17 December 2020).
- No Chance for Corrosion—Dynasylan®—the Brand for Functional Silanes. Available online: https://www.dynasylan.com/product/dynasylan/en/pages/article.aspx?articleId=26025 (accessed on 17 December 2020).
- Eco-Friendly Coatings for Transportation by Eco Smart CoatingsTM | EcoOnyx TM | SmartArmRTM | AmortizeTM Rubberized. Available online: https://ecosmartcoatings.com/transportation_coatings.html (accessed on 17 December 2020).
- Making the Switch to Eco-Friendly Coatings. Available online: https://www.solvay.com/en/article/eco-friendly-waterborne-solutions (accessed on 17 December 2020).
- Steel Bridges. Available online: http://legacy.jotun.com/us/en/b2b/paintsandcoatings/bridges/Steel-Bridges.aspx?q=Solutions (accessed on 17 December 2020).
- Anti-Corrosive Pigments for Water-Based Coatings. Available online: https://www.heubachcolor.com/news/anti-corrosive-pigments-for-water-based-coatings/ (accessed on 17 December 2020).



| Class Definition | Type of Structure That Needs an Anticorrosive Coating |
|---|---|
| C1, very low | Offices, shops, schools, hotels. |
| C2, low | Rural areas, depots, sport halls. |
| C3, medium | Urban, industrial and coastal atmospheres with low salinity, production rooms with high humidity and some air pollution (e.g., food processing plants, laundries, breweries, dairies). |
| C4, high | Industrial and coastal areas with moderate salinity (e.g., chemical plants, swimming pools, coastal ships, boatyards). |
| C5, very high | Industrial areas with high humidity and aggressive atmospheres and coastal areas with high salinity, metallic structures with almost permanent concentration and high pollution. |
| CX, extreme | Offshore areas with high salinity and industrial areas with extreme humidity, aggressive atmosphere, and sub-tropical and tropical atmospheres. Industrial areas with extreme humidity and aggressive atmosphere. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. https://doi.org/10.3390/app11083446
Faccini M, Bautista L, Soldi L, Escobar AM, Altavilla M, Calvet M, Domènech A, Domínguez E. Environmentally Friendly Anticorrosive Polymeric Coatings. Applied Sciences. 2021; 11(8):3446. https://doi.org/10.3390/app11083446
Chicago/Turabian StyleFaccini, Mirko, Lorenzo Bautista, Laura Soldi, Ana M. Escobar, Manuela Altavilla, Martí Calvet, Anna Domènech, and Eva Domínguez. 2021. "Environmentally Friendly Anticorrosive Polymeric Coatings" Applied Sciences 11, no. 8: 3446. https://doi.org/10.3390/app11083446
APA StyleFaccini, M., Bautista, L., Soldi, L., Escobar, A. M., Altavilla, M., Calvet, M., Domènech, A., & Domínguez, E. (2021). Environmentally Friendly Anticorrosive Polymeric Coatings. Applied Sciences, 11(8), 3446. https://doi.org/10.3390/app11083446







