Polyisocyanurate Foam Pyrolysis and Flame Spread Modeling
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Milligram-Scale Experiments
2.1.1. TGA and DSC
2.1.2. MCC
2.2. Gram-Scale Experiments
2.2.1. CAPA II Tests
2.2.2. Cone Calorimetry
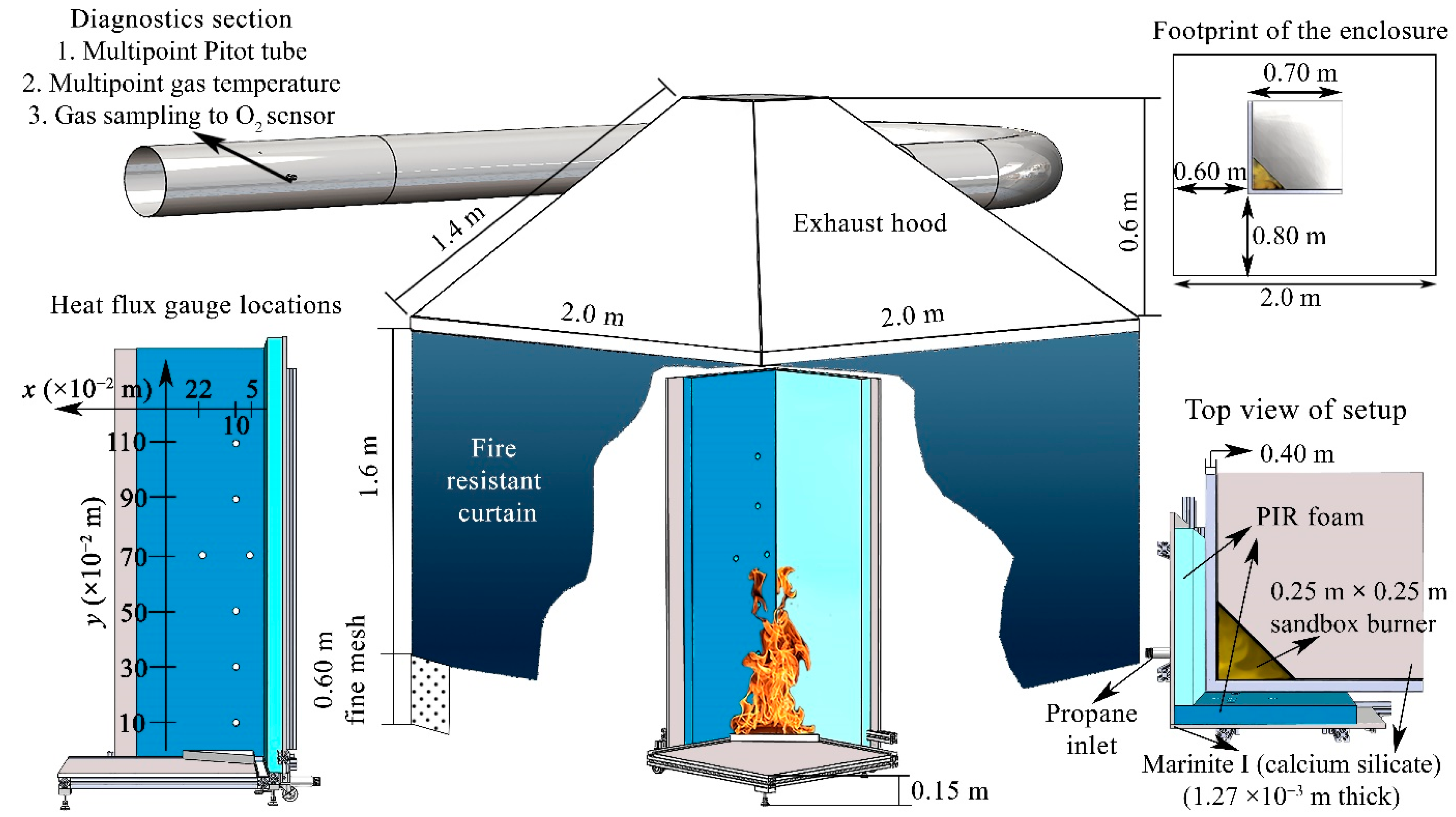
2.3. Full-Scale Experiments
2.4. Modeling
2.4.1. Modeling of Milligram-Scale Experiments
2.4.2. Modeling of Gram-Scale Experiments
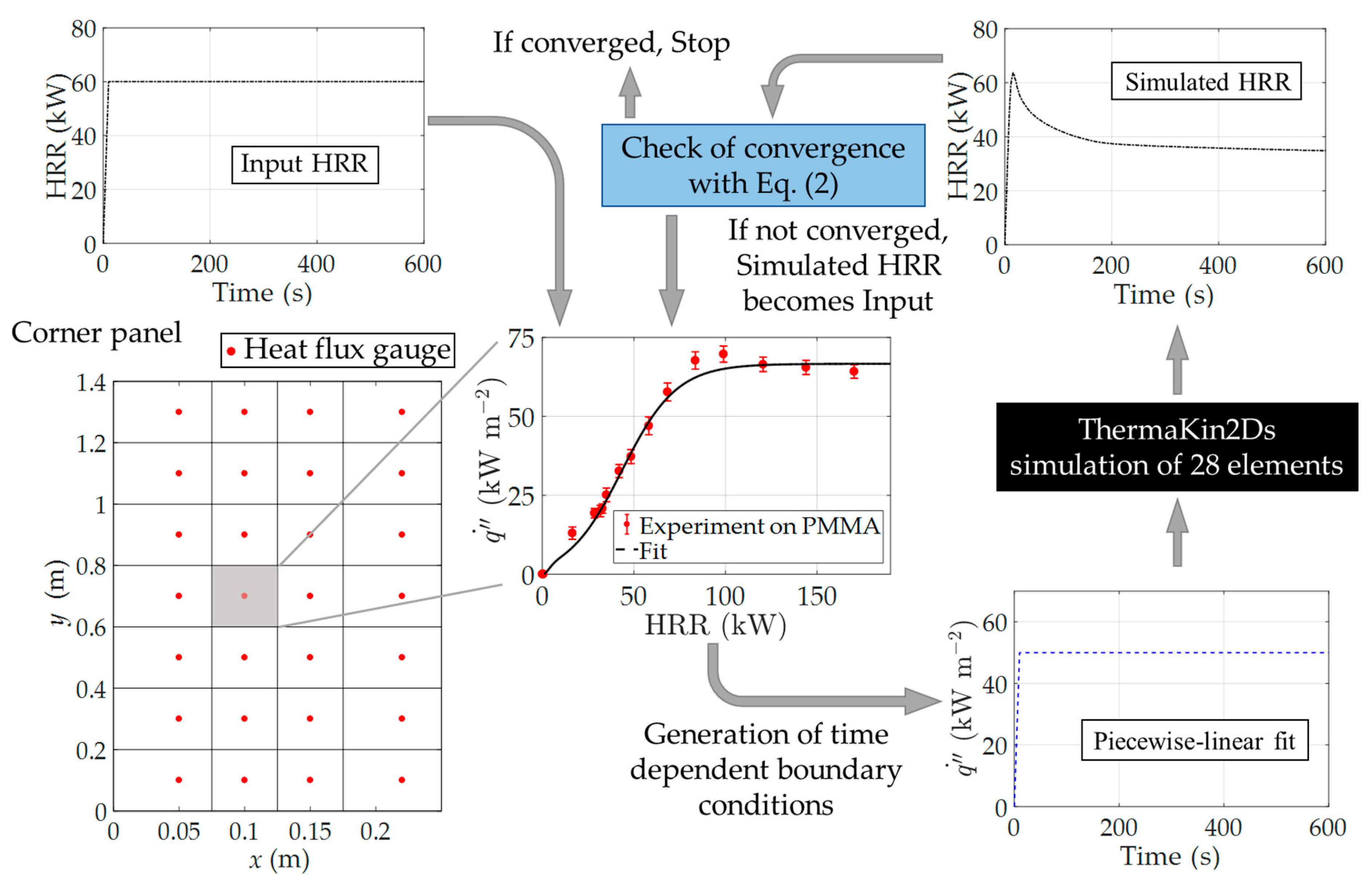
2.4.3. Modeling of Full-Scale Experiments
3. Results and Discussion
3.1. TGA and DSC Experimental Results and Modeling
3.2. MCC Experimental Results and Modeling
3.3. CAPA II Experimental Results and Modeling
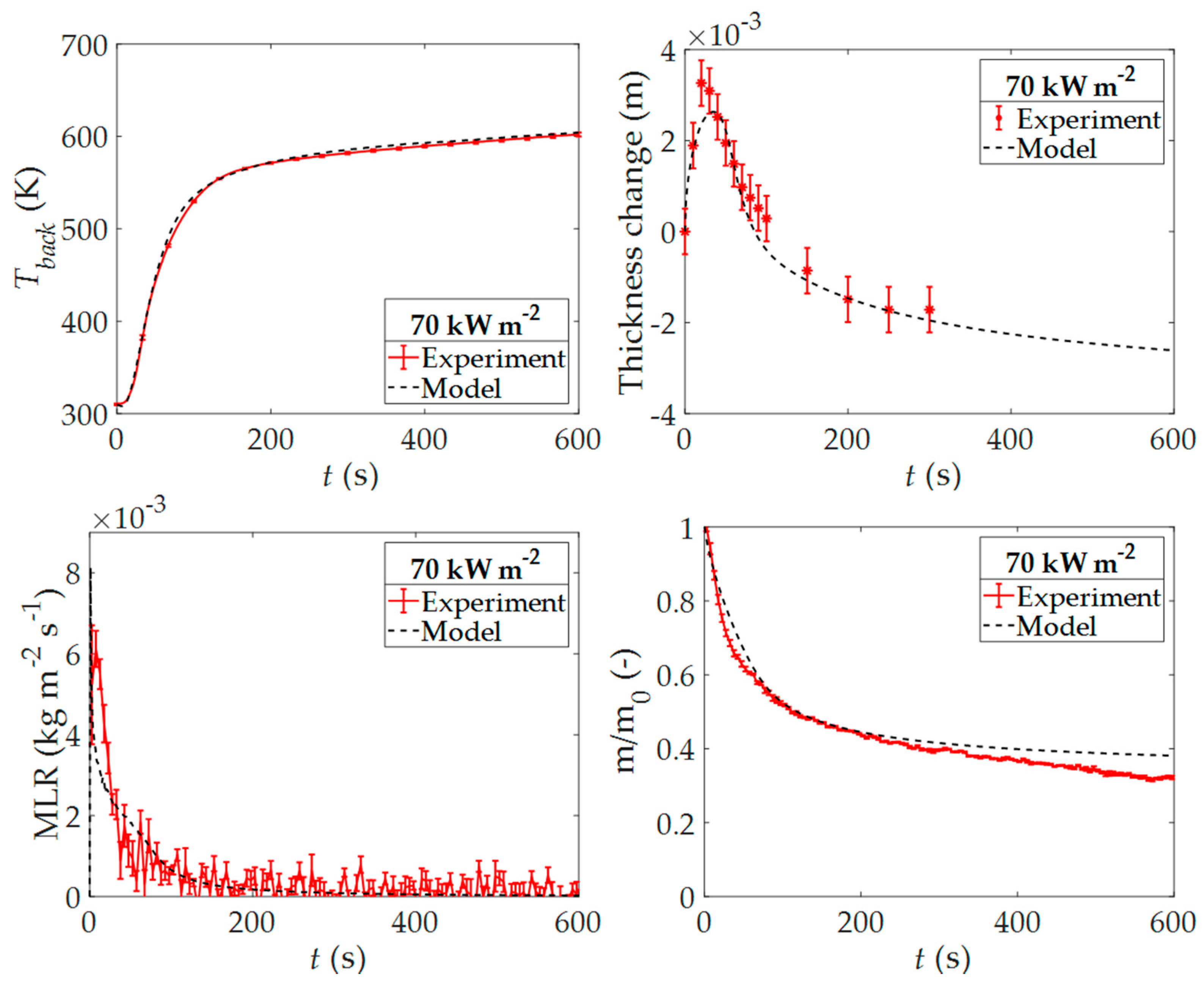
3.4. Cone Calorimetry Experimental Results and Modeling
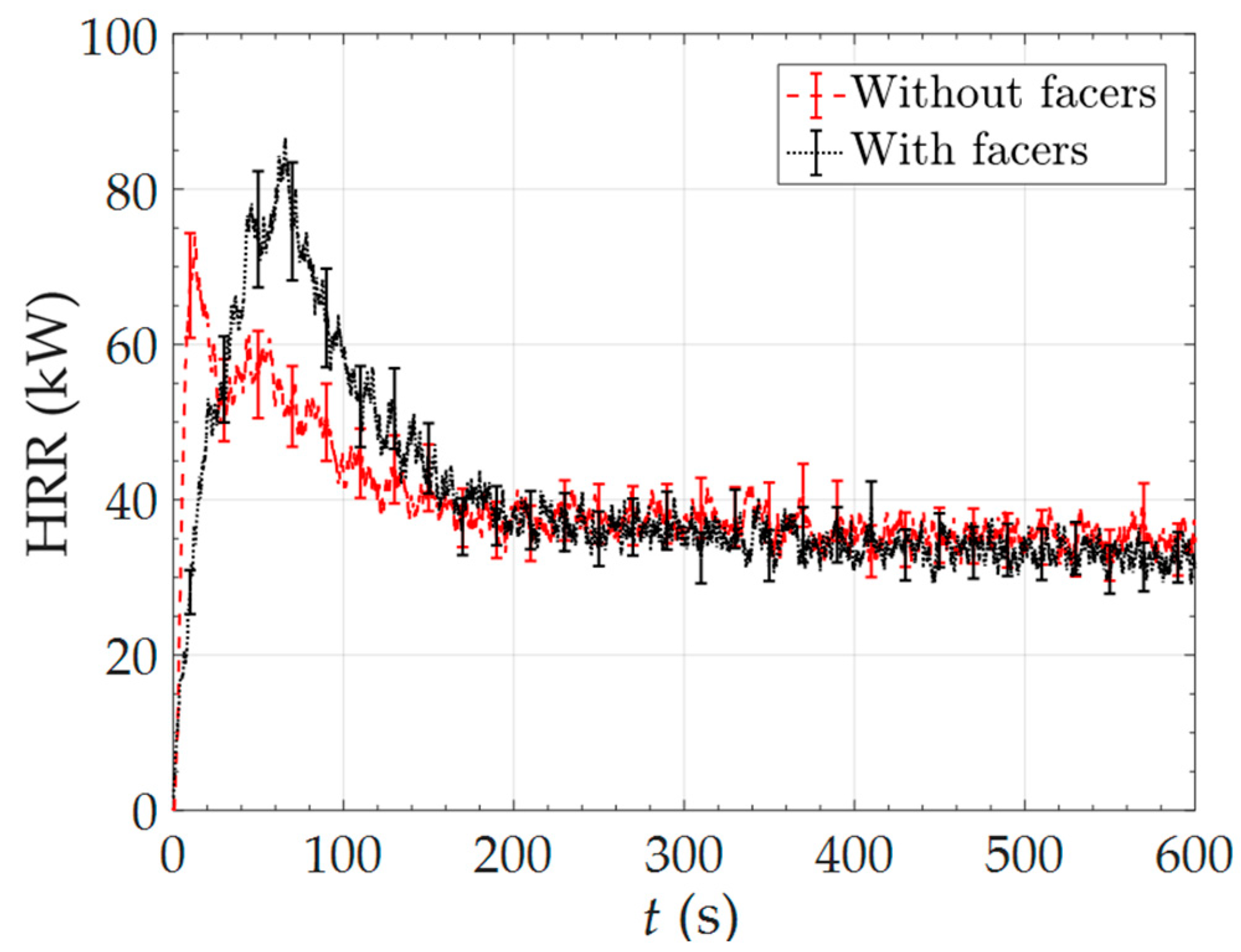
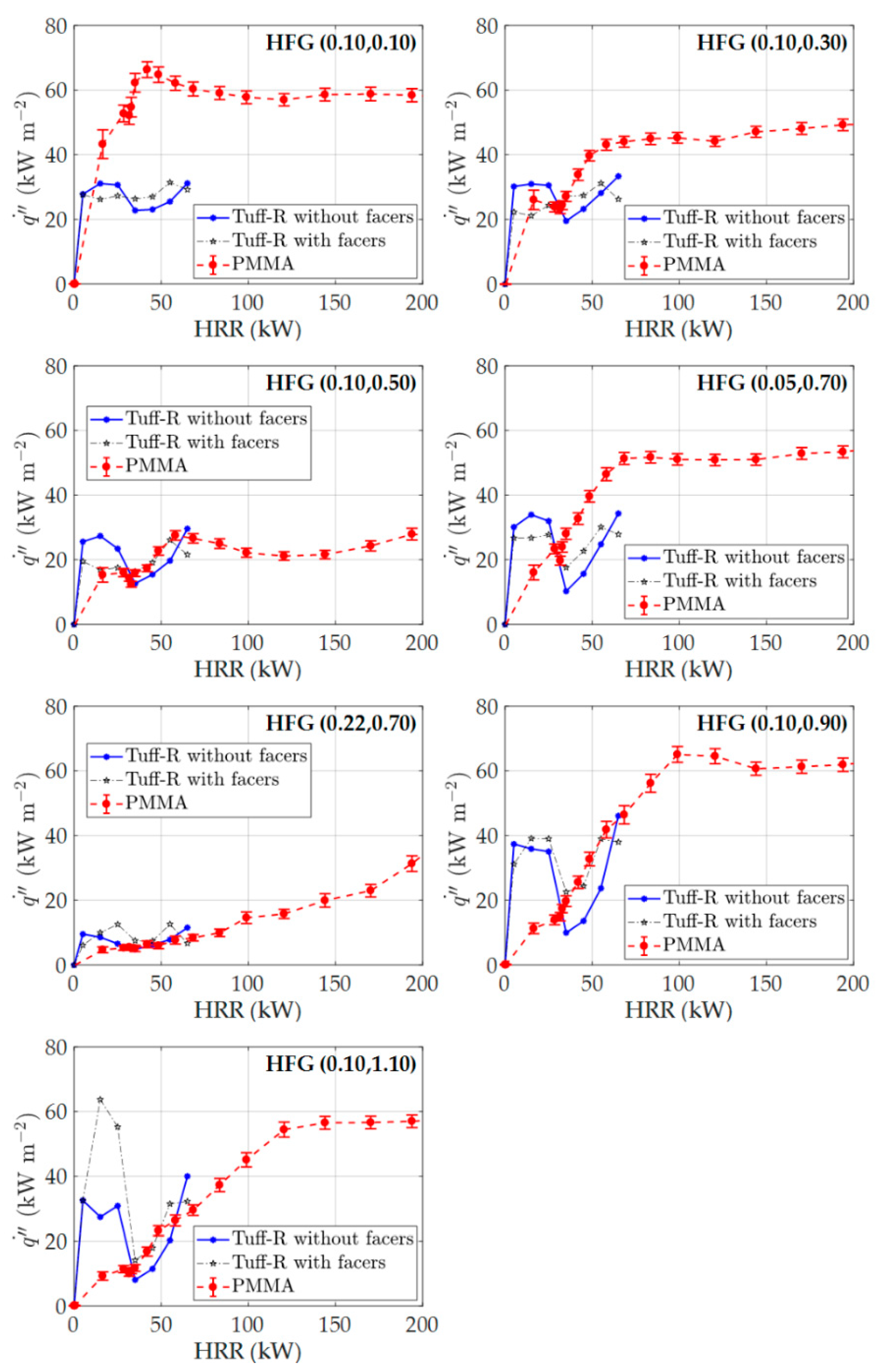
3.5. Full-Scale Experimental Results and Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Biswas, K.; Shrestha, S.S.; Bhandari, M.S.; Desjarlais, A.O. Insulation materials for commercial buildings in North America: An assessment of lifetime energy and environmental impacts. Energy Build. 2016, 112, 256–269. [Google Scholar] [CrossRef]
- McLaggan, M.S.; Hadden, R.M.; Gillie, M. Flammability assessment of phase change material wall lining and insulation materials with different weight fractions. Energy Build. 2017, 153, 439–447. [Google Scholar] [CrossRef]
- Brown, A.; Bruns, M.; Gollner, M.; Hewson, J.; Maragkos, G.; Marshall, A.; McDermott, R.; Merci, B.; Rogaume, T.; Stoliarov, S.; et al. Proceedings of the first workshop organized by the IAFSS Working Group on Measurement and Computation of Fire Phenomena (MaCFP). Fire Saf. J. 2018, 101, 1–17. [Google Scholar] [CrossRef]
- Rogaume, T. Thermal decomposition and pyrolysis of solid fuels: Objectives, challenges and modelling. Fire Saf. J. 2019, 106, 177–188. [Google Scholar] [CrossRef]
- Dick, C.; Dominguez-Rosado, E.; Eling, B.; Liggat, J.; Lindsay, C.; Martin, S.; Mohammed, M.; Seeley, G.; Snape, C. The flammability of urethane-modified polyisocyanurates and its relationship to thermal degradation chemistry. Polymer 2001, 42, 913–923. [Google Scholar] [CrossRef]
- Dominguez-Rosado, E.; Liggat, J.; Snape, C.; Eling, B.; Pichtel, J. Thermal degradation of urethane modified polyisocyanurate foams based on aliphatic and aromatic polyester polyol. Polym. Degrad. Stab. 2002, 78, 1–5. [Google Scholar] [CrossRef]
- Hidalgo, J.P.; Torero, J.L.; Welch, S. Experimental Characterisation of the Fire Behaviour of Thermal Insulation Materials for a Performance-Based Design Methodology. Fire Technol. 2017, 53, 1201–1232. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Casu, A.; Morone, V.; Costa, C. Reaction kinetics and morphological changes of a rigid polyurethane foam during combustion. Thermochim. Acta 2003, 399, 127–137. [Google Scholar] [CrossRef]
- Marquis, D.M.; Batiot, B.; Guillaume, E.; Rogaume, T. Influence of reaction mechanism accuracy on the chemical reactivity prediction of complex charring material in fire condition. J. Anal. Appl. Pyrolysis 2016, 118, 231–248. [Google Scholar] [CrossRef]
- Günther, M.; Lorenzetti, A.; Schartel, B. Fire Phenomena of Rigid Polyurethane Foams. Polymer 2018, 10, 1166. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A. Flame retardancy of polyisocyanurate–polyurethane foams: Use of different charring agents. Polym. Degrad. Stab. 2002, 78, 341–347. [Google Scholar] [CrossRef]
- Vitkauskienė, I.; Makuška, R.; Stirna, U.; Cabulis, U. Thermal Properties of Polyurethane-Polyisocyanurate Foams Based on Poly(ethylene terephthalate) Waste. Mater. Sci. 2011, 17, 249–253. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Auguścik, M.; Prociak, A.; Ryszkowska, J.; Kirpluks, M. Bio-based polyurethane-polyisocyanurate composites with an intumescent flame retardant. Polym. Degrad. Stab. 2016, 127, 11–19. [Google Scholar] [CrossRef]
- Cao, Z.-J.; Dong, X.; Fu, T.; Deng, S.-B.; Liao, W.; Wang, Y.-Z. Coated vs. naked red phosphorus: A comparative study on their fire retardancy and smoke suppression for rigid polyurethane foams. Polym. Degrad. Stab. 2017, 136, 103–111. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Li, J. Parameterization and Validation of Pyrolysis Models for Polymeric Materials. Fire Technol. 2015, 52, 79–91. [Google Scholar] [CrossRef]
- Ding, Y.; Stoliarov, S.I.; Kraemer, R.H. Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate. Polymer 2018, 10, 1137. [Google Scholar] [CrossRef]
- Ding, Y.; Kwon, K.; Stoliarov, S.I.; Kraemer, R.H. Development of a semi-global reaction mechanism for thermal decomposition of a polymer containing reactive flame retardant. Proc. Combust. Inst. 2019, 37, 4247–4255. [Google Scholar] [CrossRef]
- Ding, Y.; Stoliarov, S.I.; Kraemer, R.H. Pyrolysis model development for a polymeric material containing multiple flame retardants: Relationship between heat release rate and material composition. Combust. Flame 2019, 202, 43–57. [Google Scholar] [CrossRef]
- Ding, Y.; Swann, J.D.; Sun, Q.; Stoliarov, S.I.; Kraemer, R.H. Development of a pyrolysis model for glass fiber reinforced polyamide 66 blended with red phosphorus: Relationship between flammability behavior and material composition. Compos. Part B Eng. 2019, 176, 107263. [Google Scholar] [CrossRef]
- Swann, J.D.; Ding, Y.; Stoliarov, S.I. Characterization of pyrolysis and combustion of rigid poly(vinyl chloride) using two-dimensional modeling. Int. J. Heat Mass Transf. 2019, 132, 347–361. [Google Scholar] [CrossRef]
- Swann, J.D.; Ding, Y.; Stoliarov, S.I. Comparative analysis of pyrolysis and combustion of bisphenol A polycarbonate and poly(ether ether ketone) using two-dimensional modeling: A relation between thermal transport and the physical structure of the intumescent char. Combust. Flame 2020, 212, 469–485. [Google Scholar] [CrossRef]
- Swann, J.D.; Stoliarov, S.I. Determination of pyrolysis and combustion properties of poly(vinylidene fluoride) using comprehensive modeling: Relating heat transfer to the intumescent char’s porous structure. Fire Saf. J. 2020, 120, 103086. [Google Scholar] [CrossRef]
- McKinnon, M.B.; Stoliarov, S.I.; Witkowski, A. Development of a pyrolysis model for corrugated cardboard. Combust. Flame 2013, 160, 2595–2607. [Google Scholar] [CrossRef]
- McKinnon, M.B.; Stoliarov, S.I. Pyrolysis Model Development for a Multilayer Floor Covering. Materials 2015, 8, 6117–6153. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.B.; Ding, Y.; Stoliarov, S.I.; Crowley, S.; Lyon, R.E. Pyrolysis model for a carbon fiber/epoxy structural aerospace composite. J. Fire Sci. 2017, 35, 36–61. [Google Scholar] [CrossRef]
- McKinnon, M.B.; Martin, G.E.; Stoliarov, S.I. Pyrolysis model for multiple compositions of a glass reinforced unsaturated polyester composite. J. Appl. Polym. Sci. 2020, 137, 47697. [Google Scholar] [CrossRef]
- Swann, J.D.; Ding, Y.; McKinnon, M.B.; Stoliarov, S.I. Controlled atmosphere pyrolysis apparatus II (CAPA II): A new tool for analysis of pyrolysis of charring and intumescent polymers. Fire Saf. J. 2017, 91, 130–139. [Google Scholar] [CrossRef]
- Stoliarov, S.; Lyon, R. Thermo-Kinetic Model of Burning for Pyrolyzing Materials. Fire Saf. Sci. 2008, 9, 1141–1152. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Leventon, I.T.; Lyon, R.E. Two-dimensional model of burning for pyrolyzable solids. Fire Mater. 2013, 38, 391–408. [Google Scholar] [CrossRef]
- Fiola, G.J.; Chaudhari, D.M.; Stoliarov, S.I. Comparison of Pyrolysis Properties of Extruded and Cast Poly(methyl methacrylate). Fire Saf. J. 2020, 120, 103083. [Google Scholar] [CrossRef]
- European Standard EN 13823. Reaction to Fire Tests for Building Products-Building Products Excluding Floorings Exposed to the Thermal Attack by A Single Burning Item; National Standards Authority of Ireland: Dublin, Ireland, 2014. [Google Scholar]
- Chaudhari, D.M.; Fiola, G.J.; Stoliarov, S.I. Experimental analysis and modeling of Buoyancy-driven flame spread on cast poly(methyl methacrylate) in corner configuration. Polym. Degrad. Stab. 2020, 183, 109433. [Google Scholar] [CrossRef]
- Safety Data Sheet. TUFF-RTM C 2.00 Inch Commerial Insulation Sheathing; Dow Chemical Company: Midland, MI, USA, 2015. [Google Scholar]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I.; Safronava, N. Principles and Practice of Microscale Combustion Calorimetry; Federal Aviation Administration: Atlantic City, NJ, USA, 2013.
- ASTM Standard E1354. Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Swann, J.D. A Comprehensive Characterization of Pyrolysis and Combustion of Intumescent and Charring Polymers using Two-dimensional modeling: A Relationship between Thermal Transport and the Physical Structure of Intumescent Char. Ph.D. Dissertation, University of Maryland, College Park, MD, USA, 2019. [Google Scholar]
- Lyon, R.; Quintiere, J. Criteria for piloted ignition of combustible solids. Combust. Flame 2007, 151, 551–559. [Google Scholar] [CrossRef]
- Mccoy, C.G.; Tilles, J.L.; Stoliarov, S.I. Empirical Model of flame heat feedback for simulation of cone calorimetry. Fire Saf. J. 2019, 103, 38–48. [Google Scholar] [CrossRef]
- MatWeb- Material Property Data, Thermal Ceramics Kaowool PM Low Temperature Board. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=59a8251a2e6849f0b98ea96e228df036 (accessed on 7 July 2019).
- BNZ materials Inc. Marinite I Refractory Product Data Sheet. Available online: https://www.bnzmaterials.com/wp-con-tent/uploads/2013/03/Mar-I.pdf (accessed on 15 June 2019).
- MatWeb-Material Property Data, E-glass Fiber, Generic. Available online: http://www.matweb.com/search/datasheet.aspx?MatGUID=d9c18047c49147a2a7c0b0bb1743e812 (accessed on 8 May 2019).
- Nist Chemistry Webbook: Nist Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 15 November 2019).
- Axelsson, J.; Andersson, P.; Lönnermark, A.; Van Hees, P.; Wetterlund, I. Uncertainties in Measuring Heat and Smoke Re-Lease Rates in the Room/Corner Test and the SBI; SP Rapport 2001:04; Statens Provningsanstalt: Borås, Sweden, 2001. [Google Scholar]
- ASTM Standard E2058-19. Standard Test Method for Measurement of Synthetic Polymer Material Flammability Using a Fire Propagation Apparatus; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]




| Reaction # | Reaction Equation | Ar (s−1) | Er (J mol−1) | ∆Hr (J kg−1) |
|---|---|---|---|---|
| 1 | PIR → 0.992 PIR_Int_1 + 0.008 Gas_1 | 2.42 × 108 | 8.09 × 104 | 0 |
| 2 | PIR_Int_1 → 0.96 PIR_Int_2 + 0.04 Gas_2 | 2.27 × 107 | 8.96 × 104 | 2.16 × 104 |
| 3 | PIR_Int_2 → 0.80 PIR_Int_3 + 0.20 Gas_3 | 5.63 × 108 | 1.24 × 105 | 2.15 × 105 |
| 4 | PIR_Int_3 → 0.92 PIR_Int_4 + 0.08 Gas_4 | 1.00 × 108 | 1.19 × 105 | −1.60 × 105 |
| 5 | PIR_Int_4 → 0.83 PIR_Int_5 + 0.17 Gas_5 | 2.84 × 107 | 1.24 × 105 | 9.37 × 103 |
| 6 | PIR_Int_5 → 0.71 PIR_Int_6 + 0.29 Gas_6 | 6.85 × 103 | 8.85 × 104 | 1.06 × 105 |
| 7 | PIR_Int_6 → 0.73 PIR_Int_7 + 0.27 Gas_7 | 5.47 × 101 | 6.89 × 104 | 2.06 × 105 |
| Component | Cp (J kg−1 K−1) |
|---|---|
| Glass fiber | 1.24 × T + 442 |
| PIR | 4.86 × T − 357 |
| PIR_Int_1 | 1684 |
| PIR_Int_2 | 1684 |
| PIR_Int_3 | 1684 |
| PIR_Int_4 | 1684 |
| PIR_Int_5 | 1684 |
| PIR_Int_6 | 1684 |
| PIR_Int_7 | 0.41 × T − 883 |
| Component | (J kg−1) |
|---|---|
| Gas_1 | 0 |
| Gas_2 | 1.5 × 107 |
| Gas_3 | 1.5 × 107 |
| Gas_4 | 0.8 × 107 |
| Gas_5 | 2.4 × 107 |
| Gas_6 | 2.0 × 107 |
| Gas_7 | 3.3 × 107 |
| Component | (-) | (m−1) | (kg m−3) | k (W m−1 K−1) |
|---|---|---|---|---|
| Glass fiber | 0.81 | 4160 | 2600 | 0.36 |
| PIR | 0.95 | 3200 | 31.4 | 0.05 |
| PIR_Int_1 | 0.95 | 3200 | 15 | 0.09 |
| PIR_Int_2 | 0.95 | 3200 | 30 | 0.04 |
| PIR_Int_3 | 0.95 | 3200 | 18 | 0.08 |
| PIR_Int_4 | 0.95 | 3200 | 17 | 0.06 |
| PIR_Int_5 | 0.95 | 3200 | 16 | 0.14 |
| PIR_Int_6 | 0.95 | 3200 | 14 | 0.15 |
| PIR_Int_7 | 0.95 | 3200 | 14 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhari, D.M.; Stoliarov, S.I.; Beach, M.W.; Suryadevara, K.A. Polyisocyanurate Foam Pyrolysis and Flame Spread Modeling. Appl. Sci. 2021, 11, 3463. https://doi.org/10.3390/app11083463
Chaudhari DM, Stoliarov SI, Beach MW, Suryadevara KA. Polyisocyanurate Foam Pyrolysis and Flame Spread Modeling. Applied Sciences. 2021; 11(8):3463. https://doi.org/10.3390/app11083463
Chicago/Turabian StyleChaudhari, Dushyant M., Stanislav I. Stoliarov, Mark W. Beach, and Kali A. Suryadevara. 2021. "Polyisocyanurate Foam Pyrolysis and Flame Spread Modeling" Applied Sciences 11, no. 8: 3463. https://doi.org/10.3390/app11083463
APA StyleChaudhari, D. M., Stoliarov, S. I., Beach, M. W., & Suryadevara, K. A. (2021). Polyisocyanurate Foam Pyrolysis and Flame Spread Modeling. Applied Sciences, 11(8), 3463. https://doi.org/10.3390/app11083463






