Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments
Abstract
1. Introduction
2. Food Quality Indicators
2.1. Oxygen and Carbon Dioxide
2.2. Humidity
2.3. pH Changes
2.4. Temperature
2.5. Nitrogen Related Compounds
3. Methods for Real-Time Food Monitoring
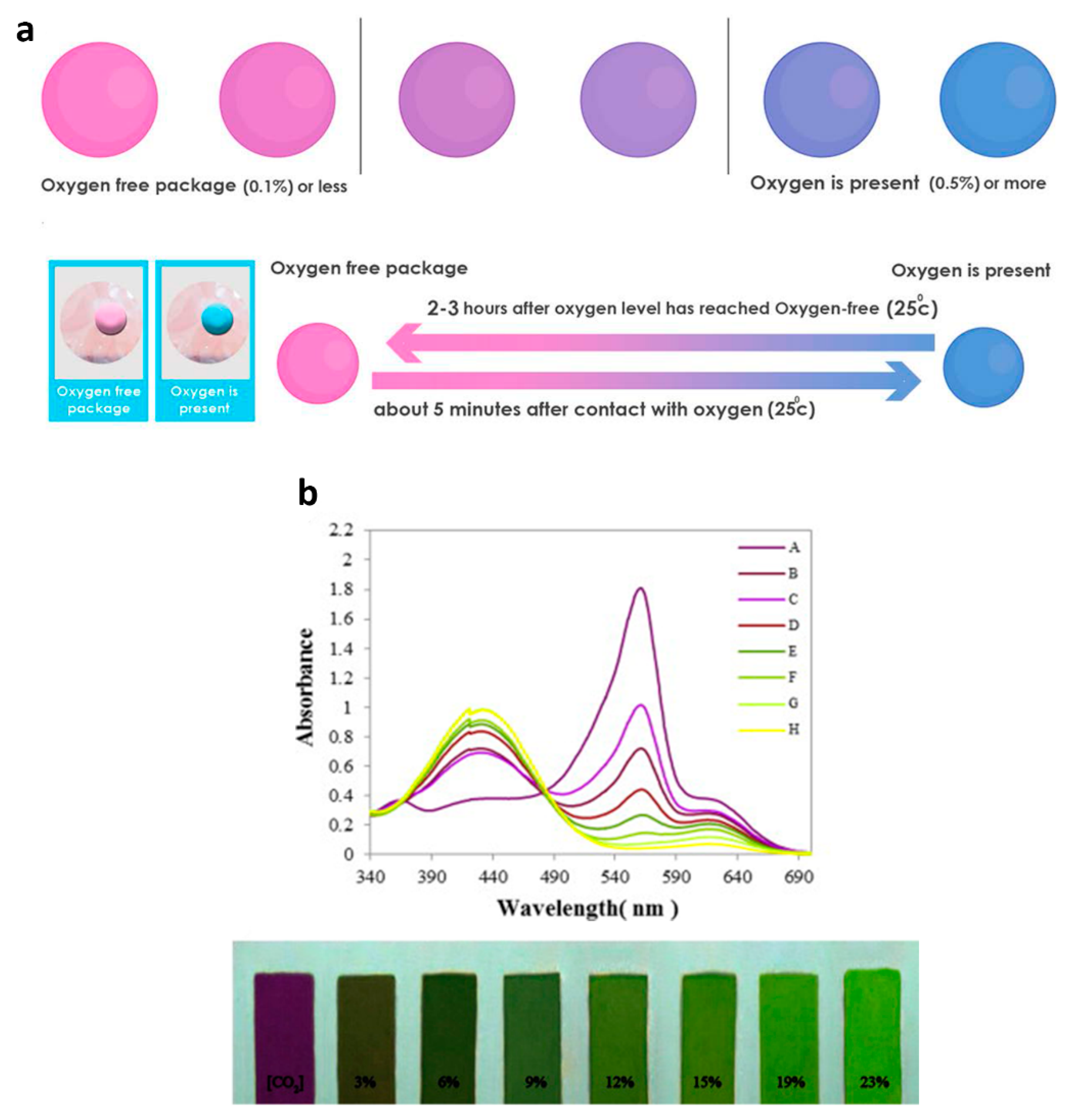
3.1. O2 Detectors
3.2. CO2 Detectors
3.3. Specific Chemicals and pH Changes
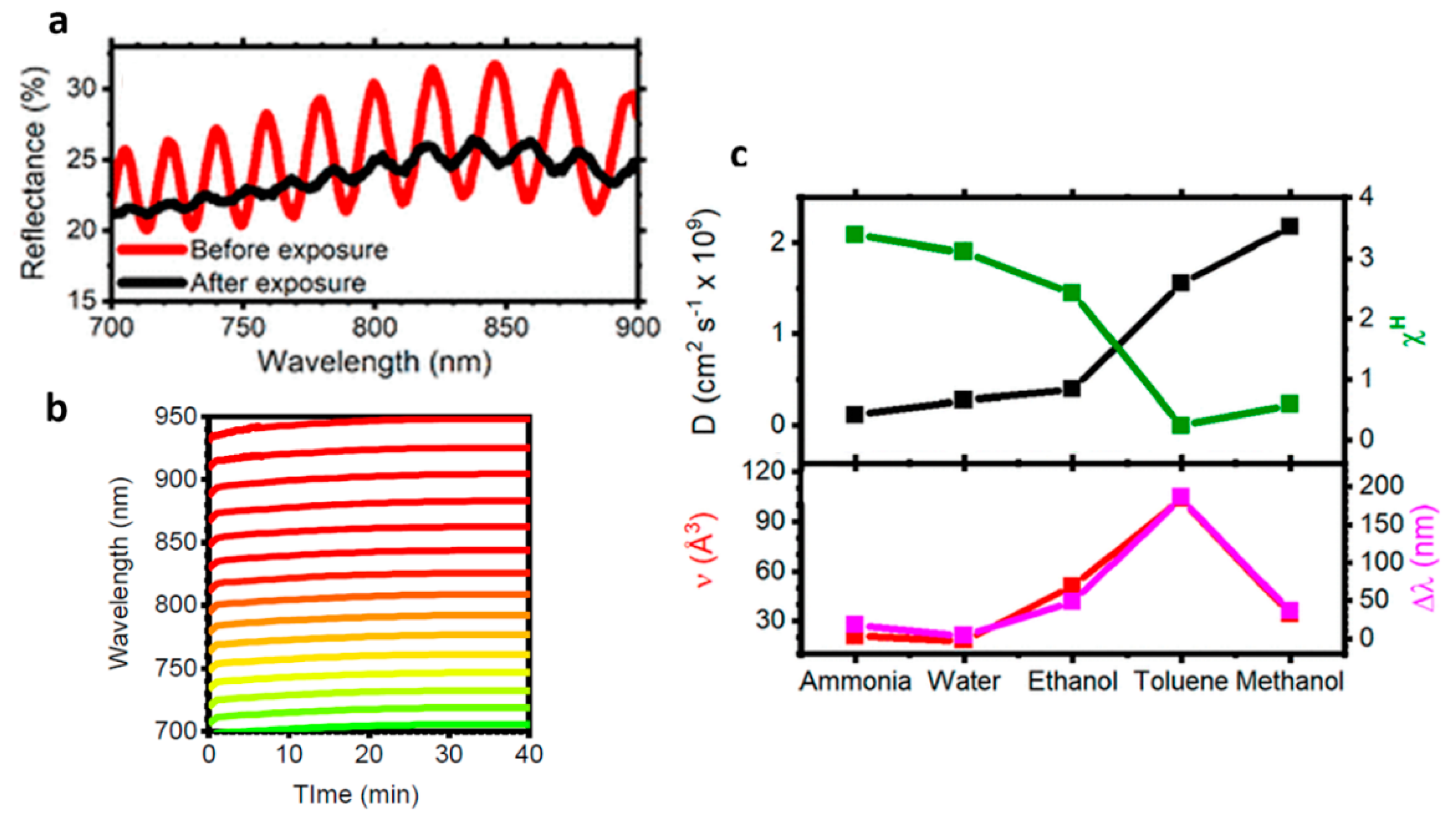
3.4. Humidity Sensors
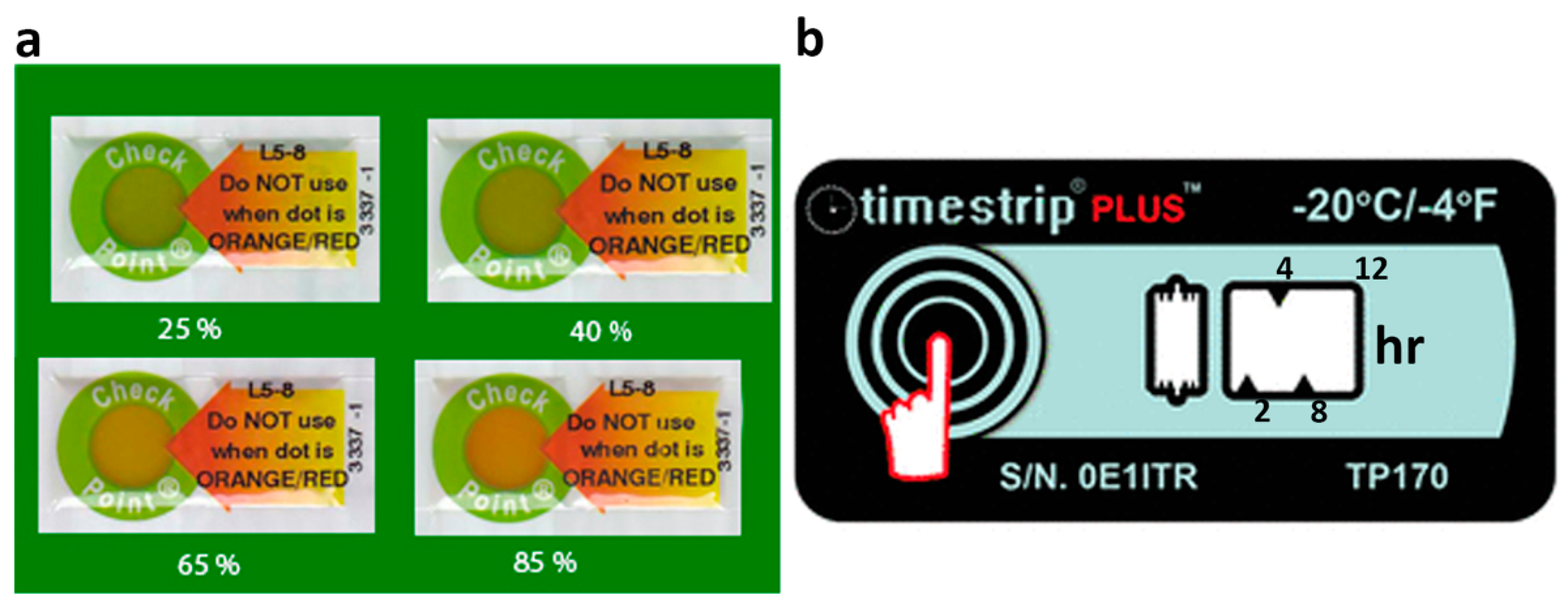
3.5. Time-Temperature Sensors
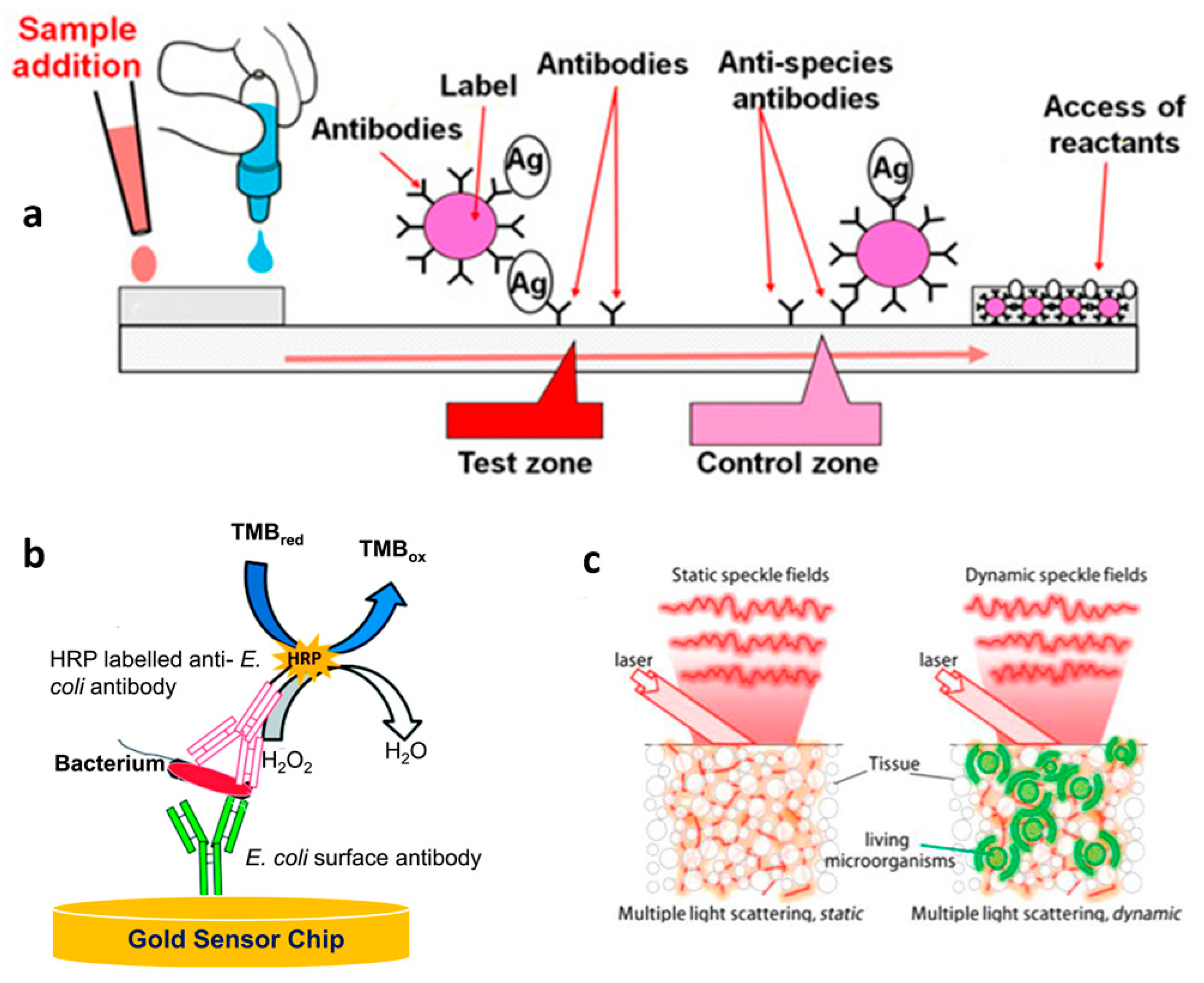
3.6. Biosensors for Bacteria Detection
4. Future Perspectives and Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 16 February 2021).
- Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 5 March 2021).
- Kotsanopoulos, K.V.; Arvanitoyannis, I.S. The Role of Auditing, Food Safety, and Food Quality Standards in the Food Industry: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, C.; Liu, F.; Qiu, Z.; He, Y. Application of Deep Learning in Food: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1793–1811. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Zhan, Y.; Alam, S.S.; Tse, Y.K.; Tan, K.H. Food supply chain integrity: The need to go beyond certification. Ind. Manag. Data Syst. 2017, 117, 1589–1611. [Google Scholar] [CrossRef]
- Callao, M.P.; Ruisánchez, I. An overview of multivariate qualitative methods for food fraud detection. Food Control 2018, 86, 283–293. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.; Cheung, W.M. Smart Packaging: Opportunities and Challenges. Procedia CIRP 2018, 72, 1022–1027. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The role of smart packaging system in food supply chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Han, J.-W.W.; Ruiz-Garcia, L.; Qian, J.-P.P.; Yang, X.-T.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Dobrucka, R.; Przekop, R. New perspectives in active and intelligent food packaging. J. Food Process. Preserv. 2019, 43, e14194. [Google Scholar] [CrossRef]
- Lydekaityte, J.; Tambo, T. Smart packaging: Definitions, models and packaging as an intermediator between digital and physical product management. Int. Rev. Retail. Distrib. Consum. Res. 2020, 30, 377–410. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Lova, P.; Soci, C. Black GaAs: Gold-Assisted Chemical Etching for Light Trapping and Photon Recycling. Micromachines 2020, 11, 573. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Megahd, H.; Oldani, C.; Radice, S.; Lanfranchi, A.; Patrini, M.; Lova, P.; Comoretto, D. Aquivion–Poly (N -vinylcarbazole) Holistic Flory–Huggins Photonic Vapor Sensors. Adv. Opt. Mater. 2021, 9, 2002006. [Google Scholar] [CrossRef]
- Lova, P.; Megahd, H.; Comoretto, D. Thin Polymer Films: Simple Optical Determination of Molecular Diffusion Coefficients. ACS Appl. Polym. Mater. 2020, 2, 563–568. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Bastianini, C.; Mennucci, C.; Buatier De Mongeot, F.; Servida, A.; Comoretto, D. Flory-Huggins Photonic Sensors for the Optical Assessment of Molecular Diffusion Coefficients in Polymers. ACS Appl. Mater. Interfaces 2019, 11, 16872–16880. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Migas, D.B.; Panahandeh-Fard, M.; Chen, S.; Wang, Z.; Lova, P.; Soci, C. Charge redistribution at GaAs/P3HT heterointerfaces with different surface polarity. J. Phys. Chem. Lett. 2013, 4, 3303–3309. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Liegeard, J.; Manning, L. Use of intelligent applications to reduce household food waste. Crit. Rev. Food Sci. Nutr. 2020, 60, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and intelligent packaging: The indication of quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Lozano, M.G.; García, Y.P.; Gonzalez, J.A.S.; Bañuelos, C.V.O.; Escareño, M.P.L.; Balagurusamy, N. Biosensors for food quality and safety monitoring: Fundamentals and applications. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 691–709. ISBN 9780128132807. [Google Scholar]
- Kuswandi, B.; Jumina. Active and intelligent packaging, safety, and quality controls. In Fresh-Cut Fruits and Vegetables: Technologies and Mechanisms for Safety Control; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 243–294. ISBN 9780128161845. [Google Scholar]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. Analysis of Protein Oxidation in Food and Feed Products. J. Agric. Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef]
- Hu, K.; Huyan, Z.; Ding, S.; Dong, Y.; Yu, X. Investigation on food packaging polymers: Effects on vegetable oil oxidation. Food Chem. 2020, 315, 126299. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2020, 1, 3. [Google Scholar] [CrossRef]
- Silva, E.K.; Guimarães, J.T.; Costa, A.L.R.; Cruz, A.G.; Meireles, M.A.A. Non-thermal processing of inulin-enriched soursop whey beverage using supercritical carbon dioxide technology. J. Supercrit. Fluids 2019, 154, 104635. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Mohi Alden, K.; Omid, M.; Rajabipour, A.; Tajeddin, B.; Soltani Firouz, M. Quality and shelf-life prediction of cauliflower under modified atmosphere packaging by using artificial neural networks and image processing. Comput. Electron. Agric. 2019, 163, 104861. [Google Scholar] [CrossRef]
- Zhu, R.; Desroches, M.; Yoon, B.; Swager, T.M. Wireless oxygen sensors enabled by Fe(II)-polymer wrapped carbon nanotubes. ACS Sens. 2017, 2, 1044–1050. [Google Scholar] [CrossRef]
- Kelly, C.; Yusufu, D.; Okkelman, I.; Banerjee, S.; Kerry, J.P.; Mills, A.; Papkovsky, D.B. Extruded phosphorescence based oxygen sensors for large-scale packaging applications. Sens. Actuators B Chem. 2020, 304, 127357. [Google Scholar] [CrossRef]
- Jalali, A.; Seiiedlou, S.; Linke, M.; Mahajan, P. A comprehensive simulation program for modified atmosphere and humidity packaging (MAHP) of fresh fruits and vegetables. J. Food Eng. 2017, 206, 88–97. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; de Quintans, J.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Jalali, A.; Rux, G.; Linke, M.; Geyer, M.; Pant, A.; Saengerlaub, S.; Mahajan, P. Application of humidity absorbing trays to fresh produce packaging: Mathematical modeling and experimental validation. J. Food Eng. 2019, 244, 115–125. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Q.; Mugambi Mariga, A.; Cao, C.; Yang, W. Effect of nano packaging on preservation quality of Nanjing 9108 rice variety at high temperature and humidity. Food Chem. 2018, 239, 23–31. [Google Scholar] [CrossRef]
- Opara, U.L.; Caleb, O.J.; Belay, Z.A. Modified atmosphere packaging for food preservation. In Food Quality and Shelf Life; Elsevie: Amsterdam, The Netherlands, 2019; pp. 235–259. [Google Scholar]
- Bai, J.; Baldwin, E.; Tsantili, E.; Plotto, A.; Sun, X.; Wang, L.; Kafkaletou, M.; Wang, Z.; Narciso, J.; Zhao, W.; et al. Modified humidity clamshells to reduce moisture loss and extend storage life of small fruits⋆. Food Packag. Shelf Life 2019, 22, 100376. [Google Scholar] [CrossRef]
- Saliu, F.; Della Pergola, R. Carbon dioxide colorimetric indicators for food packaging application: Applicability of anthocyanin and poly-lysine mixtures. Sens. Actuators B Chem. 2018, 258, 1117–1124. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural Biomaterial-Based Edible and pH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Kuswandi, B.; Asih, N.P.N.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible pH sensor based on immobilized red cabbage anthocyanins into bacterial cellulose membrane for intelligent food packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Tsang, Y.P.; Choy, K.L.; Wu, C.H.; Ho, G.T.S.; Lam, H.Y.; Tang, V. An intelligent model for assuring food quality in managing a multi-temperature food distribution centre. Food Control 2018, 90, 81–97. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Temperature sensitive smart packaging for monitoring the shelf life of fresh beef. J. Food Eng. 2018, 234, 41–49. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Comi, G. Spoilage of Meat and Fish. In The Microbiological Quality of Food: Foodborne Spoilers; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 179–210. ISBN 9780081005033. [Google Scholar]
- Liu, B.; Gurr, P.A.; Qiao, G.G. Irreversible Spoilage Sensors for Protein-Based Food. ACS Sens. 2020, 5, 2903–2908. [Google Scholar] [CrossRef]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.E.; Darwish, H.W.; Darwish, I.A.; Saad, A.S. Solid-state potentiometric sensor for the rapid assay of the biologically active biogenic amine (tyramine) as a marker of food spoilage. Food Chem. 2021, 346, 128911. [Google Scholar] [CrossRef]
- Torre, R.; Costa-Rama, E.; Nouws, H.P.A.; Delerue-Matos, C. Screen-Printed Electrode-Based Sensors for Food Spoilage Control: Bacteria and Biogenic Amines Detection. Biosensors 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-32009R0450-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0450 (accessed on 16 February 2021).
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 343–374. [Google Scholar]
- Mirza Alizadeh, A.; Masoomian, M.; Shakooie, M.; Zabihzadeh Khajavi, M.; Farhoodi, M. Trends and applications of intelligent packaging in dairy products: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–15. [Google Scholar] [CrossRef]
- Gregor-Svetec, D. Intelligent packaging. In Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–247. ISBN 9780323512718. [Google Scholar]
- Wang, L.; Wu, Z.; Cao, C. Technologies and Fabrication of Intelligent Packaging for Perishable Products. Appl. Sci. 2019, 9, 4858. [Google Scholar] [CrossRef]
- Megahd, H.; Lova, P.; Comoretto, D. Universal Design Rules for Flory–Huggins Polymer Photonic Vapor Sensors. Adv. Funct. Mater. 2021, 31, 2009626. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z.; Chen, H. Recent Application of Modified Atmosphere Packaging (MAP) in Fresh and Fresh-Cut Foods. Food Rev. Int. 2015, 31, 172–193. [Google Scholar] [CrossRef]
- Mills, A. Oxygen indicators and intelligent inks for packaging food. Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Cruz-Romero, M.; Kerry, J.; Papkovsky, D. Stability and Safety Assessment of Phosphorescent Oxygen Sensors for Use in Food Packaging Applications. Chemosensors 2018, 6, 38. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. [Google Scholar] [CrossRef]
- Zhao, H.; Zang, L.; Wang, L.; Qin, F.; Zhang, Z.; Cao, W. Luminescence ratiometric oxygen sensor based on gadolinium labeled porphyrin and filter paper. Sens. Actuators B Chem. 2015, 215, 405–411. [Google Scholar] [CrossRef]
- Urriza-Arsuaga, I.; Ielasi, G.; Bedoya, M.; Orellana, G. Luminescence-Based Sensors for Bioprocess Applications; Springer: Cham, Switzerland, 2019; pp. 1–38. [Google Scholar]
- Şen, F.B.; Bener, M.; Bekdeşer, B.; Apak, R. Redox-based colorimetric sensing of H2O2 after removal of antioxidants with ABTS radical oxidation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119266. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A.B.; Zhou, X.; Liang, K.; Jiang, Y.F.; Xu, A.W. Oxygen-Deficient TiO2-X/Methylene Blue Colloids: Highly Efficient Photoreversible Intelligent Ink. Langmuir 2016, 32, 8980–8987. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity in foods. In Handbook of Antioxidants for Food Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 287–333. ISBN 9781782420972. [Google Scholar]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT 2015, 61, 258–262. [Google Scholar] [CrossRef]
- AGELESS EYE, Oxygen Indicator|Products|Mitsubishi Gas Chemical Company, Inc. Available online: https://www.mgc.co.jp/eng/products/sc/ageless-eye.html (accessed on 5 March 2021).
- Dodero, A.; Lova, P.; Vicini, S.; Castellano, M.; Comoretto, D. Sodium alginate cross-linkable planar 1d photonic crystals as a promising tool for Pb2+ detection in water. Chemosensors 2020, 8, 37. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Lova, P.; Alloisio, M.; Castellano, M. Nanocomposite alginate-based electrospun membranes as novel adsorbent systems. Int. J. Biol. Macromol. 2020, 165, 1939–1948. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological properties of sodium alginate solutions in the presence of added salt: An application of Kulicke equation. Rheol. Acta 2020, 59. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer Alginate-Polycaprolactone Electrospun Membranes as Skin Wound Patches with Drug Delivery Abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Vicini, S.; Castellano, M. Depolymerization of sodium alginate in saline solutions via ultrasonic treatments: A rheological characterization. Food Hydrocoll. 2020, 109, 106128. [Google Scholar] [CrossRef]
- Dodero, A.; Donati, I.; Scarfì, S.; Mirata, S.; Alberti, S.; Lova, P.; Comoretto, D.; Alloisio, M.; Vicini, S.; Castellano, M. Effect of sodium alginate molecular structure on electrospun membrane cell adhesion. Mater. Sci. Eng. C 2021, 124, 112067. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Won, K. Novel water-resistant UV-activated oxygen indicator for intelligent food packaging. Food Chem. 2013, 140, 52–56. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Puligundla, P.; Ko, S. Proof-of-concept study of chitosan-based carbon dioxide indicator for food packaging applications. Food Chem. 2012, 135, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Von Bültzingslöwen, C.; McEvoy, A.K.; McDonagh, C.; MacCraith, B.D.; Klimant, I.; Krause, C.; Wolfbeis, O.S. Sol-gel based optical carbon dioxide sensor employing dual luminophore referencing for application in food packaging technology. Analyst 2002, 127, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, Z.; Yang, B.; Yang, X. Optical carbon dioxide sensor based on fluorescent capillary array. Results Phys. 2017, 7, 323–326. [Google Scholar] [CrossRef]
- Mills, A.; Chang, Q. Fluorescence plastic thin-film sensor for carbon dioxide. Analyst 1993, 118, 839–843. [Google Scholar] [CrossRef]
- Bibi, F.; Guillaume, C.; Gontard, N.; Sorli, B. Wheat gluten, a bio-polymer to monitor carbon dioxide in food packaging: Electric and dielectric characterization. Sens. Actuators B Chem. 2017, 250, 76–84. [Google Scholar] [CrossRef]
- Chocarro-Ruiz, B.; Pérez-Carvajal, J.; Avci, C.; Calvo-Lozano, O.; Alonso, M.I.; Maspoch, D.; Lechuga, L.M. A CO2 optical sensor based on self-assembled metal-organic framework nanoparticles. J. Mater. Chem. A 2018, 6, 13171–13177. [Google Scholar] [CrossRef]
- Lyu, J.S.; Choi, I.; Hwang, K.S.; Lee, J.Y.; Seo, J.; Kim, S.Y.; Han, J. Development of a BTB−/TBA+ ion-paired dye-based CO2 indicator and its application in a multilayered intelligent packaging system. Sens. Actuators B Chem. 2019, 282, 359–365. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Liu, W.; Chen, L. M-Cresol purple functionalized surface enhanced Raman scattering paper chips for highly sensitive detection of pH in the neutral pH range. Analyst 2017, 142, 2333–2337. [Google Scholar] [CrossRef]
- Zhu, M.; Kari, N.; Yan, Y.; Yimit, A. The fabrication and gas sensing application of a fast-responding m-CP-PVP composite film/potassium ion-exchanged glass optical waveguide. Anal. Methods 2017, 9, 5494–5501. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Colorimetric Sensor Array for Monitoring, Modelling and Comparing Spoilage Processes of Different Meat and Fish Foods. Foods 2020, 9, 684. [Google Scholar] [CrossRef]
- Xiao-wei, H.; Xiao-bo, Z.; Ji-yong, S.; Zhi-hua, L.; Jie-wen, Z. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Mills, A. Optical Sensors for Carbon Dioxide and Their Applications. In Sensors for Environment, Health and Security; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 347–370. [Google Scholar]
- Siripongpreda, T.; Siralertmukul, K.; Rodthongkum, N. Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide. Food Chem. 2020, 329, 127165. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Mazur, F.; Chandrawati, R. Monitoring of Food Spoilage Using Polydiacetylene- and Liposome-Based Sensors. In Smart Sensors for Environmental and Medical Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 81–102. [Google Scholar]
- Valdez, M.; Gupta, S.K.; Lozano, K.; Mao, Y. ForceSpun polydiacetylene nanofibers as colorimetric sensor for food spoilage detection. Sens. Actuators B Chem. 2019, 297, 126734. [Google Scholar] [CrossRef]
- Sudalaimani, S.; Esokkiya, A.; Hansda, S.; Suresh, C.; Tamilarasan, P.; Giribabu, K. Colorimetric Sensing of Putrescine and Cadaverine Using Ninhydrin as a Food Spoilage Detection Reagent. Food Anal. Methods 2020, 13, 629–636. [Google Scholar] [CrossRef]
- Lova, P.; Comoretto, D. Label-free vapor selectivity by polymer-inorganic composite photonic crystals sensors. AIP Conf. Proc. 2018, 1981, 020097. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Jafari, S.M. Detection of food spoilage and adulteration by novel nanomaterial-based sensors. Adv. Colloid Interface Sci. 2020, 286, 102297. [Google Scholar] [CrossRef] [PubMed]
- Blana, V.A.; Lianou, A.; Nychas, G.-J.E. Quorum sensing and microbial ecology of foods. In Quantitative Microbiology in Food Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 600–616. [Google Scholar]
- Mishra, G.; Barfidokht, A.; Tehrani, F.; Mishra, R. Food Safety Analysis Using Electrochemical Biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Sun, Z.; Li, Z.; Sun, Y.; Li, Y.; Wang, X.; Holmes, M.; et al. Amine-responsive bilayer films with improved illumination stability and electrochemical writing property for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2020, 302, 127130. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef]
- Bhadra, S.; Narvaez, C.; Thomson, D.J.; Bridges, G.E. Non-destructive detection of fish spoilage using a wireless basic volatile sensor. Talanta 2015, 134, 718–723. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Development of a novel colorimetric food package label for monitoring lean pork freshness. LWT 2019, 99, 43–49. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Liu, C.; Gao, G.; Zhang, Y.; Wang, L.; Wang, J.; Song, Y. The Naked-Eye Detection of NH3-HCl by Polyaniline-Infiltrated TiO2 Inverse Opal Photonic Crystals. Macromol. Rapid Commun. 2012, 33, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qin, M.; Hu, X.; Li, F.; Wang, Y.; Huang, Y.; Su, M.; Li, W.; Qian, X.; Tang, K.L.; et al. Bioinspired Synergy Sensor Chip of Photonic Crystals-Graphene Oxide for Multiamines Recognition. Anal. Chem. 2018, 90, 6371–6375. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jin, Y.; Su, L.; Chu, H.; Zhang, W. A two-dimensional molecularly imprinted photonic crystal sensor for highly efficient tetracycline detection. Anal. Methods 2020, 12, 1374–1379. [Google Scholar] [CrossRef]
- Galstyan, V.; Bhandari, M.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Choi, J.; Chen, Y.; Abbel, R.; Visagie, I.; Parker, K. Flexible humidity sensors for wireless monitoring based on electrospun sulfonated polyether ether ketone (SPEEK) nanofibres. Sens. Actuators B Chem. 2020, 324, 128704. [Google Scholar] [CrossRef]
- Jiang, X.; Valdeperez, D.; Nazarenus, M.; Wang, Z.; Stellacci, F.; Parak, W.J.; del Pino, P. Future Perspectives Towards the Use of Nanomaterials for Smart Food Packaging and Quality Control. Part. Part. Syst. Charact. 2015, 32, 408–416. [Google Scholar] [CrossRef]
- He, H.; Fu, Y.; Liu, S.; Cui, J.; Xu, W. Research progress and application of flexible humidity sensors for smart packaging: A review. In Proceedings of the Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2020; Volume 600, pp. 429–435. [Google Scholar]
- Tan, E.L.; Ng, W.N.; Shao, R.; Pereles, B.D.; Ong, K.G. A wireless, passive sensor for quantifying packaged food quality. Sensors 2007, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Frank, E.U. Static Dielectric Constant of Water and Steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Amin, Y.; Chen, Q.; Zheng, L.R.; Tenhunen, H. “Green” wideband log-spiral antenna for RFID sensing and wireless applications. J. Electromagn. Waves Appl. 2012, 26, 2043–2050. [Google Scholar] [CrossRef]
- Deng, F.; He, Y.; Li, B.; Song, Y.; Wu, X. Design of a slotted chipless RFID humidity sensor tag. Sens. Actuators B Chem. 2018, 264, 255–262. [Google Scholar] [CrossRef]
- Vena, A.; Perret, E.; Kaddour, D.; Baron, T. Toward a reliable chipless RFID humidity sensor tag based on silicon nanowires. IEEE Trans. Microw. Theory Tech. 2016, 64, 2977–2985. [Google Scholar] [CrossRef]
- Borgese, M.; Dicandia, F.A.; Costa, F.; Genovesi, S.; Manara, G. An Inkjet Printed Chipless RFID Sensor for Wireless Humidity Monitoring. IEEE Sens. J. 2017, 17, 4699–4707. [Google Scholar] [CrossRef]
- Sipilä, E.; Virkki, J.; Sydänheimo, L.; Ukkonen, L. Experimental study on brush-painted passive RFID-based humidity sensors embedded into plywood structures. Int. J. Antennas Propag. 2016, 2016. [Google Scholar] [CrossRef]
- Pichorim, S.F.; Gomes, N.J.; Batchelor, J.C. Two solutions of soil moisture sensing with rfid for landslide monitoring. Sensors 2018, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Burratti, L.; De Matteis, F.; Casalboni, M.; Francini, R.; Pizzoferrato, R.; Prosposito, P. Polystyrene photonic crystals as optical sensors for volatile organic compounds. Mater. Chem. Phys. 2018, 212, 274–281. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Bonam, R.K.; Hartley, J.G.; Starkey, T.A.; Vukusic, P.; Vasudev, M.; Bunning, T.; Naik, R.R.; Tang, Z.; Palacios, M.A.; et al. Towards outperforming conventional sensor arrays with fabricated individual photonic vapour sensors inspired by Morpho butterflies. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Yang, H.; Pan, L.; Han, Y.; Ma, L.; Li, Y.; Xu, H.; Zhao, J. A visual water vapor photonic crystal sensor with PVA/SiO 2 opal structure. Appl. Surf. Sci. 2017, 423, 421–425. [Google Scholar] [CrossRef]
- Sobhanimatin, M.B.; Pourmahdian, S.; Tehranchi, M.M. Colorimetric Monitoring of Humidity by Opal Photonic Hydrogel. Polym. Test. 2020, 106999. [Google Scholar] [CrossRef]
- Ndraha, N.; Hsiao, H.I.; Vlajic, J.; Yang, M.F.; Lin, H.T.V. Time-temperature abuse in the food cold chain: Review of issues, challenges, and recommendations. Food Control 2018, 89, 12–21. [Google Scholar] [CrossRef]
- Göransson, M.; Nilsson, F.; Jevinger, A. Temperature performance and food shelf-life accuracy in cold food supply chains—Insights from multiple field studies. Food Control 2018, 86, 332–341. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Alves, V.; Khmelinskii, I.; Vieira, M.C. New Food Packaging Systems. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–85. [Google Scholar]
- Mijanur Rahman, A.; Kim, D.; Jang, H.; Yang, J.; Lee, S. Preliminary Study on Biosensor-Type Time-Temperature Integrator for Intelligent Food Packaging. Sensors 2018, 18, 1949. [Google Scholar] [CrossRef]
- Hsiao, H.-I.; Chang, J.-N. Developing a microbial time-temperature indicator to monitor total volatile basic nitrogen change in chilled vacuum-packed grouper fillets. J. Food Process. Preserv. 2017, 41, e13158. [Google Scholar] [CrossRef]
- Tsironi, T.; Stamatiou, A.; Giannoglou, M.; Velliou, E.; Taoukis, P.S. Predictive modelling and selection of Time Temperature Integrators for monitoring the shelf life of modified atmosphere packed gilthead seabream fillets. LWT-Food Sci. Technol. 2011, 44, 1156–1163. [Google Scholar] [CrossRef]
- Nuin, M.; Alfaro, B.; Cruz, Z.; Argarate, N.; George, S.; Le Marc, Y.; Olley, J.; Pin, C. Modelling spoilage of fresh turbot and evaluation of a time-temperature integrator (TTI) label under fluctuating temperature. Int. J. Food Microbiol. 2008, 127, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, N.; Vasantha Jayakantha Raja, R.; Vigneswaran, D.; Lakshmi, B.; Sumathi, M.; Porsezian, K. Highly efficient compact temperature sensor using liquid infiltrated asymmetric dual elliptical core photonic crystal fiber. Opt. Mater. (Amst). 2017, 64, 574–582. [Google Scholar] [CrossRef]
- De, M.; Gangopadhyay, T.K.; Singh, V.K. Prospects of Photonic Crystal Fiber as Physical Sensor: An Overview. Sensors 2019, 19, 464. [Google Scholar] [CrossRef]
- Li, J.X.; Tong, Z.R.; Jing, L.; Zhang, W.H.; Qin, J.; Liu, J. wei Fiber temperature and humidity sensor based on photonic crystal fiber coated with graphene oxide. Opt. Commun. 2020, 467, 125707. [Google Scholar] [CrossRef]
- TTI Label › Vitsab International AB. Available online: http://vitsab.com/en/tti-label/ (accessed on 6 March 2021).
- Timestrip Plus|Timestrip. Available online: https://timestrip.com/products/timestrip-plus/#tech (accessed on 6 March 2021).
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Pennanen, K.; Focas, C.; Kumpusalo-Sanna, V.; Keskitalo-Vuokko, K.; Matullat, I.; Ellouze, M.; Pentikäinen, S.; Smolander, M.; Korhonen, V.; Ollila, M. European Consumers’ Perceptions of Time-Temperature Indicators in Food Packaging. Packag. Technol. Sci. 2015, 28, 303–323. [Google Scholar] [CrossRef]
- Choi, D.Y.; Jung, S.W.; Lee, D.S.; Lee, S.J. Fabrication and Characteristics of Microbial Time Temperature Indicators from Bio-Paste Using Screen Printing Method. Packag. Technol. Sci. 2014, 27, 303–312. [Google Scholar] [CrossRef]
- Kreyenschmidt, J.; Christiansen, H.; Hübner, A.; Raab, V.; Petersen, B. A novel photochromic time-temperature indicator to support cold chain management. Int. J. Food Sci. Technol. 2010, 45, 208–215. [Google Scholar] [CrossRef]
- Mohebi, E.; Marquez, L. Intelligent packaging in meat industry: An overview of existing solutions. J. Food Sci. Technol. 2015, 52, 3947–3964. [Google Scholar] [CrossRef]
- Sadilek, A.; Caty, S.; DiPrete, L.; Mansour, R.; Schenk, T.; Bergtholdt, M.; Jha, A.; Ramaswami, P.; Gabrilovich, E. Machine-learned epidemiology: Real-time detection of foodborne illness at scale. NPJ Digit. Med. 2018, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Legese, M.H.; Weldearegay, G.M.; Asrat, D. Extended-spectrum beta-lactamase- and carbapenemase-producing Enterobacteriaceae among ethiopian children. Infect. Drug Resist. 2017, 10, 27–34. [Google Scholar] [CrossRef]
- Gupta, V.; Gulati, P.; Bhagat, N.; Dhar, M.S.; Virdi, J.S. Detection of Yersinia enterocolitica in food: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Safenkova, I.V.; Zaitsev, I.A.; Varitsev, Y.A.; Byzova, N.A.; Drenova, N.V.; Zherdev, A.V.; Dzantiev, B.B. Development of a lateral flow immunoassay for rapid diagnosis of potato blackleg caused by Dickeya species. Anal. Bioanal. Chem. 2017, 409, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Gokduman, K.; Dilek Avsaroglu, M.; Cakiris, A.; Ustek, D.; Candan Gurakan, G. Recombinant plasmid-based quantitative Real-Time PCR analysis of Salmonella enterica serotypes and its application to milk samples. J. Microbiol. Methods 2016, 122, 50–58. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Y.; Alt, R.R.; Xie, X.; Li, F. Emerging techniques for ultrasensitive protein analysis. Analyst 2016, 141, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Gales, A.C.; Monteiro, J.; Silbert, S.; Chagas-Neto, T.; Machado, A.M.O.; Carvalhaes, C.G. Evaluation of a rapid immunochromatographic test for detection of distinct variants of Klebsiella pneumoniae carbapenemase (KPC) in Enterobacteriaceae. J. Microbiol. Methods 2017, 142, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T. Enhanced sensitivity of lateral-flow test strip immunoassays using colloidal palladium nanoparticles and horseradish peroxidase. LWT-Food Sci. Technol. 2017, 86, 566–570. [Google Scholar] [CrossRef]
- Tominaga, T. Rapid detection of Klebsiella pneumoniae, Klebsiella oxytoca, Raoultella ornithinolytica and other related bacteria in food by lateral-flow test strip immunoassays. J. Microbiol. Methods 2018, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A.M.; Fatanat Didar, T.; Veres, T.; Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 2012, 12, 3249–3266. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Ali, M.; Filipe, C.D.M.; Didar, T.F. Producing Covalent Microarrays of Amine-Conjugated DNA Probes on Various Functional Surfaces to Create Stable and Reliable Biosensors. Adv. Mater. Interfaces 2018, 5, 1800659. [Google Scholar] [CrossRef]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Basu, P.K.; Indukuri, D.; Keshavan, S.; Navratna, V.; Vanjari, S.R.K.; Raghavan, S.; Bhat, N. Graphene based E. coli sensor on flexible acetate sheet. Sens. Actuators B Chem. 2014, 190, 342–347. [Google Scholar] [CrossRef]
- DuVall, J.A.; Borba, J.C.; Shafagati, N.; Luzader, D.; Shukla, N.; Li, J.; Kehn-Hall, K.; Kendall, M.M.; Feldman, S.H.; Landers, J.P. Optical imaging of paramagnetic bead-DNA aggregation inhibition allows for low copy number detection of infectious pathogens. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Loutfi, H.; Pellen, F.; Le Jeune, B.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Real-time monitoring of bacterial growth kinetics in suspensions using laser speckle imaging. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ebrahimi, A.; Zhou, K.; Zhou, C.; Sapre, A.; Pavlock, J.H.; Weaver, A.; Muralidharan, R.; Noble, J.; Chung, T.; et al. Dynamic laser speckle imaging meets machine learning to enable rapid antibacterial susceptibility testing (DYRAST). ACS Sens. 2020, 5, 3140–3149. [Google Scholar] [CrossRef]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, L.D.; Puzzo, D.P.; Breslav, S.; Willey, B.M.; McGeer, A.; Ozin, G.A. Towards the Photonic Nose: A Novel Platform for Molecule and Bacteria Identification. Adv. Mater. 2010, 22, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Inan, H.; Poyraz, M.; Inci, F.; Lifson, M.A.; Baday, M.; Cunningham, B.T.; Demirci, U. Photonic crystals: Emerging biosensors and their promise for point-of-care applications. Chem. Soc. Rev. 2017, 46, 366–388. [Google Scholar] [CrossRef]
- Paternò, G.M.; Moscardi, L.; Donini, S.; Ariodanti, D.; Kriegel, I.; Zani, M.; Parisini, E.; Scotognella, F.; Lanzani, G. Hybrid one-dimensional plasmonic-photonic crystals for optical detection of bacterial contaminants. J. Phys. Chem. Lett. 2019, 10, 4980–4986. [Google Scholar] [CrossRef]
- Paternò, G.M.; Moscardi, L.; Donini, S.; Ross, A.M.; Pietralunga, S.M.; Dalla Vedova, N.; Normani, S.; Kriegel, I.; Lanzani, G.; Scotognella, F. Integration of bio-responsive silver in 1D photonic crystals: Towards the colorimetric detection of bacteria. Faraday Discuss. 2020, 223, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Painam, B.; Kaler, R.S.; Kumar, M. On-Chip Oval-Shaped Nanocavity Photonic Crystal Waveguide Biosensor for Detection of Foodborne Pathogens. Plasmonics 2018, 13, 445–449. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Cai, L. A porous silicon optical microcavity for sensitive bacteria detection. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Lova, P.; Megahd, H.; Stagnaro, P.; Alloisio, M.; Patrini, M.; Comoretto, D. Strategies for Dielectric Contrast Enhancement in 1D Planar Polymeric Photonic Crystals. Appl. Sci. 2020, 10, 4122. [Google Scholar] [CrossRef]
- Smart Packaging Market|Growth, Trends, and Forecast (2020–2025). Available online: https://www.mordorintelligence.com/industry-reports/smart-packaging-market (accessed on 19 February 2021).
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Joardder, M.U.H.; Hasan Masud, M.; Joardder, M.U.H.; Masud, M.H. Challenges and Mistakes in Food Preservation. In Food Preservation in Developing Countries: Challenges and Solutions; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–198. [Google Scholar]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef]
- Landaluce, H.; Arjona, L.; Perallos, A.; Falcone, F.; Angulo, I.; Muralter, F. A Review of IoT Sensing Applications and Challenges Using RFID and Wireless Sensor Networks. Sensors 2020, 20, 2495. [Google Scholar] [CrossRef]
- Bibi, F.; Guillaume, C.; Gontard, N.; Sorli, B. A review: RFID technology having sensing aptitudes for food industry and their contribution to tracking and monitoring of food products. Trends Food Sci. Technol. 2017, 62, 91–103. [Google Scholar] [CrossRef]
- Mondal, S.; Wijewardena, K.P.; Karuppuswami, S.; Kriti, N.; Kumar, D.; Chahal, P. Blockchain inspired RFID-based information architecture for food supply chain. IEEE Internet Things J. 2019, 6, 5803–5813. [Google Scholar] [CrossRef]
- Alfian, G.; Rhee, J.; Ahn, H.; Lee, J.; Farooq, U.; Ijaz, M.F.; Syaekhoni, M.A. Integration of RFID, wireless sensor networks, and data mining in an e-pedigree food traceability system. J. Food Eng. 2017, 212, 65–75. [Google Scholar] [CrossRef]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent Progress in Manufacturing Techniques of Printed and Flexible Sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; López-Villanueva, J.A. Recent Advances in Printed Capacitive Sensors. Micromachines 2020, 11, 367. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Food packaging and nanotechnology: Safeguarding consumer health and safety. Nutr. Food Sci. 2019, 49, 1164–1179. [Google Scholar] [CrossRef]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Meherishi, L.; Narayana, S.A.; Ranjani, K.S. Sustainable packaging for supply chain management in the circular economy: A review. J. Clean. Prod. 2019, 237, 117582. [Google Scholar] [CrossRef]
- Boz, Z.; Korhonen, V.; Koelsch Sand, C. Consumer Considerations for the Implementation of Sustainable Packaging: A Review. Sustainability 2020, 12, 2192. [Google Scholar] [CrossRef]
- Abhijith, R.; Ashok, A.; Rejeesh, C.R. Sustainable packaging applications from mycelium to substitute polystyrene: A review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Aramyan, L.; Grainger, M.; Logatcheva, K.; Piras, S.; Setti, M.; Stewart, G.; Vittuari, M. Food waste reduction in supply chains through innovations: A review. Meas. Bus. Excell. 2020. [Google Scholar] [CrossRef]

| Sensing Indicator | Working Principle | Main Applications |
|---|---|---|
| O2 | Luminescence-based Redox-based Colorimetric assay-based | Modified atmosphere packages |
| CO2 | Luminescence-based Colorimetric assay-based | Modified atmosphere packages |
| Humidity | Inductor and capacitor-based Colorimetric assay-based Photonic crystal-based | Modified atmosphere packages Dry food products |
| pH | Colorimetric assay-based Electrochemical assay-based Photonic crystal-based | Meat, fish, and dairy products |
| Temperature | Colorimetric assay-based | Frozen food products |
| Nitrogen | Antibodies-based Colorimetric assay-based | Meat and fish products |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Appl. Sci. 2021, 11, 3532. https://doi.org/10.3390/app11083532
Dodero A, Escher A, Bertucci S, Castellano M, Lova P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Applied Sciences. 2021; 11(8):3532. https://doi.org/10.3390/app11083532
Chicago/Turabian StyleDodero, Andrea, Andrea Escher, Simone Bertucci, Maila Castellano, and Paola Lova. 2021. "Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments" Applied Sciences 11, no. 8: 3532. https://doi.org/10.3390/app11083532
APA StyleDodero, A., Escher, A., Bertucci, S., Castellano, M., & Lova, P. (2021). Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Applied Sciences, 11(8), 3532. https://doi.org/10.3390/app11083532








