The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Setup
2.2. Sediment Sampling and Analysis
2.3. Germination Tests
2.4. Plant Sampling and TE Analysis
- (1)
- BCF ext = [TE] plant/[TE] sediment extractable
- (2)
- BCF tot = [TE] plant/[TE] sediment total
2.5. Biogas Production and Energy Value
2.6. Statistical Analysis
3. Results and Discussion
3.1. Main Physico–Chemical Properties of the Sediments from the Three Studied Areas
3.2. Biomass Yield of Sorghum on the TE-Contaminated Site
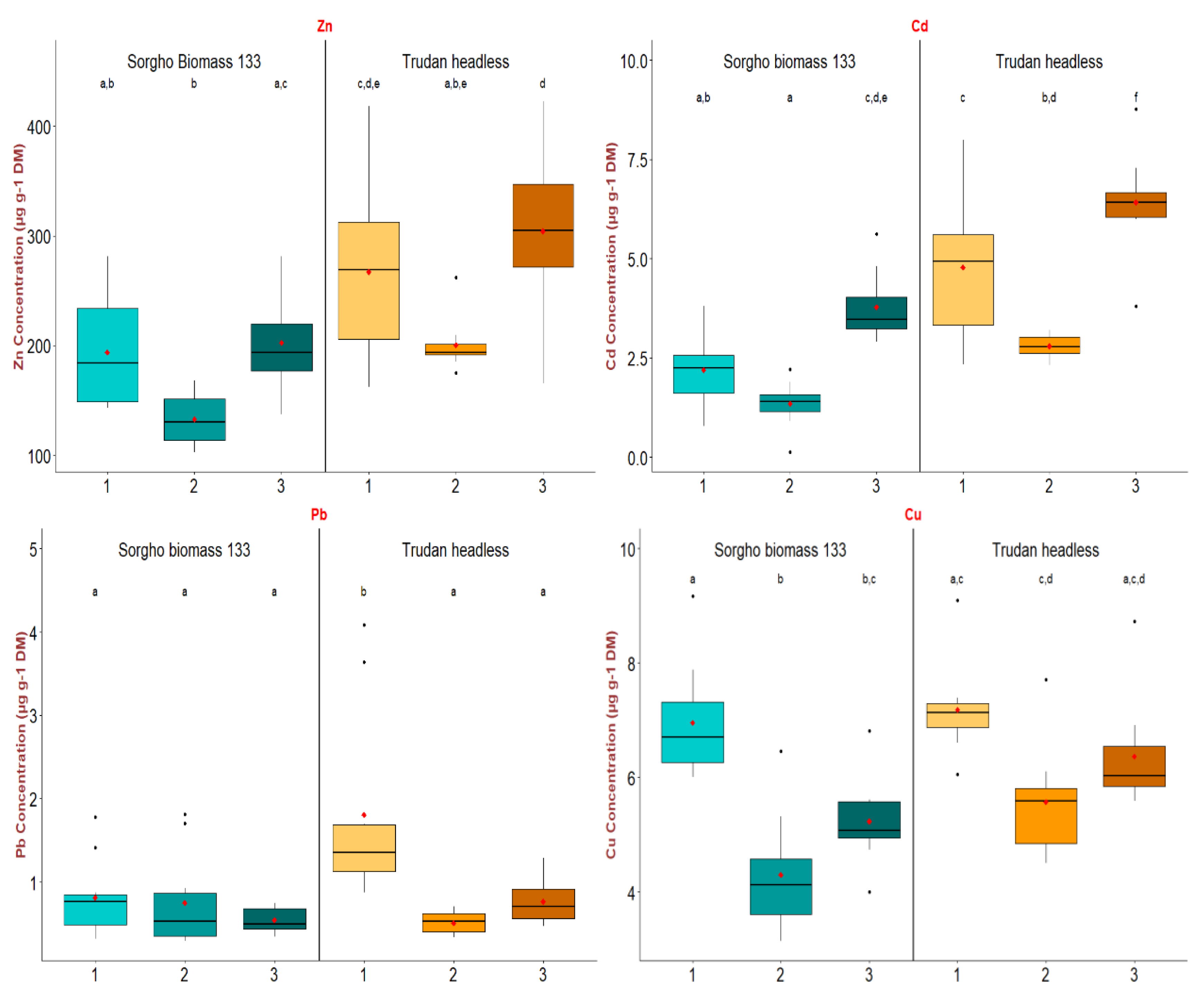
3.3. Sorghum Metal Transfer on the TE-Contaminated Site and Biomass Quality
3.3.1. Sorghum Effect on the TE Extractable Fraction
3.3.2. TE Transfer in the Aerial Biomass of the Sorghum
3.4. Valorization Options for Sorghum
3.4.1. Biogas and Energy Production
3.4.2. Other Biomass Valorization Options
3.5. Sorghum Cultivar Adequation as a Phytoattenuation Option
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the contaminations of cultivated soils eighteen trace elements around smelters in the north of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidin, S.; Fontaine, J.; Muchembled, J.; Lounès-Hadj Sahraoui, A.; Fourrier, H.; et al. Metal accumulation and shoot yield of Miscanthus x giganteus growing in contaminated agricultural soils: Insight into agronomic practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Witters, N.; Van Slycken, S.; Ruttens, A.; Adriaensen, K.; Meers, E.; Meiresonne, L.; Tack, F.M.G.; Thewys, T.; Laes, E.; Vangronsveld, J. Short-Rotation Coppice of Willow for Phytoremediation of a Metal-Contaminated Agricultural Area: A Sustainability Assessment. BioEnergy Res. 2009, 2, 144–152. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications (Review). Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Cundy, A.B.; Bardos, R.P.; Puschenreiter, M.; Menchn, M.; Bert, V.; Friesl-Hanl, W.; Müller, I.; Li, X.N.; Weyens, N.; Witters, N.; et al. Brownfields to green fields: Realising wider benefits from practical contaminant phytomanagement strategies. J. Environ. Manag. 2016, 184, 67–77. [Google Scholar] [CrossRef]
- Kidd, P.; Mench, M.; Álvarez-López, V.; Bert, V.; Dimitriou, I.; Friesl-Hanl, W.; Herzig, R.; Janssen, J.O.; Kolbas, A.; Müller, I.; et al. Agronomic Practices for improving gentle remediation of trace element-contaminated soils. Int. J. Phytoremediation 2015, 17, 1005–1037. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Bert, V.; Dimitriou, I.; Eriksson, J.; Friesl-Hanl, W.; Galazka, R.; Herzig, R.; Janssen, J.; Kidd, P.; Mench, M.; et al. Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Sci. Total Environ. 2014, 496, 510–522. [Google Scholar] [CrossRef]
- Conesa, H.M.; Evangelou, M.W.H.; Robinson, B.H.; Schulin, R. A Critical View of Current State of Phytotechnologies to Remediate Soils: Still a Promising Tool? Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Zhuang, P.; Shu, W.; Li, Z.; Liao, B.; Li, J.; Shao, J. Removal of metals by sorghum plant from contaminated land. J. Environ. Sci. 2009, 21, 1432–1437. [Google Scholar] [CrossRef]
- .Robinson, B.H.; Banuelos, G.; Conesa, H.M.; Evangelou, M.W.; Schulin, R. The phytomanagement of Trace Elements in soil. Crit. Rev. Plant Sci. 2009, 28, 140–166. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I.; Kidd, P.; Epelde, L.; Mench, M. Keep and promote biodiversity at polluted sites under phytomanagement. Environ. Sci. Pollut. Res. 2020, 27, 44820–44834. [Google Scholar] [CrossRef] [PubMed]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a phytomanagement plan by the phytostabilization of mining wastes. Sci. Afr. 2020, 10, 2468–2476. [Google Scholar]
- Cheng, S.F.; Huang, C.Y.; Chen, K.L.; Lin, S.C.; Lin, Y.C. Phytoattenuation of lead-contaminated agricultural land using Miscanthus floridulus—An in situ case study. Desalination Water Treat. 2016, 57, 7773–7779. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Deram, A. Phytomanagement: A Realistic Approach to Soil remediating Phytotechnologies with New Challenges for Plant Science. Int. J. Plant Biol. Res. 2014, 2, 1023. [Google Scholar]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Witters, N.; Thewys, T.; Tack, F.M.G. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef]
- Deyris, P.A.; Bert, V.; Diliberto, S.; Boulanger, C.; Petit, E.; Legrand, Y.M.; Grison, C. Biosourced polymetallic catalysis: A surprising ans efficient means to promote the knoevenagel condensation. Front. Chem. 2018, 6, 6–48. [Google Scholar] [CrossRef] [PubMed]
- Pogrzeba, M.; Krzyżak, J.; Rusinowski, S.; McCalmont, J.P.; Jensen, E. Energy Crop at Heavy Metal-Contaminated Arable Land as an Alternative for Food and Feed Production: Biomass Quantity and Quality. In Plant Metallomics and Functional Omics; Sablok, G., Ed.; Springer: Cham, Swizrland, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Delplanque, M.; Collet, S.; Del Gratta, F.; Schnuriger, B.; Gaucher, R.; Robinson, B.; Bert, V. Combustion of Salix used for phytoextraction: The ate of metals and viability of the process. Biomass Bioenergy 2013, 49, 160–170. [Google Scholar] [CrossRef]
- Bert, V.; Allemon, J.; Sajet, P.; Dieu, S.; Papin, A.; Collet, S.; Gaucher, R.; Chalot, M.; Michiels, B.; Raventos, C. Torrefaction and pyrolysis of metal-enriched poplars from phytotechnologies: Effect of temperature and biomass chlorine content on metal distribution in end-products and valorization options. Biomass Bioenergy 2017, 96, 1–11. [Google Scholar] [CrossRef]
- Grignet, A.; De Vaufleury, A.; Papin, A.; Bert, V. Urban soil phytomanagment for Zn and Cd in situ removal, greening, and Zn-rich biomass production taking care of snail exposure. Environ. Sci. Pollut. Res. 2020, 27, 3187–3201. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Menana, Z.; Chrusciel, L.; Chalot, M.; Bert, V.; Brosse, N. Steam explosion pretreatment of willow grown on phytomanaged soils for bioethanol production. Ind. Crop Prod. 2019, 140. [Google Scholar] [CrossRef]
- Asad, M.; Menana, Z.; Ziegler-Devin, I.; Bert, V.; Chalot, M.; Herzig, R.; Mench, M.; Brosse, N. Pretreatment of trace element-enriched biomasses grown on phytomanaged soils for bioethanol production. Ind. Crop Prod. 2017, 107, 63–72. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Craker, L.E.; Xing, B.; Nielsen, N.E.; Wilcox, A. Aromatic plant production on metal contaminated soils. Sci. Total Environ. 2008, 395, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Phanthavongsa, P.; Chalot, M.; Papin, A.; Lacercat-Didier, L.; Roy, S.; Blaudez, D.; Bert, V. Effect of mycorrhizal inoculation on metal accumulation by polar leaves at phytomanaged sites. Environ. Exp. Bot. 2017, 143, 72–81. [Google Scholar] [CrossRef]
- Winchell, F.; Brass, M.; Manzo, A.; Belados, A.; Perna, P.; Murphy, C.; Stevens, C.; Fuller, D.Q. On the origins and dissemination of domesticated sorghum and pearl millet across Africa and into India: A View from the Butana group of the far Eastern Sahel. Afr. Archaeol. Rev. 2018, 35, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Steduto, P.; Katerji, N.; Puertos-Molina, H.; Ünlü, M.; Mastrorilli, M.; Rana, G. Water-use efficiency of sweet sorghum under water stress conditions gas-exchange investigations at leaf and canopy scales. Field Crop Res. 1997, 54, 221–234. [Google Scholar] [CrossRef]
- Dahlberg, J.; Berenji, L.; Sikora, V.; Latkovic, D. Assessing sorghum [Sorghum bicolor (L) Moench] germplasm for new traits: Food, fuels & unique uses. Maydica 2011, 56, 165–172. [Google Scholar]
- Liu, Z.Q.; Li, H.L.; Zeng, X.J.; Lu, C.; Fu, J.Y.; Guo, L.J.; Kimani, W.M.; Yan, H.L.; He, Z.Y.; Hao, H.Q.; et al. Coupling phytoremediation of cadmium-contaminated soil with safe crop production based on a sorghum farming system. J. Clean. Prod. 2020, 275. [Google Scholar] [CrossRef]
- Marchiol, L.; Fellet, G.; Perosa, D.; Zerbi, G. Removal of trace metals by Sorghum bicolor and Helianthus annuus in a site polluted by industrial wastes: A field experience. Plant Physiol. Biochem. 2007, 45, 379–387. [Google Scholar] [CrossRef]
- Angelova, V.R.; Ivanova, R.V.; Delibaltova, V.A.; Ivanov, K.I. Use of sorghum crops for in situ Phytoremediation of polluted soils. J. Agric. Sci. Technol. 2011, 1, 691–702. [Google Scholar]
- Xiao, M.Z.; Sun, R.; Du, Z.Y.; Yang, W.B.; Sun, Z.; Yuan, T.Q. A sustainable agricultural strategy integrating Cd-contmainated soils remediation and bioethanol production using sorghum cultivars. Ind. Crop Prod. 2021, 162, 113299. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; He, L.Y.; Sheng, X.F. Increased growth and root Cu accumulation of Sorghum sudanense by endophytic Enterobacter sp. K3-2: Implications for Sorghum sudanense biomass production and phytostabilization. Ecotoxicol. Environ. Saf. 2016, 124, 163–168. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Yao, S.; Liu, C.; Yin, Y.; Li, H.; Li, N. A real filed phytoremediation of multi-metals contaminated soils by selected hybrid sweet sorghum with high biomass and high accumulation ability. Chemosphere 2019, 237, 124536. [Google Scholar] [CrossRef]
- Infoclimat.fr. Available online: https://www.infoclimat.fr/climatologie/annee/2019/lille-lesquin/valeurs/07015.html (accessed on 30 June 2020).
- Phanthavongsa, P. Etude de Deux Modalités de Phytomanagement Testées sur un Terrain de Gestion de Sédiments Contaminés par des Métaux et Métalloïdes. Ph.D. Thesis, Ecole Doctorale n°554 Environnements—Santé, Université de Bourgogne Franche-Comté, Besançon, France, April 2018. [Google Scholar]
- Semencesdeprovence.com. Technical Cultural fiche of Sorghum Biomass. Available online: https://www.semencesdeprovence.com/sorgho-ensilage-fourrager-biomasse/catalogue/biomass-133 (accessed on 24 June 2020).
- Semencesdeprovence.com. Technical Cultural fiche of Sorghum Trudan Headless. Available online: https://www.semencesdeprovence.com/sorgho-ensilage-fourrager-biomasse/catalogue/trudan-headless (accessed on 24 June 2020).
- ISO 19730:2008 (E.). Soil quality—Extraction of trace elements from soil using ammonium nitrate solution.
- Komárek, M.; Chrastný, V.; Štíchová, J. Metal/metalloid contamination and isotopic composition of lead in edible mushrooms and forest soils originating from a smelting area. Environ. Int. 2007, 33, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Laboratoire Agronomique de Normandie. Available online: http://www.lano.asso.fr/web/matiere_organique.html. (accessed on 1 February 2021). (In Franch).
- AIDA INERIS. Arrêté du 30/06/20 Modifiant L’arrêté du 9 Août 2006 Relatif aux Niveaux à Prendre en Compte Lors D’une Analyse de Rejets dans les Eaux de Surface ou de Sédiments Marins, Estuariens ou Extraits de Cours D’eau ou Canaux Relevant Respectivement des Rubriques 2.2.3.0, 3.2.1.0 et 4.1.3.0 de la Nomenclature Annexée à L’article R. 214-1 du Code de L’environnement. Available online: https://aida.ineris.fr/consultation_document/43365 (accessed on 1 February 2021).
- Toler, H.D.; Morton, J.B.; Cumming, J.R. Growth and metal accumulation of mycorrhizal sorghum exposed to elevated copper and zinc. Water Air Soil Pollut. 2005, 164, 155–172. [Google Scholar] [CrossRef]
- Arvalis Info.fr Institut du Vegetal. Available online: https://www.arvalis-infos.fr/positionner-les-applications-selon-le-type-d-adventices-@/view-15894-arvarticle.html (accessed on 9 October 2020).
- Cabelguenne, M.; Marty, J.R.; Hilaire, A. Comparaison technico-économique de la valorisation de l’irrigation par quatre culture d’été (maïs, soja, sorgho, tournesol). Agron. EDP Sci. 1982, 2, 567–576. (In French) [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH change in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group: Oxford, UK, 2011. [Google Scholar]
- Soudek, P.; Rodriguez Valseca, I.M.; Petrová, Š.; Song, J.; Vaněk, T. Characteristics of different types of biochar and effects on the toxicity of heavy metal to germinating sorghum seeds. J. Geochem. Explor. 2017, 182, 157–165. [Google Scholar] [CrossRef]
- Qiao, Y.; Crowley, D.; Wang, K.; Zhang, H.; Li, H. Effect of biochar and Arbuscular mycorrhizae on bioavailability of potentially toxic elements in an aged contaminated soil. Environ. Pollut. 2015, 206, 636–643. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation Review. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- Leung, H.M.; Wang, Z.W.; Ye, Z.H.; Yung, K.L.; Peng, X.L.; Cheung, K.C. Interactions between arbuscular mycorrhizae and plants in phytoremediation of metal-contaminated soils: A review. Pedosphere 2013, 23, 549–563. [Google Scholar] [CrossRef]
- Epelde, L.; Mijangos, I.; Becerril, J.M.; Garbisu, C. Soil microbial community as bioindicator of the recovery of soil functioning derived from metal phytoextraction with sorghm. Soil Biol. Biochem. 2009, 41, 1788–1794. [Google Scholar] [CrossRef]
- Barbanti, L.; Di Girolamo, G.; Grigatti, M.; Bertin, L.; Ciavatta, C. Anaerobic digestion of annual and multi-annual biomass crops. Ind. Crop Prod. 2014, 56, 137–144. [Google Scholar] [CrossRef]
- Frigon, J.C.; Guiot, S.R. Biomethane production from starch and lignocellulosic crops: A comparative review. Biofuels Bioprod. Biorefining 2010, 4, 447–458. [Google Scholar] [CrossRef]
- Bruni, E.; Jensen, A.P.; Pedersen, E.S.; Angelidaki, I. Anaerobic digestion of maize focusing on variety, harvest time and pretreatment. Appl. Energy 2010, 87, 2212–2217. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003.
- Alburquerque, J.A.; Gonzálvez, J.; Tortosa, G.; Baddi, G.A.; Cegarra, J. Evaluation of “alperujo” composting based on organic matter degradation, humifcation and compost quality. Biodegradation 2009, 20, 257–270. [Google Scholar] [CrossRef]
- Clemens, S. How metal hyperaccumulating plants can advance Zn biofortification. Plant Soil 2017, 411, 111–120. [Google Scholar] [CrossRef]
- Anderson, C.W.N.; Robinson, B.H.; West, D.M.; Clucas, L.; Portmann, D. Zinc-enriched and zinc-biofortified feed as a possible animal remedy in pastoral agriculture: Animal health and environmental benefits. J. Geochem. Explor. 2012, 121, 30–35. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Lucchini, P.; Dickinson, N.M.; Mosca, G. Long-term phytomanagement of metal-contaminated land with field crops: Integrated remediation and biofortification. Eur. J. Agron. 2014, 53, 56–66. [Google Scholar] [CrossRef]
- Wang, X.; Fernandes de Souza, M.; Li, H.; Tack, F.M.G.; Sik Ok, Y.; Meers, E. Zn phytoextraction and recycling of alfalfa biomass as potential Zn-biofortified feed crop. Sci. Total Environ. 2021, 760. [Google Scholar] [CrossRef] [PubMed]
- Vintila, T.; Negrea, A.; Barbu, H.; Sumalan, R.; Kovacs, K. Metal distribution in the process of lignocellulosic ethanol production from heavy metal contaminated sorghum biomass. J. Chem. Technol. Biotechnol. 2016, 91, 1607–1614. [Google Scholar] [CrossRef]
- Almeida, L.G.F.d.; Parella, R.A.d.C.; Simeone, M.L.F.; Ribeiro, P.C.d.O.; Dos Santos, A.S.; Da Costa, A.S.V.; Guimaraes, A.G.; Schaffert, R.E. Composition and growth of sorghum biomass genotypes for ethanol production. Biomass Bionergy 2019, 122, 343–348. [Google Scholar] [CrossRef]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed—Council statement. Official Journal L 140, 30/05/2002 P. 0010-0022.
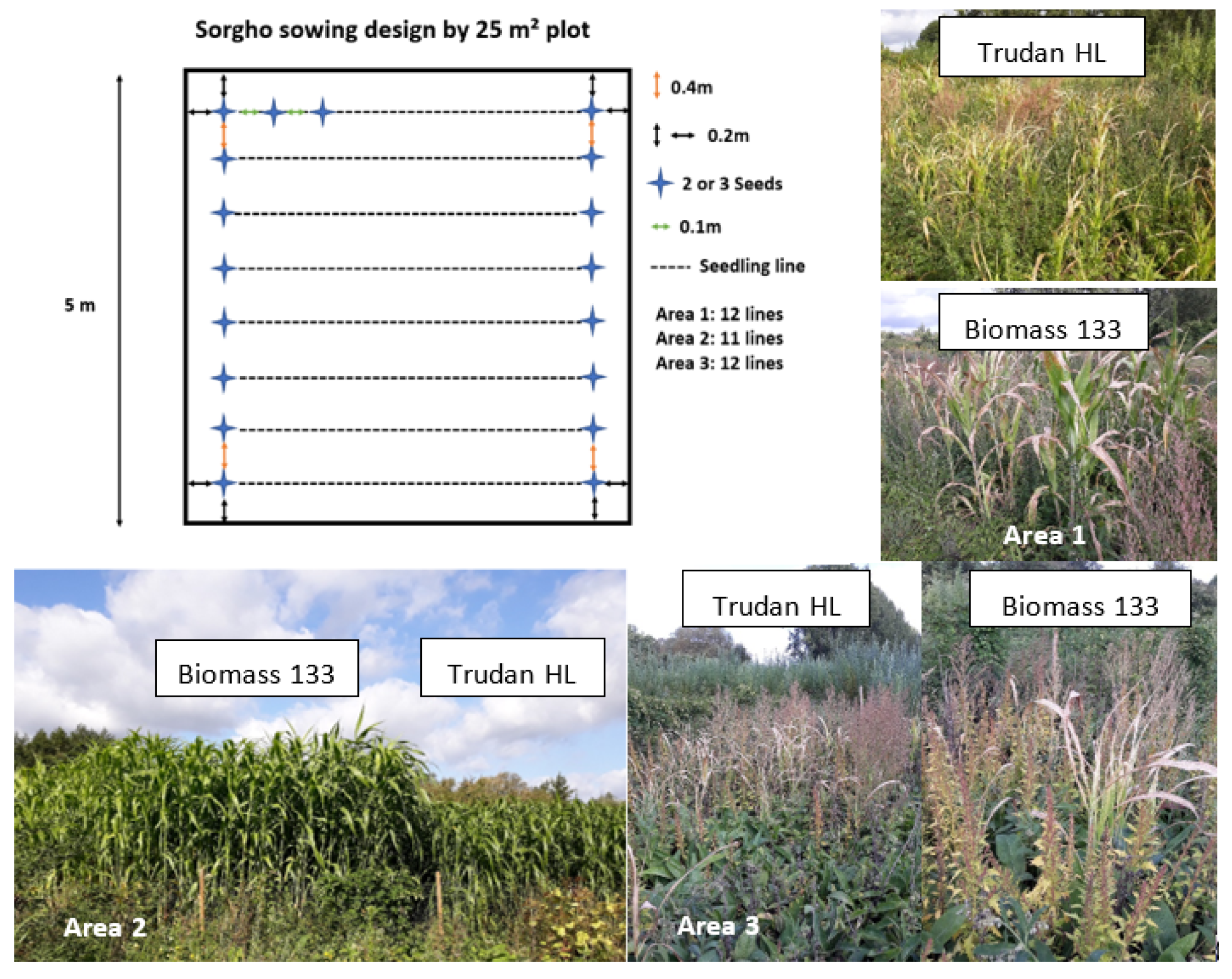

| Parameters | Area 1 | Area 2 | Area 3 | S1 | |
|---|---|---|---|---|---|
| Clay (%) | 7.7 | 10.7 | 11.7 | - | |
| Silt (%) | 69 | 70.5 | 78.6 | - | |
| Sand (%) | 23.3 | 18.8 | 9.8 | - | |
| Soil texture (USDA texture triangle) | Fine silt | Fine silt | Fine silt | - | |
| Carbon (g kg−1) | 76.3 | 82.4 | 96.8 | - | |
| Organic matter (g kg−1) | 131.3 | 141.7 | 166.5 | - | |
| C/N* | 28.4 | 28 | 31.5 | - | |
| CEC+ (meq 100 g−1) | 17.6 | 17.9 | 21.2 | - | |
| CaO++ g kg−1 | 11.7 | 11.5 | 12.5 | - | |
| N kjeldahl TNK+++ (g kg−1) | 2.69 | 2.94 | 3.07 | - | |
| Phosphorus Olsen (g kg−1) P2O5 | 0.216 | 0.259 | 0.258 | - | |
| pH-H2O | 8.08 | 8.06 | 8.12 | - | |
| Total TE (mg kg−1) | Zn | 6685 ± 509 (0.060% ± 0.004) | 6084 ± 132 (0.090% ± 0.008) | 8980 ± 340 (0.070% ± 0.012) | 300 |
| Pb | 774 ± 18 (0.007% ± 0.003) | 592 ± 12 (0.009% ± 0.003) | 1043 ± 12 (0.007% ± 0.006) | 100 | |
| Cd | 6.3 ± 0.1 (0.47% ± 0.07) | 5.0 ± 0.1 (0.62% ± 0.08) | 9.0 ± 0.1 (0.48% ± 0.12) | 2 | |
| Cu | 87.0 ± 2.3 (0.53% ± 0.07) | 76.0 ± 1.5 (0.59% ± 0.09) | 101.0 ± 1.3 (0.52% ± 0.09) | 100 | |
| As | 33.0 ± 1.0 (0.043% ± 0.004) | 29.0 ± 0.1 (0.050% ± 0.008) | 43.0 ± 1.2 (0.030% ± 0.007) | 30 | |
| Cr | 116 ± 12 (0.020% ± 0.002) | 114 ± 3 (0.040% ± 0.007) | 100 ± 4 (0.030% ± 0.004) | 150 | |
| Ni | 52 ± 1 (0.19% ± 0.05) | 51 ± 1 (0.21% ± 0.03) | 53 ± 1 (0.20% ± 0.04) | 50 | |
| Area | Average Dry Weight Per Plant (g) | |
|---|---|---|
| Trudan Headless | 1 | 16 ± 9 a |
| 2 | 43 ± 16 a | |
| 3 | 16 ± 8 a | |
| Biomass 133 | 1 | 125 ± 88 b |
| 2 | 247 ± 98 c | |
| 3 | 16 ± 15 a |
| Area 2 | Yield (kg/25m2) FM | Yield (t ha−1) FM | Yield (t ha−1) DM | Duration of Growth (Day) | Height (m) |
|---|---|---|---|---|---|
| Sorghum Biomass 133 | 137.43 | 54.97 | 21.75 | 131 | 3.39 (±0.21) |
| Reference Biomass 133 [40] | - | - | 20.7 | 105–125 | 3.5 |
| Sorghum Trudan HL | 80.97 | 32.38 | 9.85 | 105 | - |
| Reference Trudan HL [41] | - | - | 10–15 | 60 first cut and 30 s cut (90) | - |
| Sorghum Biomass 133 | Sorghum Trudan HL | |
|---|---|---|
| Biogas production (Nm3 t−1 FM) | 205 ± 2 | 325 ± 11 |
| Biogas production (Nm3 t−1 ODM) | 329 ± 20 | 398 ± 7 |
| Average CH4 level % | 53 | 56 |
| Average H2S level ppm | 22 | 18 |
| Electricity production (kWhe t−1 FM, 41% efficiency) | 439 ± 2 | 737 ± 16 |
| Heat production (kWht t−1 FM, 44% efficiency) | 469 ± 2 | 787 ± 18 |
| Potential CH4 production (Nm3 CH4 ha−1) | 5924 | 5862 |
| Area 2 | Cd Removal (kg ha−1 y−1) | Cu Removal (kg ha−1 y−1) | Pb Removal (kg ha−1 y−1) | Zn Removal (kg ha−1 y−1) |
|---|---|---|---|---|
| Sorghum Biomass 133 | 0.0292 | 0.0935 | 0.0163 | 2.888 |
| Sorghum Trudan HL | 0.0275 | 0.0549 | 0.0050 | 1.973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlein, A.; Bert, V.; Desannaux, O.; Fernandes de Souza, M.; Papin, A.; Gaucher, R.; Zdanevitch, I.; Meers, E. The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site. Appl. Sci. 2021, 11, 3471. https://doi.org/10.3390/app11083471
Perlein A, Bert V, Desannaux O, Fernandes de Souza M, Papin A, Gaucher R, Zdanevitch I, Meers E. The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site. Applied Sciences. 2021; 11(8):3471. https://doi.org/10.3390/app11083471
Chicago/Turabian StylePerlein, Alexandre, Valérie Bert, Océane Desannaux, Marcella Fernandes de Souza, Arnaud Papin, Rodolphe Gaucher, Isabelle Zdanevitch, and Erik Meers. 2021. "The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site" Applied Sciences 11, no. 8: 3471. https://doi.org/10.3390/app11083471
APA StylePerlein, A., Bert, V., Desannaux, O., Fernandes de Souza, M., Papin, A., Gaucher, R., Zdanevitch, I., & Meers, E. (2021). The Use of Sorghum in a Phytoattenuation Strategy: A Field Experiment on a TE-Contaminated Site. Applied Sciences, 11(8), 3471. https://doi.org/10.3390/app11083471







