Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poonam Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Kim, H.-J.; Krishna, T.; Zeb, K.; Rajangam, V.; Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Obaidat, I.M. A Comprehensive Review of Li-Ion Battery Materials and Their Recycling Techniques. Electronics 2020, 9, 1161. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Simon, P. MATERIALS SCIENCE: Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Murray, D.B.; Hayes, J.G. Cycle Testing of Supercapacitors for Long-Life Robust Applications. IEEE Trans. Power Electron. 2015, 30, 2505–2516. [Google Scholar] [CrossRef]
- Panda, P.K.; Grigoriev, A.; Mishra, Y.K.; Ahuja, R. Progress in Supercapacitors: Roles of Two Dimensional Nanotubular Materials. Nanoscale Adv. 2020, 2, 70–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2016, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Luryi, S. Quantum capacitance devices. Appl. Phys. Lett. 1988, 52, 501–503. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Supercapacitor performance of activated carbon derived from rotten carrot in aqueous, organic and ionic liquid based electrolytes. J. Saudi Chem. Soc. 2018, 22, 993–1002. [Google Scholar] [CrossRef]
- Piñeiro-Prado, I.; Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Design of Activated Carbon/Activated Carbon Asymmetric Capacitors. Front. Mater. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Pina, A.C.; Amaya, A.; Marcuzzo, J.S.; Rodrigues, A.C.; Baldan, M.R.; Tancredi, N.; Cuña, A. Supercapacitor Electrode Based on Activated Carbon Wool Felt. C J. Carbon Res. 2018, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Jiang, Y.; Lin, H.; Wang, L.; Liu, A.; Cao, Y.; Sheng, R.; Guo, Y.; Fan, C.; Zhang, S.; et al. Boosting the Supercapacitor Performance of Activated Carbon by Constructing Overall Conductive Networks Using Graphene Quantum Dots. J. Mater. Chem. A 2019, 7, 6021–6027. [Google Scholar] [CrossRef]
- Paek, E.; Pak, A.J.; Hwang, G.S. A computational study of the interfacial structure and capacitance of graphene in [BMIM][PF6] ionic liquid. J. Electrochem. Soc. 2013, 160, A1–A10. [Google Scholar] [CrossRef] [Green Version]
- Paek, E.; Pak, A.J.; Kweon, K.E.; Hwang, G.S. On the origin of the enhanced supercapacitor performance of nitrogen-doped graphene. J. Phys. Chem. C 2013, 117, 5610–5616. [Google Scholar] [CrossRef]
- Hirunsit, P.; Liangruksa, M.; Khanchaitit, P. Electronic structures and quantum capacitance of monolayer and multilayer graphenes influenced by Al, B, N and P doping, and monovacancy: Theoretical study. Carbon 2016, 108, 7–20. [Google Scholar] [CrossRef]
- Mousavi-Khoshdel, S.M.; Jahanbakhsh-bonab, P.; Targholi, E. Structural, electronic properties, and quantum capacitance of B, N and P-doped armchair carbon nanotubes. Phys. Lett. A 2016, 380, 3378–3383. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Ma, C.; Wang, M.; Zhou, J. Interaction and quantum capacitance of nitrogen/sulfur co-doped graphene: A theoretical calculation. J. Phys. Chem. C 2017, 121, 18344–18350. [Google Scholar] [CrossRef]
- Sun, P.; Wang, R.; Wang, Q.; Wang, H.; Wang, X. Uniform MoS2 Nanolayer with Sulfur Vacancy on Carbon Nanotube Networks as Binder-free Electrodes for Asymmetrical supercapacitor. Appl. Surf. Sci. 2019, 475, 793–802. [Google Scholar] [CrossRef]
- Miao, J.; Dong, X.; Xu, Y.; Zhai, Z.; Zhang, L.; Ren, B.; Liu, Z. Preparation and electrochemical performance of 1,4-naphthaquinone-modified carbon nanotubes as a supercapacitor material. Org. Electron. 2019, 73, 304–310. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. MnO2@Polyaniline-CNT-boron-doped graphene as a freestanding binder-free electrode material for supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 8385–8393. [Google Scholar] [CrossRef]
- Hao, H.; Xiaogang, S.; Wei, C.; Jie, W.; Xu, L.; Yapan, H.; Chengcheng, W.; Guodong, L. Electrochemical properties of supercapacitors using boron nitrogen double doped carbon nanotubes as conductive additive. Nano 2019, 14, 1950080. [Google Scholar] [CrossRef]
- Maity, C.K.; Sahoo, S.; Verma, K.; Behera, A.K.; Nayak, G.C. Facile functionalization of boron nitride (BN) for the development of high-performance asymmetric supercapacitors. New J. Chem. 2020, 44, 8106–8119. [Google Scholar] [CrossRef]
- Jung, D.; Muni, M.; Marin, G.; Ramachandran, R.; El-Kady, M.F.; Balandin, T.; Kaner, R.B.; Spokoyny, A.M. Enhancing cycling stability of tungsten oxide supercapacitor electrodes via a boron cluster-based molecular cross-linking approach. J. Mater. Chem. A 2020, 8, 18015–18023. [Google Scholar] [CrossRef]
- Banda, H.; Périé, S.; Daffos, B.; Taberna, P.-L.; Dubois, L.; Crosnier, O.; Simon, P.; Lee, D.; Paëpe, G.D.; Duclairoir, F. Sparsely Pillared Graphene Materials for High Performance Supercapacitors: Improving Ion Transport and Storage Capacity. ACS Nano 2019, 13, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ozkan, M.; Ozkan, C.S. Ultrafast high energy supercapacitors based on pillared graphene nanostructures. J. Mater. Chem. A 2016, 4, 3356–3361. [Google Scholar] [CrossRef]
- Ghosh, K.; Yue, C.Y.; Sk, M.M.; Jena, R.K. Development of 3D Urchin-Shaped Coaxial Manganese Dioxide@Polyaniline (MnO2@PANI) Composite and Self-Assembled 3D Pillared Graphene Foam for Asymmetric All-Solid-State Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 15350–15363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sheng, L.; Long, C.; Wei, T.; Fan, Z. Functional Pillared Graphene Frameworks for Ultrahigh Volumetric Performance Supercapacitors. Adv. Energy Mater. 2015, 5, 1500771. [Google Scholar] [CrossRef]
- Ordejón, P.; Artecho, E.; Soler, J.M. Self-consistent order-N density-functional calculations for very large systems. Phys. Rev. B 1996, 53, 10441. [Google Scholar] [CrossRef] [Green Version]
- Soler, J.M.; Artecho, E.; Gale, J.D.; Garcýa, A.; Junqera, J.; Ordejón, P.; Sanchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef] [Green Version]
- Naveh, Z.M.; Khajehdad, M.M.; Sarmazdeh, M.M. A theoretical study on the chirality detection of serine amino acid based on carbon nanotubes with and without Stone-Wales defects. Struct. Chem. 2019, 31, 455–464. [Google Scholar] [CrossRef]
- Juarez, F.; Dominguez-Flores, F.; Quaino, P.M.; Santos, E.; Schmickler, W. Interactions of ions across carbon nanotubes. Phys. Chem. Chem. Phys. 2020, 22, 10603–10608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Lin, M.K.; Chan, D.H.; Lin, K.B.; Kaun, C.C. Ab Initio and Theoretical Study on Electron Transport through Polyene Junctions in between Carbon Nanotube Leads of Various Cuts. Sci. Rep. 2020, 10, 8033. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.D. Modified Broyden’s method for accelerating convergence in self-consistent calculations. Phys. Rev. Lett. 1988, 38, 12807. [Google Scholar] [CrossRef] [PubMed]
- Shunaev, V.V.; Ushakov, A.V.; Glukhova, O.E. Increase of gamma-Fe2O3/CNT composite quantum capacitance by structural design for performance optimization of electrode materials. Int. J. Quantum Chem. 2020, 120, e26165. [Google Scholar] [CrossRef]
- Slepchenkov, M.M.; Shmygin, D.S.; Zhang, G.; Glukhova, O.E. Controlling anisotropic electrical conductivity in porous graphene-nanotube thin films. Carbon 2020, 165, 139–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, C.; Casillas, G.; Sun, Z.; Yan, Z.; Ruan, G.; Peng, Z.; Raji, A.R.O.; Kittrell, C.; et al. A seamless three dimensional carbon nanotube graphene hybrid material. Nat. Commun. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Kolosov, D.A.; Glukhova, O.E. A New Composite Material on the Base of Carbon Nanotubes and Boron Clusters B12 as the Base for High-Performance Supercapacitor Electrodes. C J. Carbon Res. 2021, 7, 26. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, G.; Fan, X.; Zheng, W. Improving the Quantum Capacitance of Graphene-Based Supercapacitors by the Doping and Co-Doping: First-Principles Calculations. ACS Omega 2019, 4, 13209–13217. [Google Scholar] [CrossRef] [Green Version]
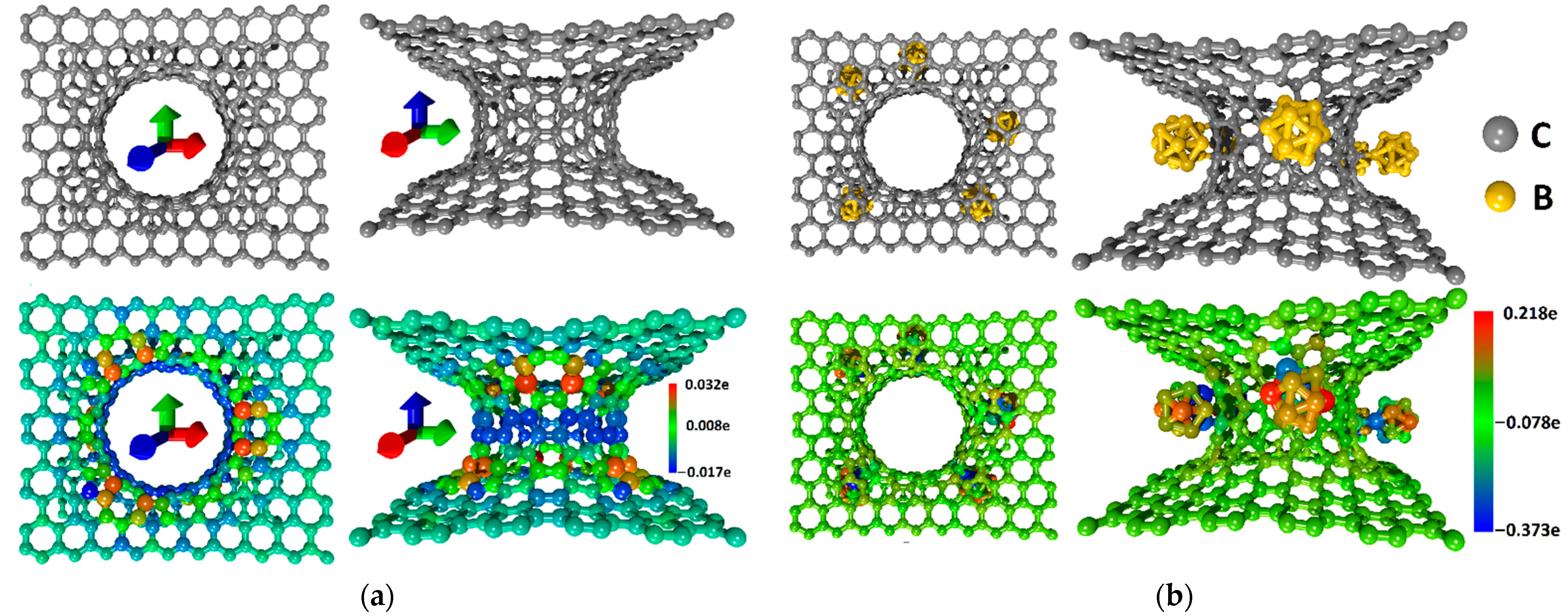
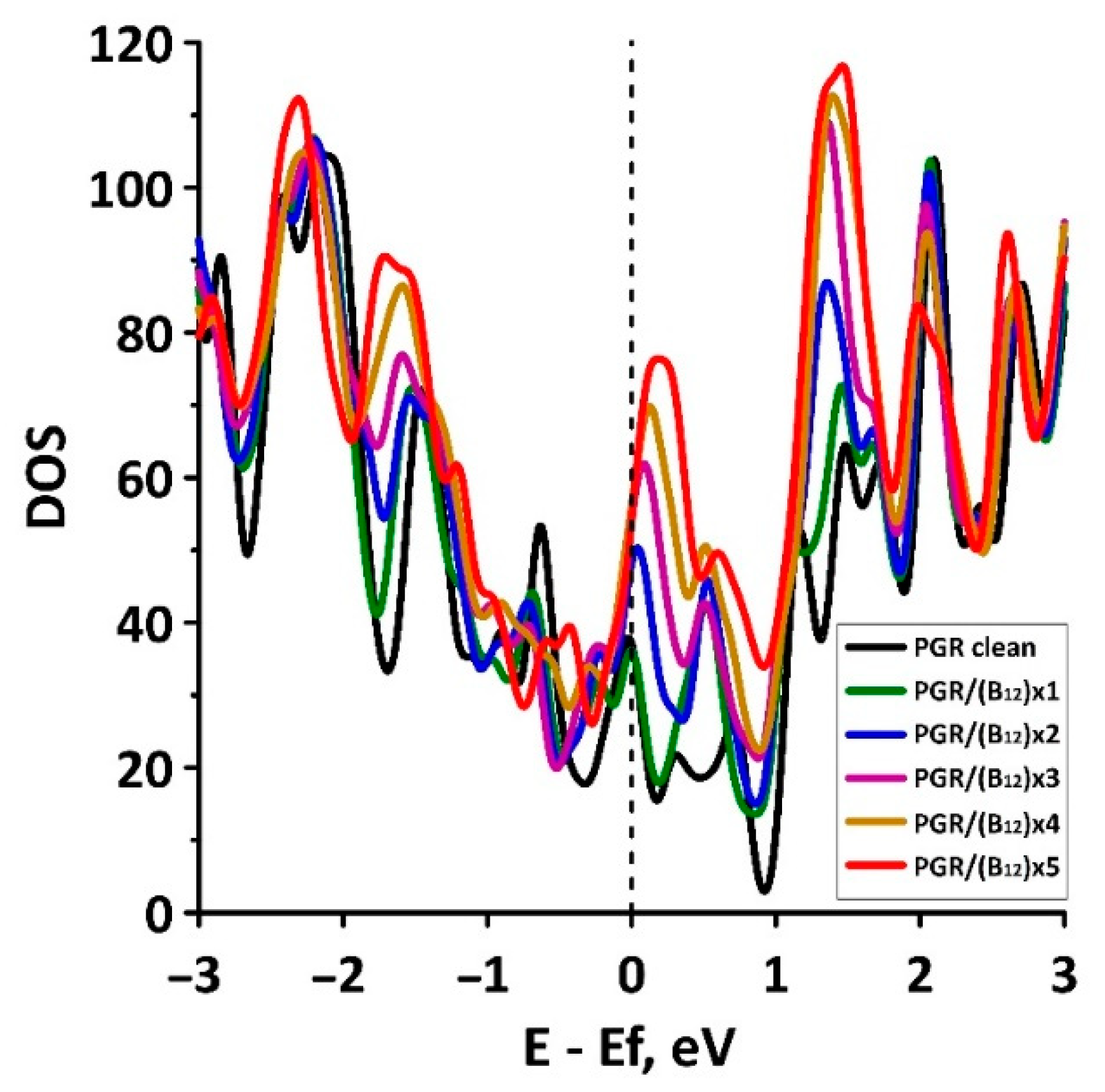
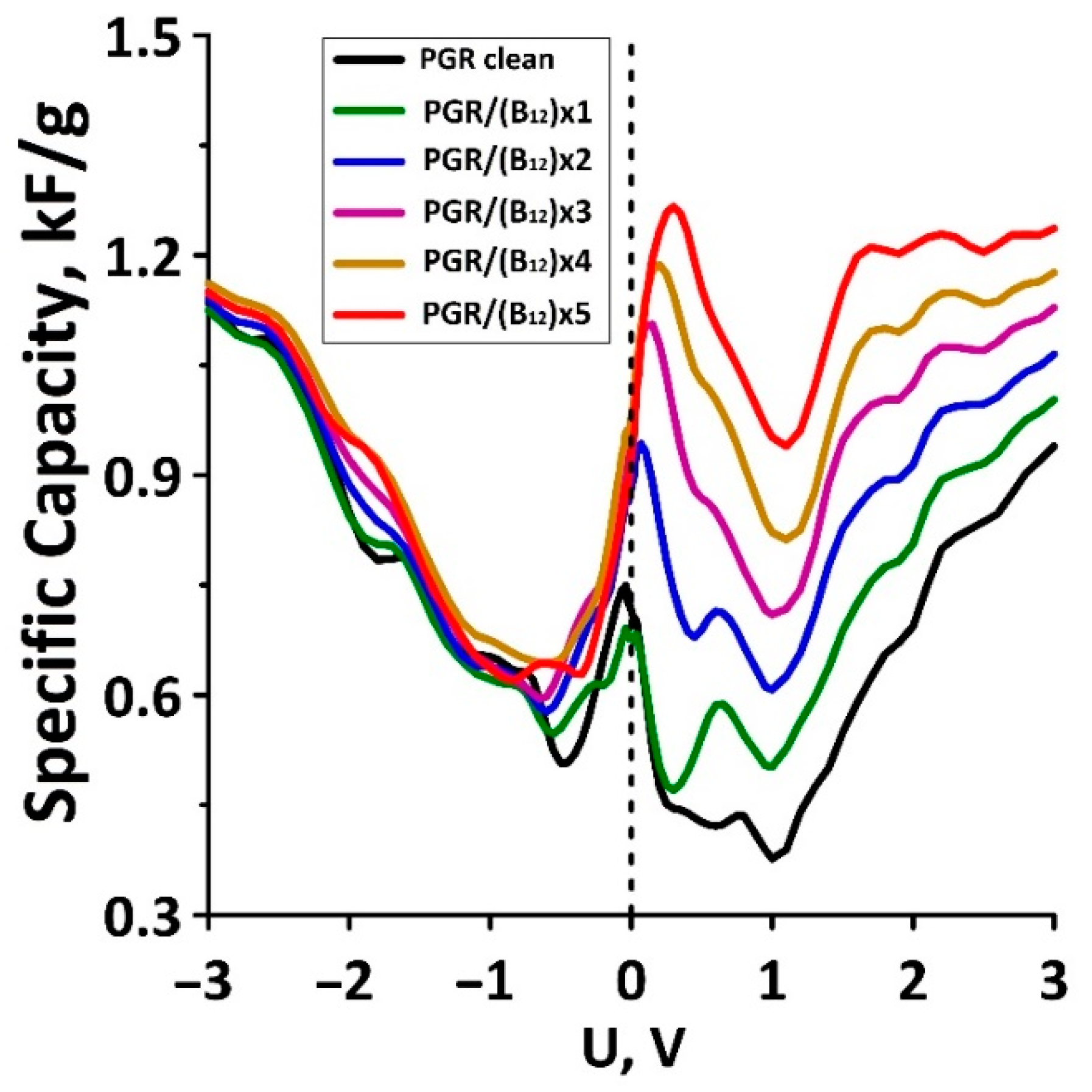

| No. | ΔH, eV | QSCD, kC/g | CQ, kF/g |
|---|---|---|---|
| Pure PGR | - | −2.384/1.748 | 0.749 |
| PGR/(B12) × 1 | −0.00607 | −2.359/2.036 | 0.691 |
| PGR/(B12) × 2 | −0.00965 | −2.466/2.418 | 0.943 |
| PGR/(B12) × 3 | −0.01271 | −2.532/2.757 | 1.105 |
| PGR/(B12) × 4 | −0.01743 | −2.608/3.030 | 1.187 |
| PGR/(B12) × 5 | −0.02272 | −2.534/3.317 | 1.266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolosov, D.A.; Glukhova, O.E. Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study. Appl. Sci. 2021, 11, 3496. https://doi.org/10.3390/app11083496
Kolosov DA, Glukhova OE. Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study. Applied Sciences. 2021; 11(8):3496. https://doi.org/10.3390/app11083496
Chicago/Turabian StyleKolosov, Dmitry A., and Olga E. Glukhova. 2021. "Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study" Applied Sciences 11, no. 8: 3496. https://doi.org/10.3390/app11083496
APA StyleKolosov, D. A., & Glukhova, O. E. (2021). Boron-Decorated Pillared Graphene as the Basic Element for Supercapacitors: An Ab Initio Study. Applied Sciences, 11(8), 3496. https://doi.org/10.3390/app11083496







