4-Hexylresorcinol Inhibits Class I Histone Deacetylases in Human Umbilical Cord Endothelial Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. HUVEC Culture
2.2. Western Blot and HDAC Inhibitory Assay
3. Results
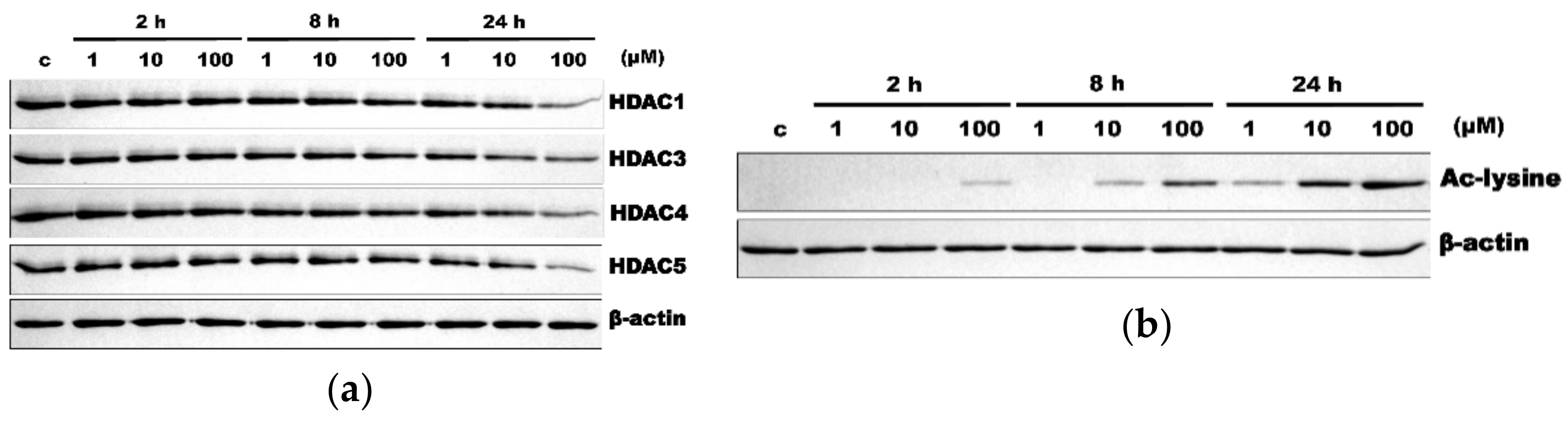
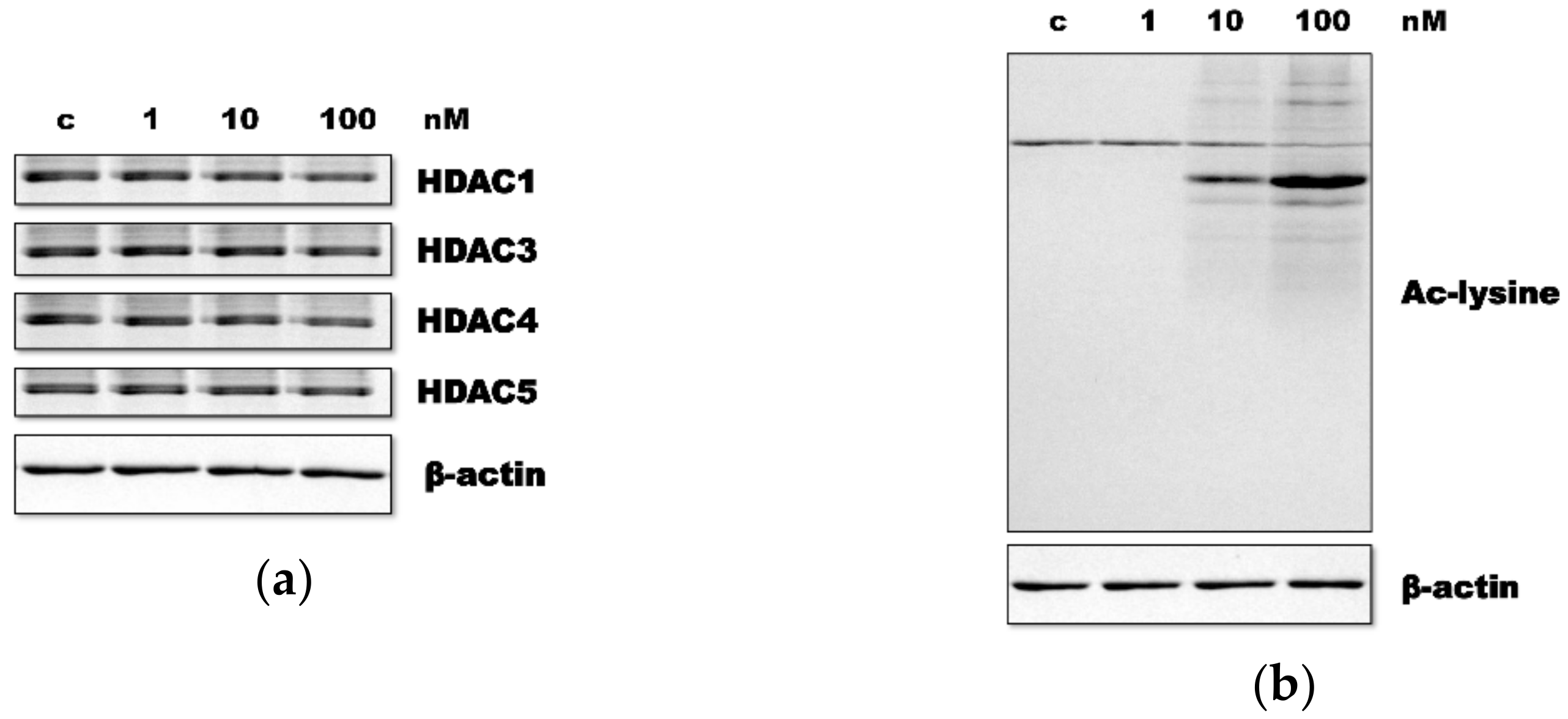
3.1. HR Decreased HDAC Expression and Increased Ac-Lys
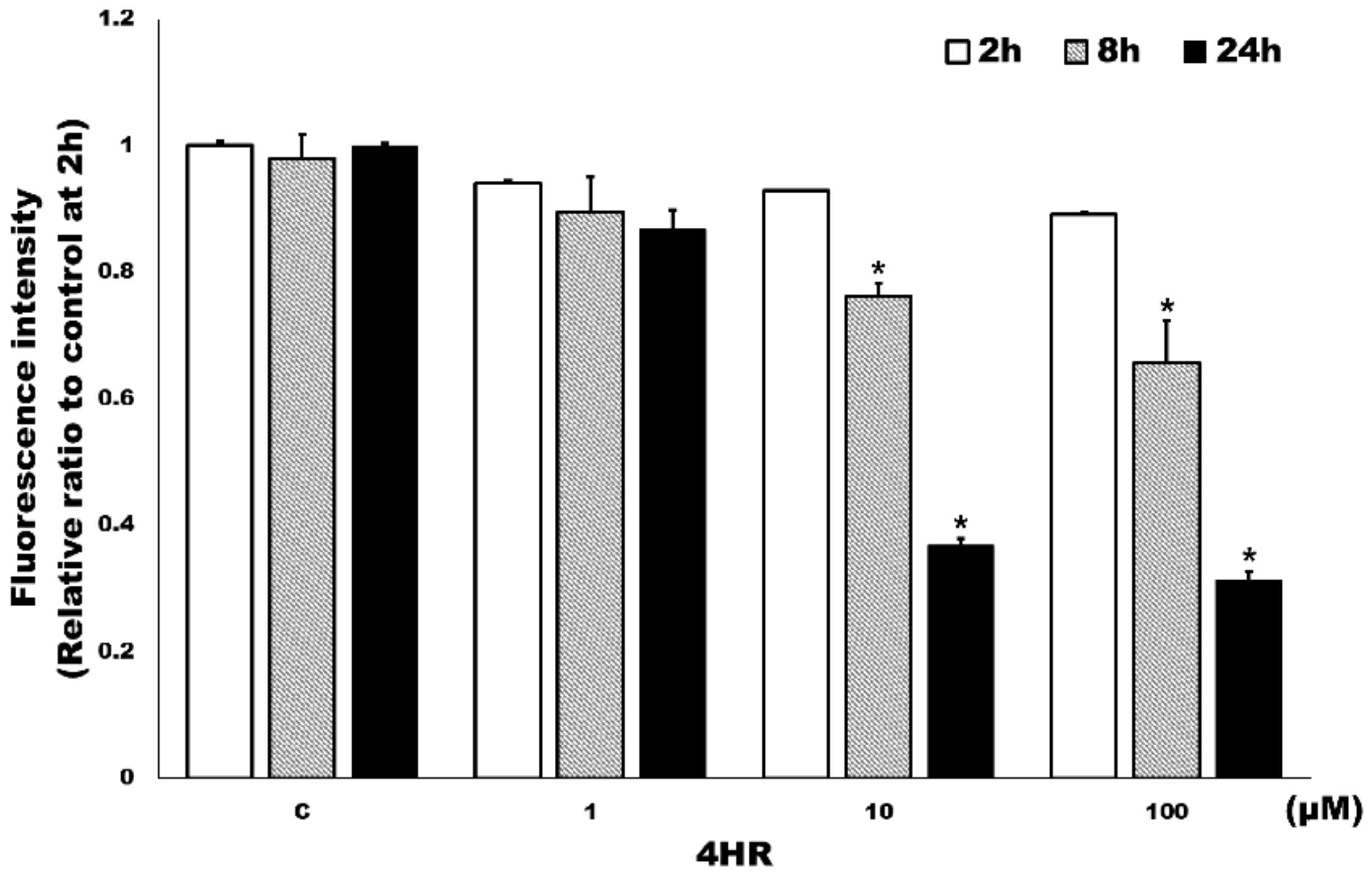
3.2. HR Inhibited Class I HDAC Activity in HUVECs
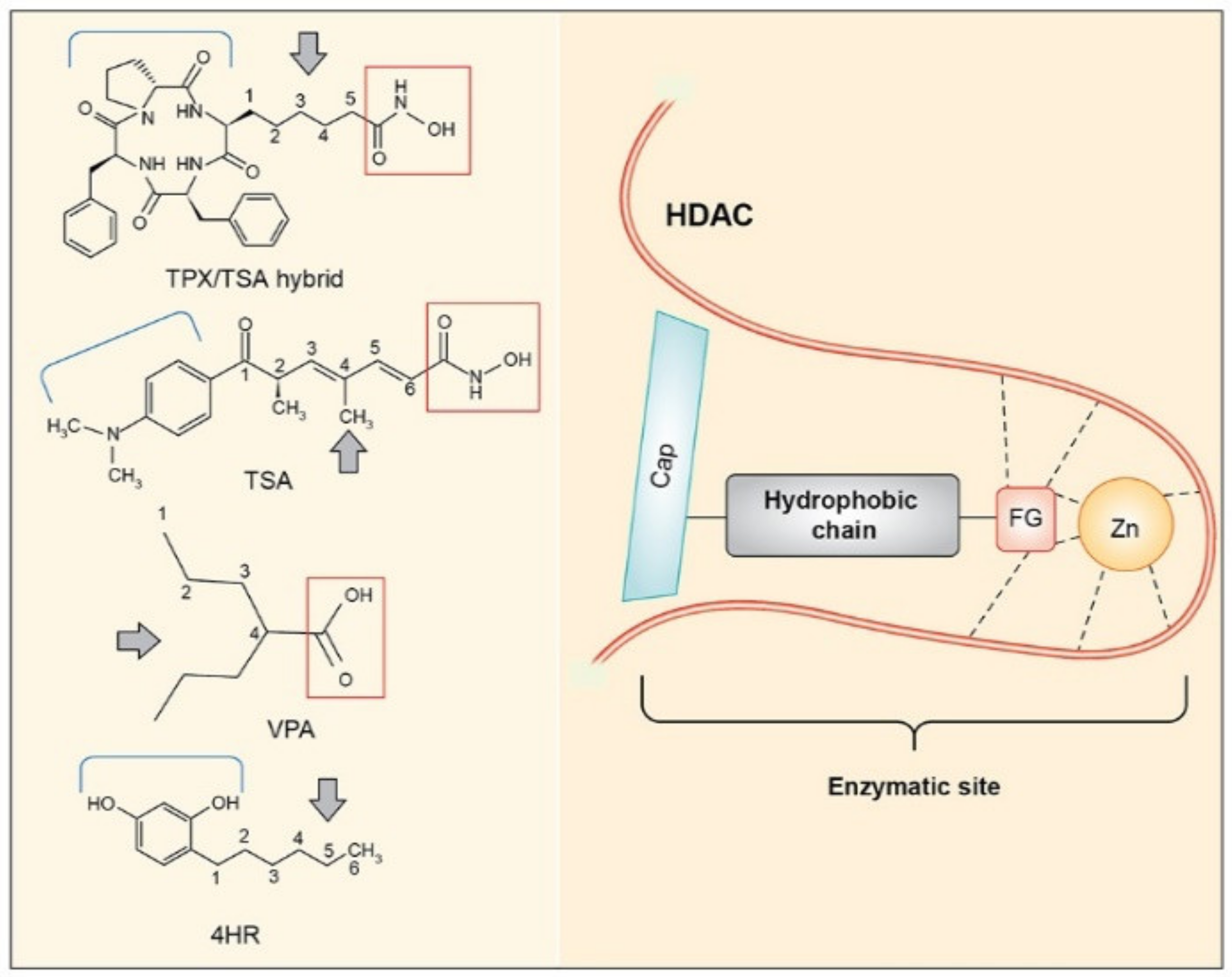
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The Many Roles of Histone Deacetylases in Development and Physiology: Implications for Disease and Therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Aka, J.A.; Kim, G.W.; Yang, X.J. Histone Deactylases: The Biology and Clinical Implication; Springer: New York, NY, USA, 2011; pp. 1–12. ISBN 978-3-642-21630-5. [Google Scholar]
- Reichert, N.; Choukrallah, M.A.; Matthias, P. Multiple Roles of Class I HDACs in Proliferation, Differentiation, and Development. Cell Mol. Life Sci. 2012, 69, 2173–2187. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, R.; Richard, C.S.; Santos, M.M. The Role of Butyrate in Surgical and Oncological Outcomes in Colorectal Cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2021. [Google Scholar] [CrossRef]
- Bartl, S.; Taplick, J.; Lagger, G.; Khier, H.; Kuchler, K.; Seiser, C. Identification of Mouse Histone Deacetylase 1 as a Growth Factor-Inducible Gene. Mol. Cell Biol. 1997, 17, 5033–5043. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, P.; Seidler, B.; Schuler, S.; Schnieke, A.; Gottlicher, M.; Schmid, R.M.; Saur, D.; Schneider, G. HDAC2 Mediates Therapeutic Resistance of Pancreatic Cancer Cells via the BH3-Only Protein NOXA. Gut 2009, 58, 1399–1409. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, C.M.; Luo, Y.; Yin, Z.; Zhang, M.; Zhu, W.; Wang, T.; Floss, T.; Goettlicher, M.; Noppinger, P.R.; Wurst, W.; et al. Hdac2 Regulates the Cardiac Hypertrophic Response by Modulating Gsk3 Beta Activity. Nat. Med. 2007, 13, 324–331. [Google Scholar] [CrossRef]
- Kook, H.; Lepore, J.J.; Gitler, A.D.; Lu, M.M.; Wing-Man, Y.W.; Mackay, J.; Zhou, R.; Ferrari, V.; Gruber, P.; Epstein, J.A. Cardiac Hypertrophy and Histone Deacetylase-Dependent Transcriptional Repression Mediated by the Atypical Homeodomain Protein Hop. J. Clin. Investig. 2003, 112, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Kee, H.J.; Sohn, I.S.; Nam, K.I.; Park, J.E.; Qian, Y.R.; Yin, Z.; Ahn, Y.; Jeong, M.H.; Bang, Y.J.; Kim, N.; et al. Inhibition of Histone Deacetylation Blocks Cardiac Hypertrophy Induced by Angiotensin II Infusion and Aortic Banding. Circulation 2006, 113, 51–59. [Google Scholar] [CrossRef]
- Iezzi, S.; Padova, M.D.; Serra, C.; Caretti, G.; Simone, C.; Maklan, E.; Minetti, G.; Zhao, P.; Hoffman, E.P.; Puri, P.L.; et al. Deacetylase Inhibitors Increase Muscle Cell Size by Promoting Myoblast Recruitment and Fusion through Induction of Follistatin. Dev. Cell 2004, 6, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.S.; Yang, C.P.; Bowen, R.C.; Bai, O.; Li, X.M.; Jiang, W.; Zhang, X. Valproic Acid Enhances Axonal Regeneration and Recovery of Motor Function after Sciatic Nerve Axotomy in Adult Rats. Brain Res. 2003, 975, 229–236. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Krausz, S.; de Jager, W.; Burakowski, T.; Groot, D.; Sanders, M.E.; Prakken, B.J.; Maslinski, W.; Eldering, E.; Tak, P.P.; et al. Histone Deacetylase Inhibitors Suppress Inflammatory Activation of Rheumatoid Arthritis Patient Synovial Macrophages and Tissue. J. Immunol. 2010, 184, 2718–2728. [Google Scholar] [CrossRef] [Green Version]
- De Caro, C.; Di Cesare Mannelli, L.; Branca, J.J.V.; Micheli, L.; Citraro, R.; Russo, E.; De Sarro, G.; Ghelardini, C.; Calignano, A.; Russo, R. Pain Modulation in WAG/Rij Epileptic Rats (A Genetic Model of Absence Epilepsy): Effects of Biological and Pharmacological Histone Deacetylase Inhibitors. Front. Pharmacol. 2020, 3, 11. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic Lipids, the Natural Non-Isoprenoid Phenolic Amphiphiles and Their Biological Activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Kushneruk, M.A.; Tugarova, A.V.; Il’chukova, A.V.; Slavkina, E.A.; Starichkova, N.I.; Bogatyrev, V.A.; Antoniuk, L.P. Factors Inducing Transition from Growth to Dormancy in Rhizobacteria Azospirillum Brasilense. Mikrobiologiia 2013, 82, 563–570. [Google Scholar] [CrossRef]
- Arias, E.; González, J.; Peiró, J.M.; Oria, R.; Lopez-Buesa, P. Browning Prevention by Ascorbic Acid and 4-Hexylresorcinol: Different Mechanisms of Action on Polyphenol Oxidase in the Presence and in the Absence of Substrates. J. Food Sci. 2007, 72, C464–G470. [Google Scholar] [CrossRef]
- Chhabra, R.S.; Huff, J.E.; Haseman, J.; Hall, A.; Baskin, G.; Cowan, M. Inhibition of Some Spontaneous Tumors by 4-Hexylresorcinol in F344/N Rats and B6C3F1 Mice. Fundam. Appl. Toxicol. 1988, 11, 685–690. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 4-Hexylresorcinol (CAS No. 136-77-6) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 1988, 330, 1–166. [Google Scholar]
- Kim, S.G.; Lee, S.W.; Park, Y.W.; Jeong, J.H.; Choi, J.Y. 4-Hexylresorcinol Inhibits NF-κB Phosphorylation and has a Synergistic Effect with Cisplatin in KB Cells. Oncol. Rep. 2011, 26, 1527–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.G.; Kim, A.S.; Jeong, J.H.; Choi, J.Y.; Kweon, H. 4-Hexylresorcinol Stimulates the Differentiation of SCC-9 Cells through the Suppression of E2F2, E2F3 and Sp3 Expression and the Promotion of Sp1 Expression. Oncol. Rep. 2012, 28, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Jeong, J.H.; Park, Y.W.; Song, J.Y.; Kim, A.S.; Choi, J.Y.; Chae, W.S. 4-Hexylresorcinol Inhibits Transglutaminase-2 Activity and has Synergistic Effects Along with Cisplatin in KB Cells. Oncol. Rep. 2011, 25, 1597–1602. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Kim, S.G.; Choi, J.Y. Inhibition of Foreign Body Giant Cell Formation by 4-Hexylresorcinol through Suppression of Diacylglycerol Kinase Delta Gene Expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, S.G.; Kim, M.K.; Kim, D.W.; Lee, J.H.; Seok, H.; Choi, J.Y. Topical Delivery of 4-Hexylresorcinol Promotes Wound Healing via Tumor Necrosis Factor-α Suppression. Burns 2016, 42, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G. Immunomodulation for Maxillofacial Reconstructive Surgery. Maxillofac. Plast. Reconstr. Surg 2020, 42, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Yoon, C.S.; Kim, S.G.; Park, Y.W.; Lee, S.K. Effects of 4-Hexylresorcinol on Protein Expressions in RAW 264.7 Cells as Determined by Immunoprecipitation High Performance Liquid Chromatography. Sci. Rep. 2019, 9, 3379. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jo, Y.Y.; Garagiola, U.; Choi, J.Y.; Kang, Y.J.; Oh, J.H.; Kim, S.G. Increased Level of Vascular Endothelial Growth Factors by 4-Hexylresorcinol is Mediated by Transforming Growth Factor-β1 and Accelerates Capillary Regeneration in the Burns in Diabetic Animals. Int. J. Mol. Sci. 2020, 21, 3473. [Google Scholar] [CrossRef] [PubMed]
- Furumai, R.; Komatsu, Y.; Nishino, N.; Khochbin, S.; Yoshida, M.; Horinouchi, S. Potent Histone Deacetylase Inhibitors Built from Trichostatin A and Cyclic Tetrapeptide Antibiotics Including Trapoxin. Proc. Natl. Acad. Sci. USA 2001, 98, 87–92. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.G.; Lee, S.K. 4-Hexylresorcinol-Induced Angiogenesis Potential in Human Endothelial Cells. Maxillofac. Plast. Reconstr. Surg 2020, 42, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, D.W.; Kim, S.G.; Lee, S.K. 4-Hexylresorcinol-Induced Protein Expression Changes in Human Umbilical Cord Vein Endothelial Cells as Determined by Immunoprecipitation High-Performance Liquid Chromatography. PLoS ONE 2020, 15, e0243975. [Google Scholar] [CrossRef]
- Xu, W.S.; Parmigiani, R.B.; Marks, P.A. Histone Deacetylase Inhibitors: Molecular Mechanisms of Action. Oncogene 2007, 26, 5541–5552. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhao, S.; Ammanamanchi, S.; Brattain, M.; Venkatasubbarao, K.; Freeman, J.W. Trichostatin A Induces Transforming Growth Factor Beta Type II Receptor Promoter Activity and Acetylation of Sp1 by Recruitment of PCAF/p300 to a Sp1.NF-Y Complex. J. Biol. Chem. 2005, 280, 10047–10054. [Google Scholar] [CrossRef] [Green Version]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Gut Commensal Bacteria Activates TGF-Beta1 Expression Through the Transcription Factor SP1 in Human Intestinal Epithelial Cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Kim, M.S.; Kwon, H.J.; Lee, Y.M.; Baek, J.H.; Jang, J.E.; Lee, S.W.; Moon, E.J.; Kim, H.S.; Lee, S.K.; Chung, H.Y.; et al. Histone Deacetylases Induce Angiogenesis by Negative Regulation of Tumor Suppressor Genes. Nat. Med. 2001, 7, 437–443. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kim, D.W.; Choi, J.Y.; Kim, S.G. 4-Hexylresorcinol and Silk Sericin Increase the Expression of Vascular Endothelial Growth Factor via Different Pathways. Sci. Rep. 2019, 9, 3448. [Google Scholar] [CrossRef] [Green Version]
- Lagger, G.; O’Carroll, D.; Rembold, M.; Khier, H.; Tischler, J.; Weitzer, G.; Schuettengruber, B.; Hauser, C.; Brunmeir, R.; Jenuwein, T.; et al. Essential Function of Histone Deacetylase 1 in Proliferation Control and CDK Inhibitor Repression. EMBO J. 2002, 21, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskara, S.; Chyla, B.J.; Amann, J.M.; Knutson, S.K.; Cortez, D.; Sun, Z.W.; Hiebert, S.W. Deletion of Histone Deacetylase 3 Reveals Critical Roles in S Phase Progression and DNA Damage Control. Mol. Cell 2008, 30, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Liu, T.; Gao, X.; Xie, Y.; Liu, M. 4-Hexylresorcinol Inhibits Osteoclastogenesis by Suppressing the NF-κB Signaling Pathway and Reverses Bone Loss in Ovariectomized Mice. Exp. Ther. Med. 2021, 21, 354. [Google Scholar] [CrossRef] [PubMed]

| Class | Members |
|---|---|
| I | HDAC1, 2, 3, 8 |
| IIA | HDAC4, 5, 7, 9 |
| IIB | HDAC6, 10 |
| III | SIRT1, 2, 3, 4, 5, 6, 7 |
| IV | HDAC11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kweon, H.-Y.; Kim, D.-W.; Choi, J.-Y.; Kim, S.-G. 4-Hexylresorcinol Inhibits Class I Histone Deacetylases in Human Umbilical Cord Endothelial Cells. Appl. Sci. 2021, 11, 3486. https://doi.org/10.3390/app11083486
Kim J-Y, Kweon H-Y, Kim D-W, Choi J-Y, Kim S-G. 4-Hexylresorcinol Inhibits Class I Histone Deacetylases in Human Umbilical Cord Endothelial Cells. Applied Sciences. 2021; 11(8):3486. https://doi.org/10.3390/app11083486
Chicago/Turabian StyleKim, Jwa-Young, Hae-Yong Kweon, Dae-Won Kim, Je-Yong Choi, and Seong-Gon Kim. 2021. "4-Hexylresorcinol Inhibits Class I Histone Deacetylases in Human Umbilical Cord Endothelial Cells" Applied Sciences 11, no. 8: 3486. https://doi.org/10.3390/app11083486








