Abstract
The quantity of polymer waste in our communities is increasing significantly. It is therefore necessary to consider reuse or recycling waste to avoid an increase in the risk to public health. This project is aimed at using pulverized low-density polyethylene (LDPE) waste as a source to reinforce and improve compressive strength, and to reduce the water absorption of geopolymer ceramics (GC). Clay:LDPE composition consisting of 5%, 10%, and 15% LDPE was geopolymerized with an NaOH/Na2SiO3 solution and cured at 30 °C and 50 °C. Characterization of the geopolymer samples was carried out using XRF and XRD. The microstructure was analyzed by SEM and chemical bonding by FTIR. The SEM micrographs showed LDPE particle pull-out on the geopolymer ceramics’ fracture surface. The result showed that the compressive strength increases with the addition of pulverized polymer waste compared to the controlled without LDPE addition. Water absorption decreased with an increase in LDPE addition in the geopolymer ceramics composite.
1. Introduction
Improper disposal of polymeric materials causes the generation and release of undesirable pollutants into the environment. Polymers in the form of plastics are generally non-biodegradable and their waste causes environmental hazards [1]. The non-biodegradable plastics continually fill water and soil space by landfill [2]. The increasing problem can be alleviated if another means of disposal other than landfills can be found. After degrading, the plastics release oxides of carbon, nitrogen, and Sulphur, which are harmful to both humans and the environment when burnt [3,4]. The increase in demand and the environmental footprint of plastics for households during the COVID-19 pandemic response is on the increase [5]. At least a sachet of pure water per day is consumed by 70 percent of Nigerian adults, resulting in about 50 to 60 million used water-sachets being disposed of daily in Nigeria [6]. To solve this problem Azeko et al. (2015) added low-density polyethylene (LDPE) to laterite soil to form bricks and observed high compressive strength [7]. Agglutinated low-density polyethylene (LDPE), crushed polyethylene terephthalate (PET), and rubber from useless tires were used for hydraulic dam repairs in concrete. Gains in mechanical properties with LDPE addition were better than PET and rubber [8].
Geopolymers are a new class of inorganic polymer materials used for fiber-reinforced composites, replacement of Ordinary Portland Cement (OPC) and waste encapsulation [9,10] and can have ceramic properties [11]. Geopolymers are generally composed of aluminosilicates from materials such as clay [12], metaclay [13], kaolin, metakaolin [14] and fly ash [15], usually in the presence of an alkaline solution [16], and cured at low temperatures lower than 100 °C [17,18,19,20]. Geopolymers cured at temperatures greater than 80 °C are more porous than those cured at lower temperatures [21,22].
Geopolymer alkali-activation of metakaolin studies has increased in recent decades. This is partly due to the lower CO2 emission during geopolymerization processes compared to the use of Portland Cement [23,24]. Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are alkali-activation solutions commonly used for geopolymerization processes at low temperatures [25,26,27,28]. Sodium hydroxide has been observed to improve the geopolymerization process more than potassium hydroxide due to its smaller sodium ions [29]. The addition of sodium hydroxide to sodium silicate increases the reaction rate in the geopolymer process [30,31]. Shriram et al. (2014) observed that the use of metakaolin rather than kaolinite significantly increases the geopolymer strength [32]. Calcined source material, such as metakaolin or metaclay, showed higher compressive strength compared to the non-calcined sources, such as kaolin or clay, due to increased geopolymerization reaction rates [33]. Geopolymer ceramics generally have a compressive strength of about 45 MPa [34]. The compressive strength of geopolymer ceramics can be improved by using particulates, fibres, granulates, organic polymers or powder for reinforcement [20,35,36,37]. Compressive strength also depends on the geopolymerization reaction between silicates and aluminates (Si/Al), and the metal oxides such as CaO and Fe2O3 present in the aluminosilicate source material. The increase in CaO percentage increases settling time, while SiO2 percent higher than 45%, Al2O3 percentage higher than 22%, and increase in Fe2O3 percentage increases the compressive strength [17,38,39].
Most studies have been done on polymer waste as a reinforcement for concrete [40,41]. Few studies on the effect of LDPE geopolymer ceramics have been reported. In this work, low-density polyethylene waste was pulverized and mixed with metaclay (MC) and metakaolin (MK) to produce geopolymer ceramics. The compressive strength and water absorption were evaluated. Geopolymers are waste encapsulation for building materials and use lower curing temperatures for high strength materials. Therefore, the aim of the present study is to elucidate the effects of polymer waste addition on the compressive strength and water absorption at a low curing temperature of geopolymer ceramics.
2. Materials and Methods
Kaolinite clay (RC and RK) was sourced from two different locations in Ushafa-Abuja, Nigeria. RC reddish was brown and RK was yellowish white in colour (Figure 1a,b). Metaclay or metakaolin, represented by MC and MK (Figure 1a,b), respectively, was formed by heat treatment of kaolinite clay at 750 °C for 3 h. Used polyethylene sachets were collected and processed by dissolving in kerosene and re-precipitated into particles (Figure 1c). Sodium silicate (water glass) was formed by adding 200 g of sodium hydroxide and 300 g of silica gel in 500 mL distilled water (Figure 1d). Kerosene was obtained from the Niger Delta Development Commission (NDDC) Abuja. Muslin cloth and sodium hydroxide (98% analytical grade) were obtained from Finlab Nigeria Limited, Nigeria. The geopolymer ceramics was cured at temperatures of 30 °C and 50 °C for both MC and MK.

Figure 1.
(a) Metaclay (MC) (b) Metakaolin (MK) (c) LDPE powder (d) Beaker with water glass.
2.1. Geopolymer Ceramics Composite
LDPE: aluminosilicate powder (MC or MK) ratio with LDPE volume fraction 5%, 10% and 15% was measured and dry mixed for 7 to 10 min for homogeneity before the addition of geopolymer activator. A total of 8 M sodium hydroxide (NaOH) was also prepared using a 98% pure Sodium pellet and distilled water. The geopolymer activator was then obtained by measuring 2.5 molar ratio of Na2SiO3/NaOH. The geopolymer activator was then added and further mixed mechanically for 20–25 min. The mixtures were poured in a mold with dimension shape of 50 mm × 50 mm × 10 mm. A vibrator was used to ensure proper molding and to reduce pores formed by air bubbles. The geopolymer ceramics were then cured at temperatures 30 °C and 50 °C in an oven for 24 h (Figure 2). The compressive strength and water absorption were measured. The geopolymer ceramics from MC was named MCGC and the geopolymer ceramics from MK was named MKGC.

Figure 2.
Geopolymer ceramics cured in oven.
2.2. Compressive Strength
The compressive strengths of geopolymer ceramics were measured with a Universal Mechanical Testing Machine series model 2810 (Figure 3) after 7 days of casting. Low curing temperatures of 30 °C and 50 °C for 24 h were chosen to reduce energy cost and to prevent initial cracks during water evaporation in the curing process [42].

Figure 3.
Compressive strength test.
For each ceramic sample, replicates of five tests were done to obtain the average compressive strength and standard deviation. The compressive strength (σ) was determined by using the formula
where
- σ is the compressive strength (MPa),
- F is the force (N) applied and A (mm2) is the area of sample.
2.3. Water Absorption (WA)
The average percent water absorption was calculated for each geopolymer sample, using the formula in Equation (2), according to the EN ISO 10545 standard
where
- M1 is mass of the dry sample, in (g)
- M2 is mass of the wet sample, in (g)
The ceramic samples were classified according to their water absorption capacity using EN ISO 10545 standard [43]. For example, if the water absorption (WA) capacity was 0% ≤ WA ≤ 0.5%, then the ceramic sample was classified as “Low water absorption”, and if the water absorption was 0.5% < WA ≤ 10.0% then the ceramic sample was classified as “Medium water absorption”. The ceramic sample was classified as “High water absorption” if WA> 10%.
2.4. Characterization
Kaolinite clay, metakaolin, LDPE and geopolymer ceramic samples were characterized using a Carl Zeiss EVO/LS10 Scanning Electron Microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (EDX). The chemical composition of both sources metaclay (MC) and metakaolin (MK) were characterized using a thermo scientific X-ray (XRF) epsilon spectrometer. The functional groups of geopolymer ceramics were characterized using a FT-IR Nicolet IS5 269-263200. X-ray diffraction was obtained using a thermo scientific model: ARL’XTRA X-ray diffractometer. The measurements were done using Cu Kα radiation at 40 mA and 45 kV.
3. Result and Discussion
3.1. Chemical Analysis: X-Ray Fluorescence Spectroscopy
Thermo Scientific X-ray (XRF) Epsilon Spectrometer with a reference material of Montana soil SRM 2710 was used to determine the chemical composition of both metaclay (MC) and metakaolin (MK). Clay samples were ground into fine powder with mortar and pestle and 2 g of each of the samples transferred to the sample holder. Samples were held for 10 min in order to get rid of oxygen and moisture. The method was calibrated using geological calibration of oxides. Sample measurements were ran in the XRF spectrometer for 10 min for each result (Table 1). The metaoxides composition predicts the compressive strength of geopolymers [39]. The percentage of SiO2/Al2O3 determines the compressive strength of geopolymer ceramics. MK has a lower SiO2/Al2O3 ratio than MC, hence a lower compressive strength was expected in MKGC compared to MCGC (Table 1). The CaO and Fe2O3 percentage in the XRF composition of Mk is less than that of MC chemical composition. In a study by Reddy et al. (2016), metal oxides having composition, SiO2 = 50.5%, Al2O3 = 26.57%, CaO + Fe2O3 = 15.9% showed higher compressive strength compared to metal oxides with composition, SiO2 = 65.77%, Al2O3 = 26.72%, CaO + Fe2O3 = 1.38% of source material [39].

Table 1.
XRF composition of MC and MK.
3.2. Effect of LDPE on Microstructure of Geopolymer Ceramics
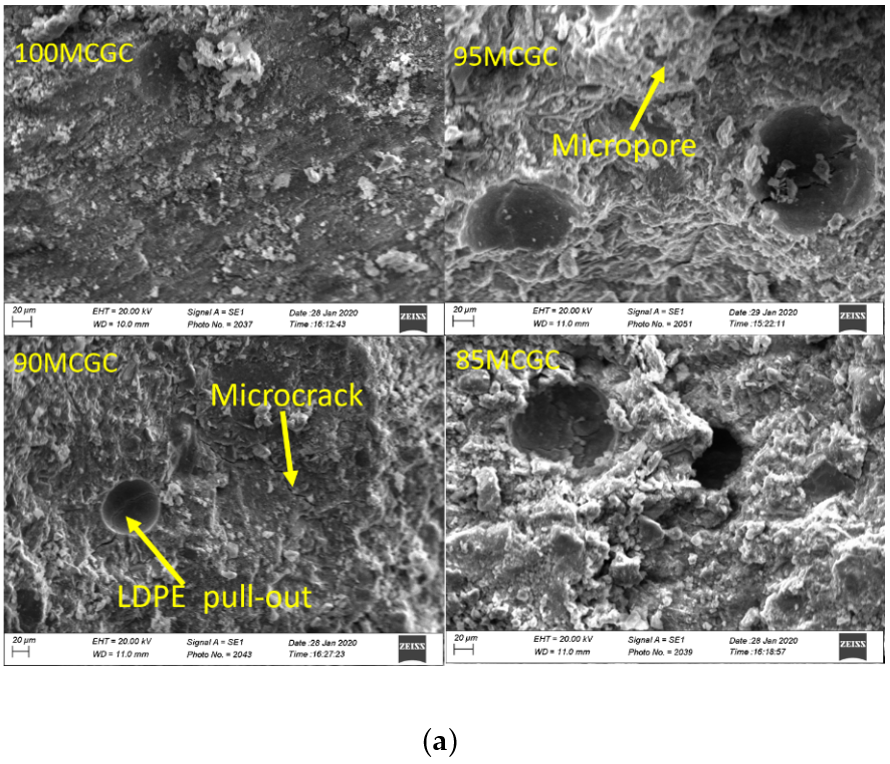
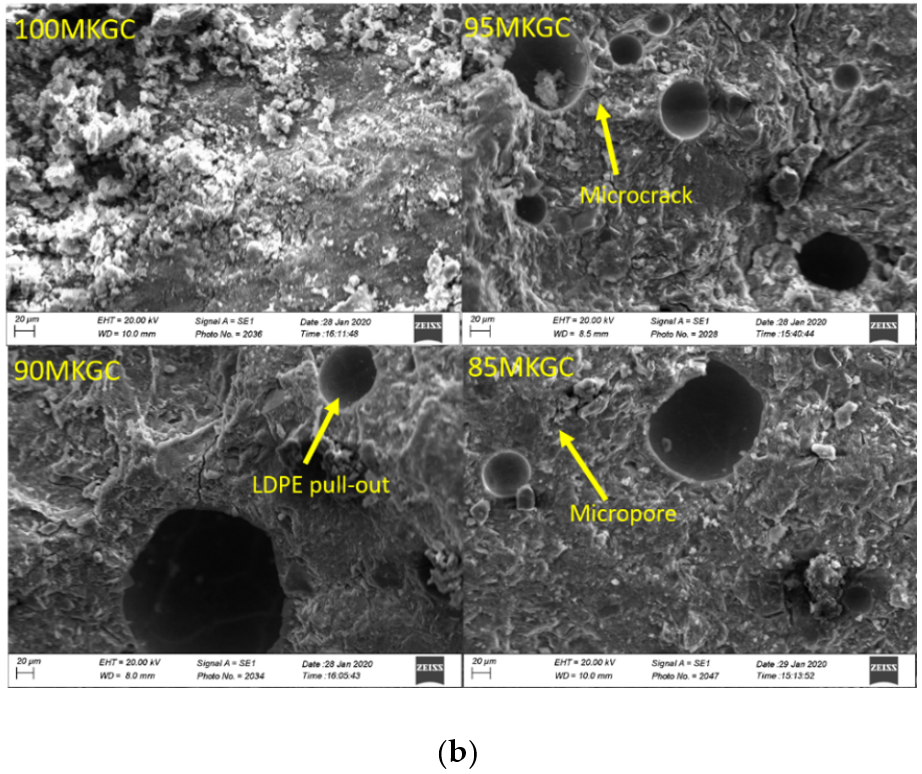
Figure 4a,b is the SEM micrograph of geopolymer ceramics samples. The Aluminum sample holders were coated with G303 colloidal graphite and the samples were coated with gold to prevent sample charge. Aluminum and silicate were the main morphological features on the SEM-EDX of geopolymer ceramics (Figure 5a,b) and agrees with the chemical composition result in XRF. SEM micrograph analysis of surface of the geopolymer ceramics samples showed evidence of microcracks, due to the pull-out of undissolved LDPE particles from composite samples (Figure 4a,b). The pull-out and undissolved LDPE particles (Figure 4b) supported the fact that particles did not take part in the geopolymerization reaction.
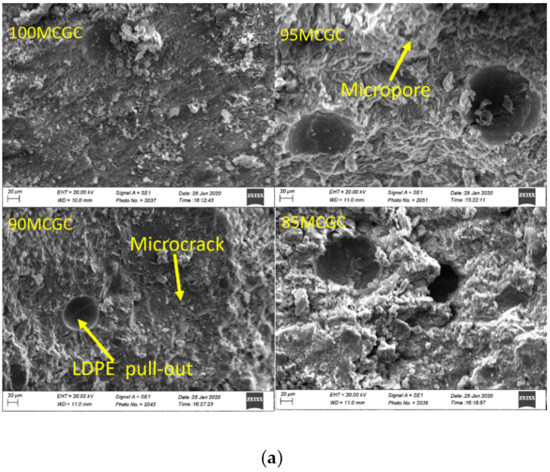
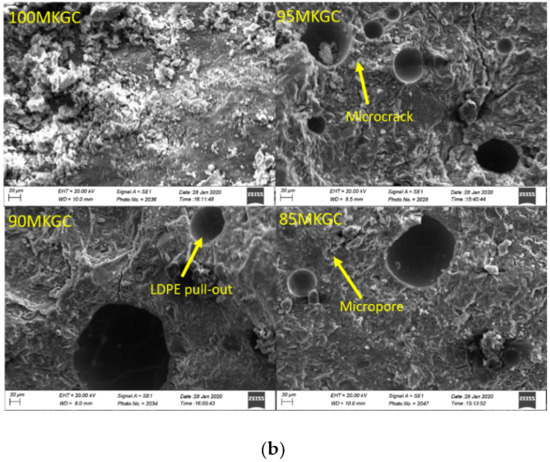
Figure 4.
(a) SEM micrographs of 100MCGC, 95MCGC, 90MCGC and 85MCGC. (b) SEM micrographs of 100MKGC, 95MKGC, 90MKGC and 85MKGC.
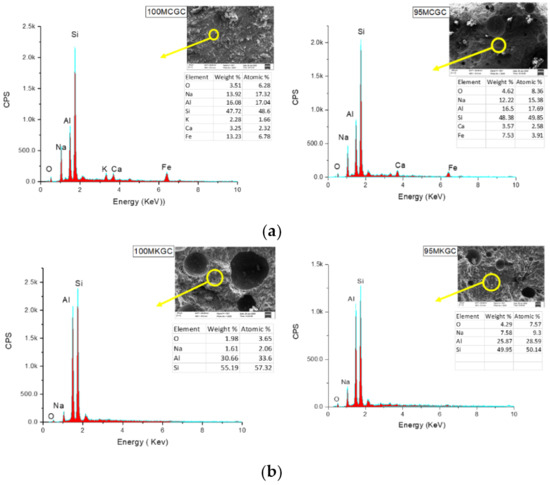
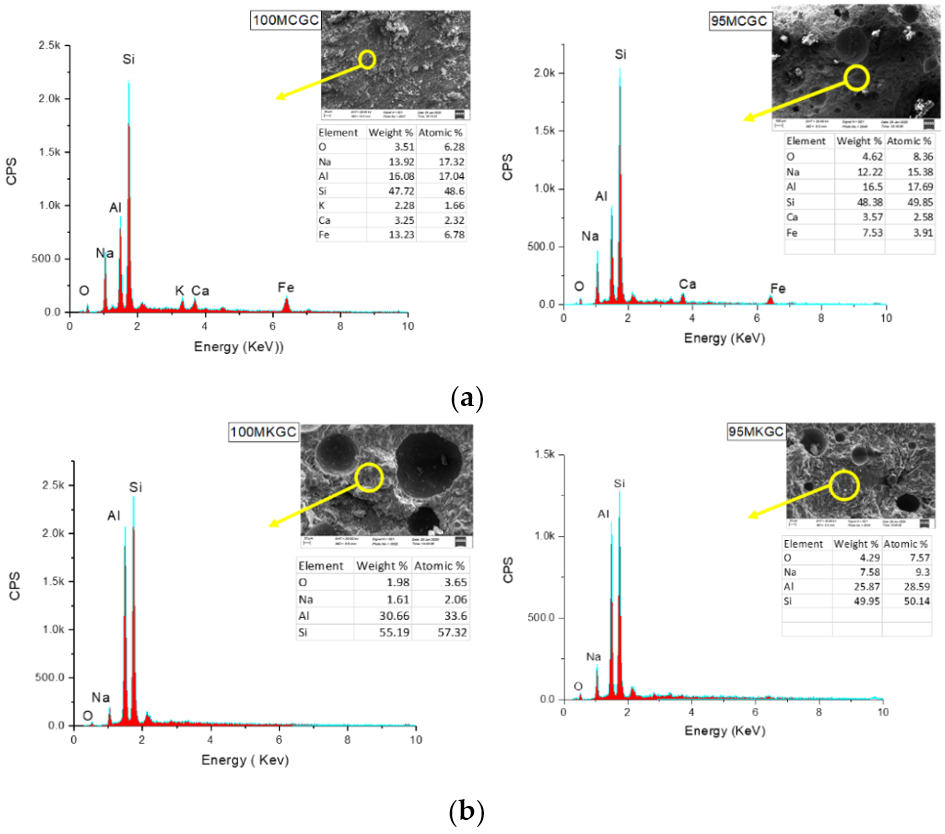
Figure 5.
(a) EDX micrographs of 100MCGC and 95MCGC. (b) EDX micrographs of 100MKGC and 95MKGC.
Figure 5a,b showed EDS micrographs of geopolymer ceramics representing the presence of alumina, silica, sodium oxide and calcium oxide. The percentage of iron oxide in the MCGC geopolymer (Figure 5a) compared to the MKGC geopolymer (Figure 5b), caused the red coloration (Figure 1a). The microcracks were arrested at the LDPE/geopolymer interface, suggesting improved toughness, with high compressive strength. The SiO2 and Al203 composition in Figure 5a,b is in agreement with the XRF results. The absence of CaO and Fe2O3 in MK EDS (Figure 5b) depicts a lower compressive strength of MKGC compared to MCGC.
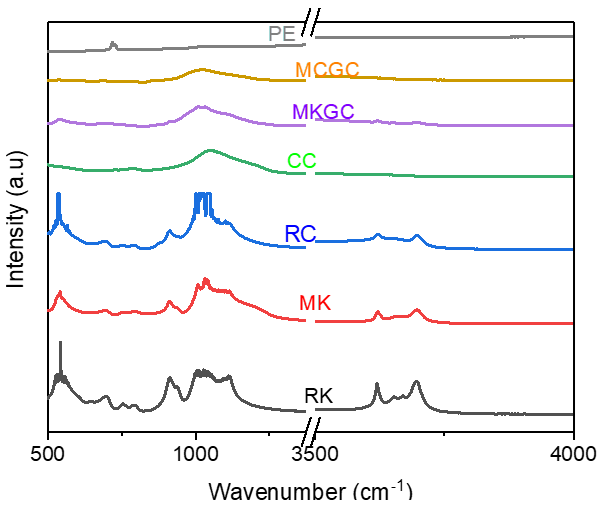
3.3. FTIR Spectra
FT-IR spectra were obtained for RC and RK before, and after calcination for MC and MK. Further, FT-IR spectra was obtained for the geopolymer ceramics (MCGC and MKGC). The measurements were obtained between 500 cm−1 and 4000 cm−1 (Figure 6). The spectrum of the raw clay (RC) and raw kaolin (RK) had bands mostly between 550 cm−1 and 1200 cm−1, and between 3600 cm−1 and 3750 cm−1, corresponding to the stretching of O-H bonds and H-O-H bonds’ vibrations [44]. The small bands between 700 cm−1 and 900 cm−1 correspond to octahedral Al-O bonds present in raw clay and calcined clay. This is associated to the increase in the aluminum quantity during curing [45]. Slight changes in IR spectra bands were found in the calcined clay (MC) and calcined kaolin (MK), corresponding to bands at 560 cm−1 and 1050 cm−1 attributed to plane and bending vibrations of Al-O/Si-O, respectively [46]. Asymmetric stretching of Si-O or Al-O between 900 cm−1 and 1100 cm−1 shows the amorphous nature of geopolymer ceramic (MCGC and MKGC) and absorbed water molecules during geopolymerization. The variation assigned to (Si, Al)–O–Si structures can be attributed to the addition of alkali solution and earth metals [44], which enhances reactivity during geopolymerization and increases the strength of the composite [38,47,48].
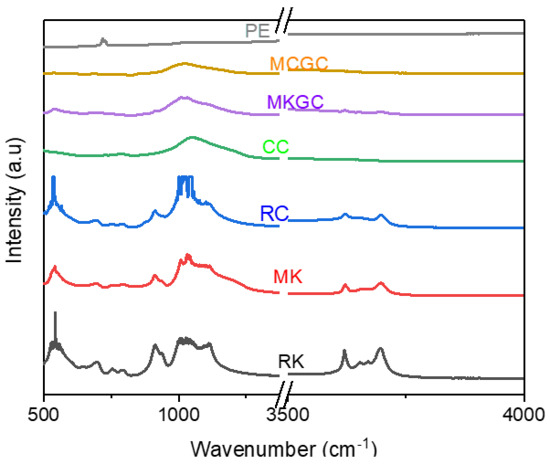
Figure 6.
FTIR spectra analysis of geopolymer ceramics.
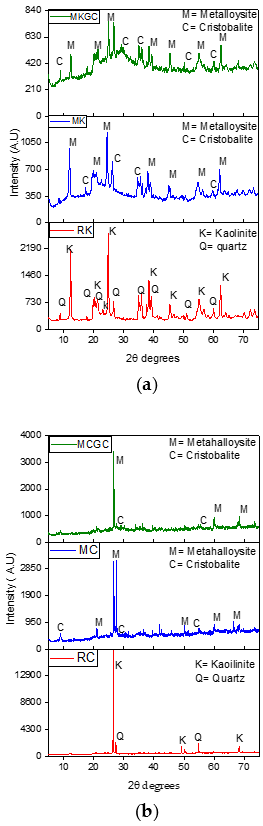
3.4. X-Ray Diffraction
Figure 7a,b show x-ray diffraction patterns of raw kaolinite clay (RC and RK), metaclay (MC) and metakaolin (MK) and metakaolin geopolymer ceramics (MCGC and MKGC). The X-ray pattern of the raw kaolin shows kaolinitic and quartz amorphous phases [49]. Characteristic kaolin peaks at 2θ values of 12.5°, 24.9°, and 27.1° had high intensities but decreased after calcination and geopolymerization (Figure 7a). Similarly, characteristic high-intensity kaolin peaks at 2θ = 27.1° were decreased after geopolymerization (Figure 7b), which suggests that the amorphous kaolinitic phase is the main constituent after geopolymerization [50,51,52].
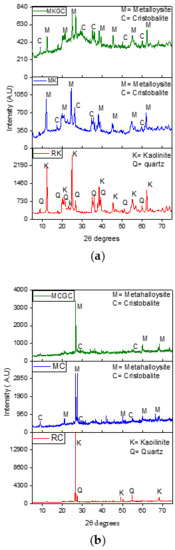
Figure 7.
(a) X-ray diffraction patterns of raw kaolin (RK), Metakaolin (MK) and Metakaolin geopolymer ceramics (MKGC). (b) X-ray diffraction patterns of raw clay (RC), Metaclay (MC) and Metaclay geopolymer ceramics (MCGC).
3.5. Effect of LDPE particles on Compressive Strength
The effect of the fraction of LDPE particles on compressive strength is presented in Table 2 and Table 3. MC and MK formed with no LDPE addition was used as a control. Geopolymer ceramic samples were cured at 30 °C and 50 °C. Clay and LDPE powder mixture was derived from the control sample, as calculated in Equation (1)
where

Table 2.
Effect of LDPE/MC geopolymer composite on compressive strength for composites formed using 0.6 mm and 1.18 mm LDPE particle size.

Table 3.
Effect of LDPE/MK geopolymer matrix ratio on compressive strength.
- ∆σ is a measure of the effect of LDPE addition to compressive strength
- σo is Compressive strength of geopolymer/LDPE composite
- σoo is Compressive strength of geopolymer or control sample
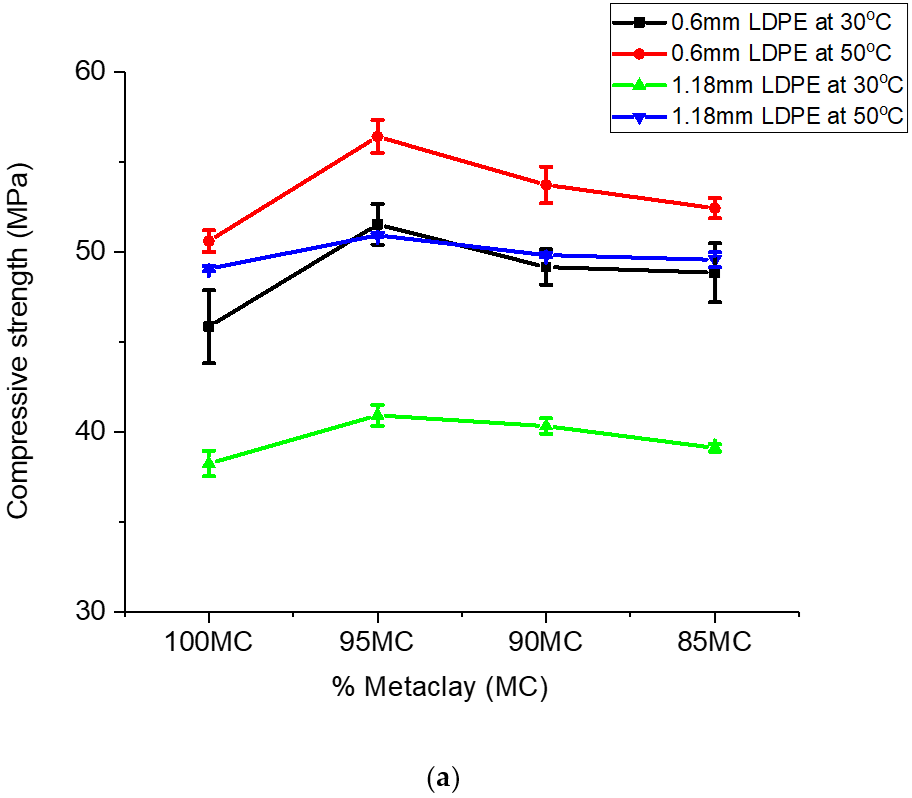
From Table 2, the compressive strengths were generally higher with the addition of LDPE particles to metaclay (MC). For composites formed with 0.6 mm-sized LDPE particles (95MCGC) and cured at 30 °C, the variation in compressive strength was 5.65 MPa higher than the control. Similarly, strength improvement was observed for geopolymers cured at 50 °C. This was the highest strength increase observed as compared to the strength increase observed for the 10% and 15% LDPE addition cured at 30 °C. Composites formed with 5% of 0.6 mm-sized LDPE addition and cured at 50 °C. Five percentage LDPE addition increased the compressive strength of the ceramics to 5.81 MPa. In both composites formed at 30 °C and 50 °C, the highest gains in LDPE/geopolymer composite strength were observed for the 5% LDPE addition.
The addition of 1.18 mm-sized LDPE also increased the compressive strength of the ceramics. Similar high gains in compressive strength made for the 5% 0.6 mm LDPE particles were observed for the 5% LDPE/geopolymer composites formed with the 1.18-mm-sized LDPE particles.
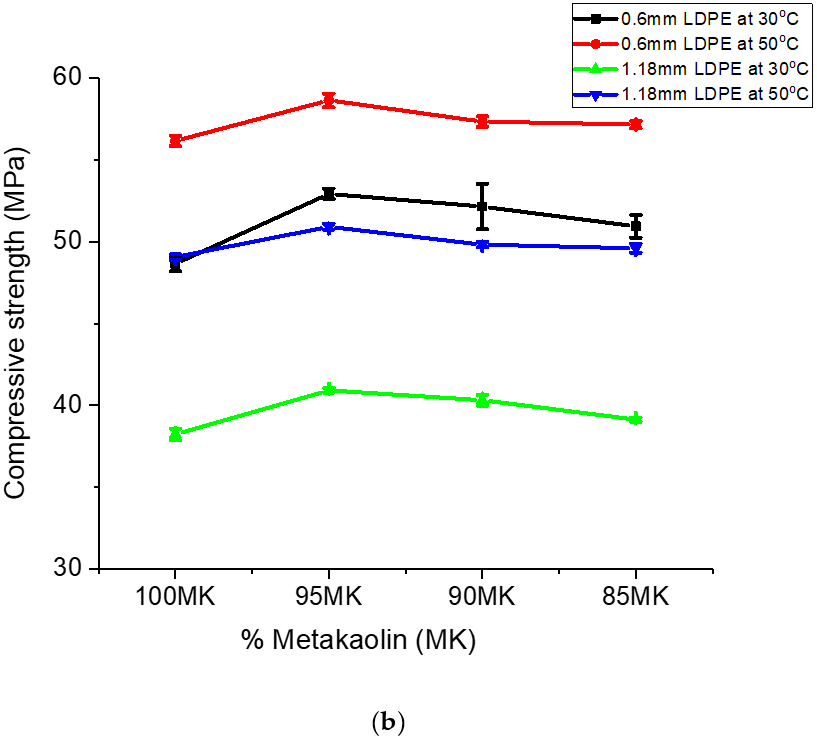
The trend in the gains in the variation in compressive strength observed for the metakaclay (Table 2) was similar to that of metakaolin (Table 3). The addition of LDPE particles to metakaolin slightly increased the compressive strength. Powder with 0.6 mm particle size and 5% LDPE contents had gains in compressive strength variation of 4.26 MPa and 2.47 MPa for geopolymer/LDPE composites cured at temperatures 30 ℃ and 50 °C, respectively. Analogous results were observed for composites formed with 5% LDPE fraction and 1.18 mm LDPE powder particle size. In summary, the data presented so far showed that the inclusion of LDPE particles in the geopolymer composites slightly improved the compressive strength of the geopolymer ceramics. A graph of compressive strength versus percent metaclay (MC) or percent metakaolin (MK) is shown in Figure 8a,b.
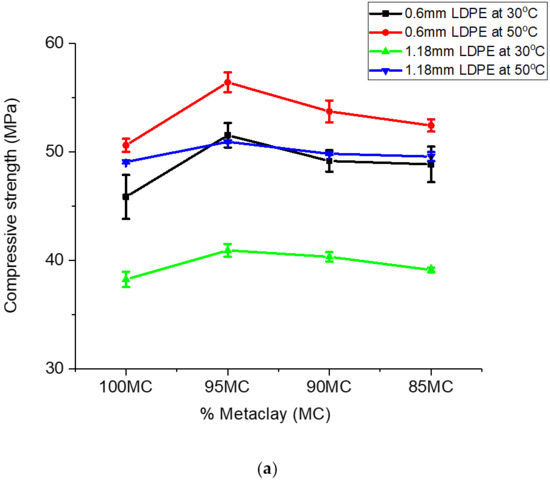
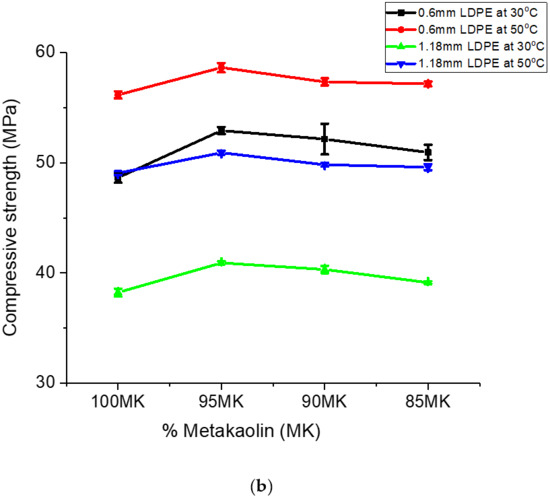
Figure 8.
(a) A plot of compressive strength versus % MC. (b) A plot of compressive strength versus % MK.
Compressive strength was found to depend on the geopolymer material, the particle size of the reinforcement material and the composition of the geopolymer [42]. The compressive strength of the geopolymer/LDPE composite formed with 0.6 mm particle size was higher than that of 1.18 mm particle size. This was true for geopolymer/LDPE composites cured at 30 °C and 50 °C (Figure 8a,b). The higher compressive strength of 0.6 mm LDPE addition is the result of smaller LDPE particles, which fill the interstitial site and absorb the stress for a longer time before crack propagation occurs. The 5% LDPE showed the highest strength for both 0.6 mm and 1.18 mm particle size; this could be due to the fact that the 5% LDPE addition spread through the geopolymer ceramics matrix while higher LDPE percentage addition (10% and 15%) may cause clusters in the matrix, and hence lower compressive strength. The compressive strength of geopolymer ceramics from metaclay (MCGC) was higher than that of geopolymer ceramics from metakaolin (MKGC). The higher percentage of CaO and Fe2O3 in MC (10.1% CaO/Fe2O3) resulted in higher compressive strength (Figure 8a). This result is in agreement with the study by Reddy al. (2016) who evaluated the role of CaO and Fe2O3 on the compressive strength of geopolymer concrete [39]. SiO2/Al2O3 ratio contributed to the increase in compressive strength. The presence of more Si-O-Si and Al-O bonds, as seen from FTIR, caused an increase in the geopolymerization reaction. A higher Si02/Al2O3 ratio in MC suggests higher Si-O-Si stronger bonds than Si-O-Al bonds in MK (Figure 8b), and hence higher compressive strength [49,53]. A higher curing temperature also affects the compressive strength of geopolymer ceramics. The curing temperature at 50 °C for both 0.6 mm and 1.18 mm LDPE was higher than curing temperature of 30 °C for both 0.6 mm and 1.18 mm LDPE addition. The increase in compressive strength at a higher curing temperature is due to the increase in geopolymerization reaction at a higher curing temperature [44].
Figure 8b is a plot of compressive strength of geopolymer ceramics formed with metakaolin. Similar to the observation made for MC samples, the addition of LDPE particles increased the compressive strength of the geopolymer ceramic. The largest increase was observed for 5% LDPE powder; an LDPE percentage greater than 5 weight percent decreased the compressive strength. Similar to the observation made for the MC samples, MK compressive strength corresponding to LDPE particle size 0.6 mm was higher compared to that of 1.18 mm. These results are in agreement with previous work [54]. Research carried out on the El-Hiswa kaolinite deposits showed a compressive strength of 44.4 MPa with 16% NaOH at 80 °C for 14 h [55]. The measured compressive strength (56.8 MPa) for geopolymer ceramics formed with NaOH, sodium silicate/NaOH after being cured at 60 °C for 24 h in this work was higher than that of Rahier et al. (2011). The higher compressive strength in his study was due to of LDPE particles added to the geopolymer.
The compressive strength of LDPE in the geopolymer ceramics can be estimated by the rule of mixture from Equation (4)
where
- is the composite strength;
- is the matrix volume fraction;
- is the matrix strength;
- is particle volume fraction;
- is the particle strength.
An example of the rule of mixture from the 95MC and 100MC of 0.6 mm at 50 °C to calculate the compressive strength of LLDPE from Equation (5) is given as follows
The compressive strength of LDPE in the composite is higher than compressive strength of the control sample 100MC. Hence, LDPE is a suitable material to reinforce geopolymer ceramics.
3.6. Water Absorption Calculation
Table 4 summarizes the water absorption study of geopolymer ceramics. Water absorption was highest for the control samples 100MCGC and 100MKGC, and water absorption decreased as LDPE content was increased. C85 and K85 had the lowest water absorption. This could be due to the hydrophobic nature of the LDPE polymer, which absorbs very little water for both particle sizes, 0.6 mm and 1.18 mm LDPE. The water absorption was higher at 30 °C compared to 50 °C as a result of lower curing temperatures in the geopolymer ceramics at 30 °C.

Table 4.
Water absorption (WA) of geopolymer ceramics.
The results of WA in this study were compared to that of Tabassum and Khadwal, (2015) that formed geopolymer using a low-calcium fly ash and alkaline solution of 8 M NaOH and Na2SiO3 [26]. The NaOH/Na2SiO3 molar ratio was 2.5 and water absorption was 3.0% and consistent with the studies reported in this work. In summary, all the geopolymer ceramics had water absorption capacity in the range 0.5% < WA ≤ 10.0%, which falls under medium water absorption of ceramics.
4. Conclusions
Alkaline-based geopolymers were formed with metaclay and metakaolin alumina-silicates. Metakaclay derived geopolymers were found to have a higher compressive strength. XRF, EDS and FTIR results suggest the higher SiO2/Al2O3 ratio and the presence of CaO and Fe2O3, in the metaclay geopolymer ceramics improved its compressive strength compared to the metakaolin derived geopolymer. The effect of addition of low-density polyethylene (LDPE) on the compressive strength and water absorption of a metaclay and metakaolin geopolymer matrix composites were investigated. The addition of LDPE particles slightly increased the compressive strength of the geopolymer ceramics. SEM fractography showed microcracks in the LDPE geopolymer composite. The microcracks were arrested at the LDPE/geopolymer interface, resulting in improved toughness, and high compressive strength. The smaller particle size of LDPE 0.6 mm increased the compressive strength of the ceramic to a higher extent compared to the larger particle size 1.18 mm. The highest compressive strength was observed for 95MCGC at a curing temperature of 50 °C due to good LDPE particle dispersion in the geopolymer matrix. Higher LDPE percentages caused LDPE to cluster in the composite, with consequent strength reduction. The contribution by LDPE to the compressive strength of geopolymer ceramics is attributed to the proper distribution of LDPE in the geopolymer matrix, particle size and curing temperature. A five percent LDPE addition was found to be the optimum for geopolymer compressive strength improvement. The water adsorption decreased with LDPE addition. The decrease in water absorption was directly related to the increase in LDPE percentage addition. According to the EN ISO 10545 classification, and the water absorption results, the LDPE reinforced clay geopolymer ceramics is classified as medium water absorption. The results were consistent with the averaged water absorption of 3.0%.
Author Contributions
The authors contributed to the following. Conceptualization N.L.B. and E.E.B.; methodology, N.L.B.; software, J.B.H.; validation, J.B.H., A.A.M. and N.L.B.; formal analysis, E.E.B.; investigation, A.A.M.; J.B.H. resources P.A.O.; data curation, A.A.M.; writing—original draft preparation, E.E.B.; writing—review and editing, A.A.M.; visualization, N.L.B.; supervision, E.E.B.; project administration, P.A.O.; funding acquisition, P.A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the African Development Bank (AfDB), grant number (NMI-AIST 2100155032824) and by the Pan African Materials Institute under the World Bank African Centers of Excellence (ACE) program at the African University of Science and Technology (AUST), Abuja, Nigeria grant number (PAMI/2015/5415-NG).
Institutional Review Board Statement
Not applicable. This study did not involve humans or animals.
Data Availability Statement
The data presented in this study are available on request from the corresponding author after obtaining permission of authorized person.
Conflicts of Interest
The authors declare no conflicts of interest with respect to the research, figures and authorship of this study.
References
- Ameer, Z.A.; Ehood, H.S. Preparation and properties of Biodegradable Blend from LDPE/PVA Mixture. Aust. J. Basic Appl. Sci. 2016, 10, 102–111. [Google Scholar]
- Verma, R.; Vinoda, K.; Papireddy, M.; Gowda, A. Toxic pollutants from plastic waste-a review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Ouiminga, S.K.; Rogaume, T.; Daho, T.; Yonli, A.H.; Koulidiati, J. Reductive and oxidative combustion of polyethylene bags: Characterization of carbonaceous and nitrogenous species. J. Anal. Appl. Pyrolysis 2012, 98, 72–78. [Google Scholar] [CrossRef]
- Kumari, K.; Kumar, S.; Rajagopal, V.; Khare, A.; Kumar, R. Emission from open burning of municipal solid waste in India. Environ. Technol. 2019, 40, 2201–2214. [Google Scholar] [CrossRef]
- Klemeš, J.J.; Fan, Y.V.; Tan, R.R.; Jiang, P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew. Sustain. Energy Rev. 2020, 127, 109883. [Google Scholar] [CrossRef]
- Edoga, M.; Onyeji, L.; Oguntosin, O. Achieving vision 20: 2020 through waste produce candle. J. Eng. Appl. Sci. 2008, 3, 642–646. [Google Scholar]
- Azeko, S.T.; Mustapha, K.; Annan, E.; Odusanya, O.S.; Soboyejo, W.O. Recycling of polyethylene into strong and tough earth-based composite building materials. J. Mater. Civ. Eng. 2016, 28, 04015104. [Google Scholar] [CrossRef]
- Galvão, J.C.A.; Portella, K.F.; Joukoski, A.; Mendes, R.; Ferreira, E.S. Use of waste polymers in concrete for repair of dam hydraulic surfaces. Constr. Build. Mater. 2011, 25, 1049–1055. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Ghadir, P.; Ranjbar, N. Clayey soil stabilization using geopolymer and Portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar] [CrossRef]
- Le Chi, H.; Louda, P.; Periyasamy, A.P.; Bakalova, T.; Kovacic, V. Flexural behavior of carbon textile-reinforced geopolymer composite thin plate. Fibers 2018, 6, 87. [Google Scholar] [CrossRef]
- Hassan, A.; Ismail, N.; Mourad, A.-H.I.; Rashid, Y.; Laghari, M.S. Preparation and characterization of expanded clay-paraffin wax-geo-polymer composite material. Materials 2018, 11, 2191. [Google Scholar] [CrossRef]
- Korniejenko, K.; Lin, W.-T.; Šimonová, H. Mechanical properties of short polymer fiber-reinforced geopolymer composites. J. Compos. Sci. 2020, 4, 128. [Google Scholar] [CrossRef]
- Hájková, P. Kaolinite claystone-based geopolymer materials: Effect of chemical composition and curing conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, Y.; Mu, Q. Preparation and microstructure of fly ash geopolymer paste backfill material. J. Clean. Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2015. [Google Scholar]
- Safari, Z.; Kurda, R.; Al-Hadad, B.; Mahmood, F.; Tapan, M. Mechanical characteristics of pumice-based geopolymer paste. Resour. Conserv. Recycl. 2020, 162, 105055. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa AlBakri, A.M.; Binhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Effect of curing profile on kaolin-based geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef]
- Korniejenko, K.; Tyliszczak, B.; Łach, M.; Mikuła, J.; Hebdowska-Krupa, M.; Mierzwiński, D. Organic Polymers Reinforced Inorganic Polymers-An Overview. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 416, p. 012090. [Google Scholar]
- Perera, D.; Uchida, O.; Vance, E.; Finnie, K. Influence of curing schedule on the integrity of geopolymers. J. Mater. Sci. 2007, 42, 3099–3106. [Google Scholar] [CrossRef]
- Aredes, F.; Campos, T.; Machado, J.; Sakane, K.; Thim, G.; Brunelli, D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Carreño-Gallardo, C.; Tejeda-Ochoa, A.; Perez-Ordonez, O.I.; Ledezma-Sillas, J.E.; Lardizabal-Gutierrez, D.; Prieto-Gomez, C.; Valenzuela-Grado, J.A.; Hernandez, F.C.R.; Herrera-Ramirez, J.M. In the CO2 emission remediation by means of alternative geopolymers as substitutes for cements. J. Environ. Chem. Eng. 2018, 6, 4878–4884. [Google Scholar] [CrossRef]
- Lopes, A.V.; Lopes, S.M.; Pinto, I. Influence of the Composition of the Activator on Mechanical Characteristics of a Geopolymer. Appl. Sci. 2020, 10, 3349. [Google Scholar] [CrossRef]
- Samarakoon, M.; Ranjith, P.; Duan, W.H.; de Silva, V. Properties of one-part fly ash/slag-based binders activated by thermally-treated waste glass/NaOH blends: A comparative study. Cem. Concr. Compos. 2020, 112, 103679. [Google Scholar] [CrossRef]
- Rao, F.; Liu, Q. Geopolymerization and its potential application in mine tailings consolidation: A review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, C.; Duan, P.; Liu, Y.; Zhang, Z.; Qiu, X.; Li, D. A comparative study of high-and low-Al2O3 fly ash based-geopolymers: The role of mix proportion factors and curing temperature. Mater. Des. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash–metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Shriram, H.; Mohitkar, V.; Ravi, K. Effect of metakaolin on fresh and hardened propertios of self compacting concrete. Int. J. Civ. Eng. Technol. 2014, 5, 137–145. [Google Scholar]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Assaedi, H.; Shaikh, F.; Low, I.M. Effect of nano-clay on mechanical and thermal properties of geopolymer. J. Asian Ceram. Soc. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Sakulich, A.R. Reinforced geopolymer composites for enhanced material greenness and durability. Sustain. Cities Soc. 2011, 1, 195–210. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; MohdSam, A.R.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Ding, Y.; Bai, Y.-L. Fracture properties and softening curves of steel fiber-reinforced slag-based geopolymer mortar and concrete. Materials 2018, 11, 1445. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 2016, 234, 12–23. [Google Scholar] [CrossRef]
- Islam, M.J.; Meherier, M.S.; Islam, A.R. Effects of waste PET as coarse aggregate on the fresh and harden properties of concrete. Constr. Build. Mater. 2016, 125, 946–951. [Google Scholar] [CrossRef]
- Taaffe, J.; O’Sullivan, S.; Rahman, M.E.; Pakrashi, V. Experimental characterisation of Polyethylene Terephthalate (PET) bottle Eco-bricks. Mater. Des. 2014, 60, 50–56. [Google Scholar] [CrossRef]
- Amari, S.; Darestani, M.; Millar, G.J.; Rintoul, L.; Samali, B. Microchemistry and microstructure of sustainable mined zeolite-geopolymer. J. Clean. Prod. 2019, 234, 1165–1177. [Google Scholar] [CrossRef]
- Vieira, A.W.; de Mello Innocentini, M.D.; Mendes, E.; Gomes, T.; Demarch, A.; Montedo, O.R.K.; Angioletto, E. Comparison of methods for determining the water absorption of glazed porcelain stoneware ceramic tiles. Mater. Res. 2017, 20, 637–643. [Google Scholar] [CrossRef]
- Prasanphan, S.; Wannagon, A.; Kobayashi, T.; Jiemsirilers, S. Reaction mechanisms of calcined kaolin processing waste-based geopolymers in the presence of low alkali activator solution. Constr. Build. Mater. 2019, 221, 409–420. [Google Scholar] [CrossRef]
- Aseniero, J.P.J.; Opiso, E.M.; Banda, M.H.T.; Tabelin, C.B. Potential utilization of artisanal gold-mine tailings as geopolymeric source material: Preliminary investigation. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2018, 172, 2892–2898. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Merabtene, M.; Kacimi, L.; Clastres, P. Elaboration of geopolymer binders from poor kaolin and dam sludge waste. Heliyon 2019, 5, e01938. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Sun, W.; Li, Z.; Liu, Z. Preparation of metakaolin based geopolymer and its three-dimensional pore structural characterization. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 550–555. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Bazan, P.; Kozub, B.; Łach, M.; Korniejenko, K. Evaluation of Hybrid Melamine and Steel Fiber Reinforced Geopolymers Composites. Materials 2020, 13, 5548. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.; Lin, K.L.; Cheng, T.W.; Zhang, B.X. The influence of sapphire substrate silicon carbide sludge on structural properties of metakaolin-based geopolymers. Environ. Prog. Sustain. Energy 2020, 39, 13305. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. Mater. J. 2004, 101, 467–472. [Google Scholar]
- Rahier, H.; Esaifan, M.; Wastiels, J. Geopolymer products from Jordan for sustainability of the environment. Adv. Mater. Sci. Environ. Nucl. Technol. II 2011, 227, 289. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).