Biofilm Formation by Multidrug-Resistant Serotypes of Salmonella Isolated from Fresh Products: Effects of Nutritional and Environmental Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Susceptibility Testing
2.3. Determination of Morphotype
2.4. Development of Mono-Species Biofilms by Salmonella Serotypes
2.4.1. Polystyrene Biofilm Formation Assays
2.4.2. Conditions and Quantification of Biofilm Formation on Polypropylene Type B Surfaces
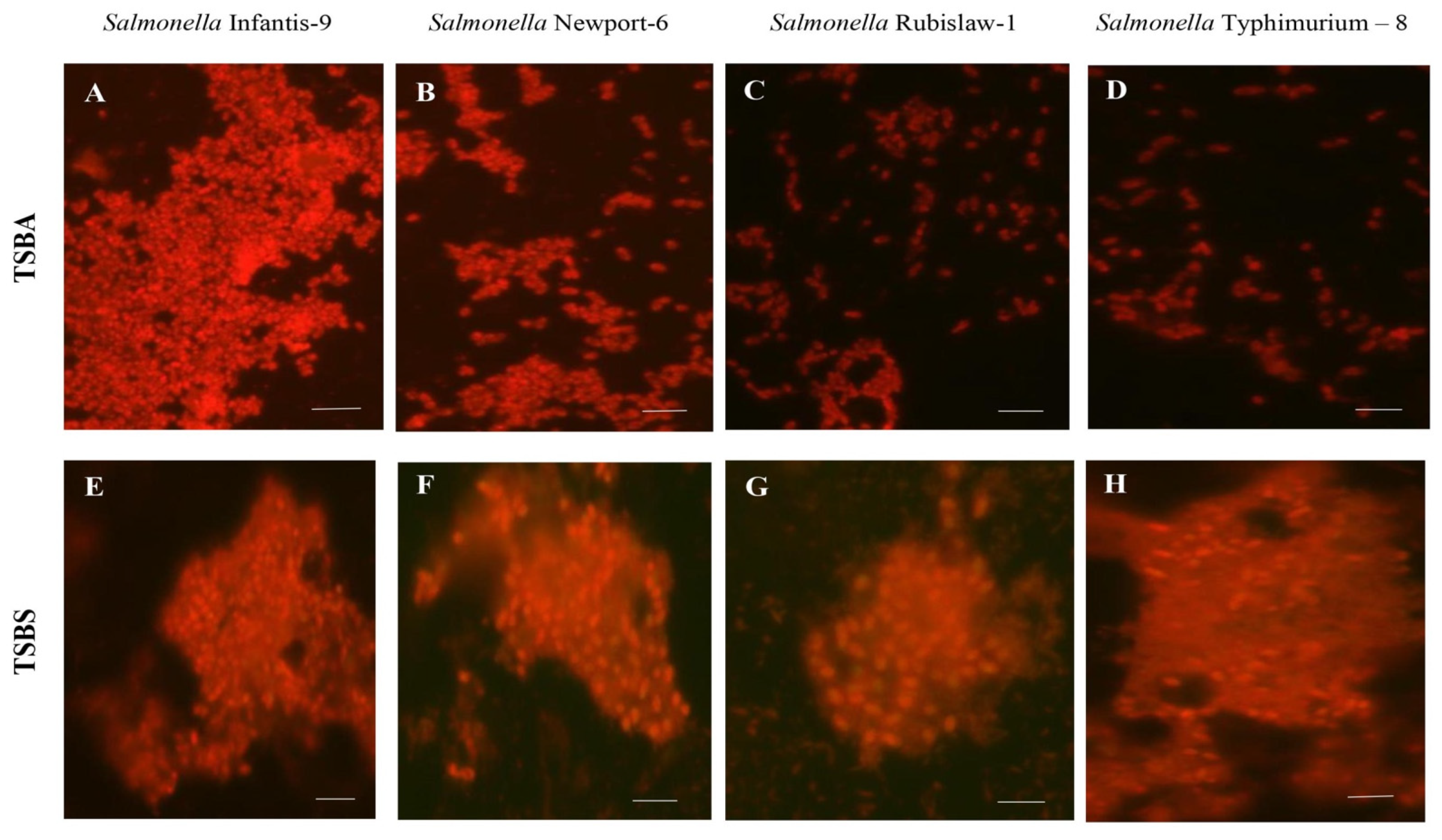
2.4.3. Epifluorescence Microscopy
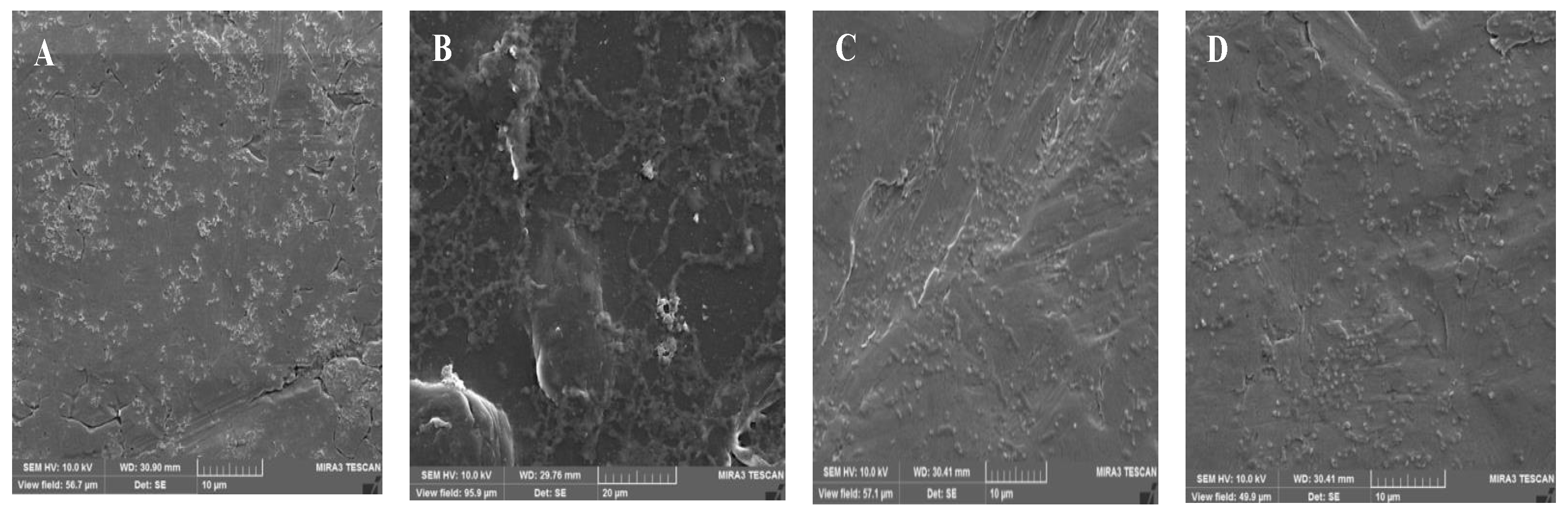
2.4.4. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results
3.1. Antimicrobial Susceptibility Testing
3.2. Ability to Form Mono-Species Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC (Centers for Disease Control and Prevention). Top 6 Salmonella Serotypes. 2019. Available online: https://www.cdc.gov/foodnet/reports/prelim-data-2019.html (accessed on 8 June 2020).
- CDC (Centers for Disease Control and Prevention). Reports of Salmonella Outbreak Investigations from 2006–2019. 2020. Available online: https://www.cdc.gov/salmonella/outbreaks.html (accessed on 8 June 2020).
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Dev-Kumar, G.; Williams, R.C.; Sriranganathan, N.; Boyer, R.R.; Eifert, J.D. Survival of tomato outbreak associated Salmonella serotypes in soil and water and the role of biofilms in abiotic surface attachment. Foodborne Pathog. Dis. 2018, 15, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- De Oliveira, D.C.V.; Fernandes Júnior, A.; Kaneno, R.; Silva, M.G.; Araújo Júnior, J.P.; Silva, N.C.C.; Rall, V.L.M. Ability of Salmonella spp. to produce biofilm is dependent on temperature and surface material. Foodborne Pathog. Dis. 2014, 11, 478–483. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 8 June 2020).
- Boyd, D.; Peters, G.A.; Cloeckaert, A.; Boumedine, K.S.; Chaslus-Dancla, E.; Imberechts, H.; Mulvey, M.R. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar typhimurium DT104 and its identification in phage type DT120 and serovar agona. J. Bacteriol. 2001, 183, 5725–5732. [Google Scholar] [CrossRef]
- Malcova, M.; Hradecka, H.; Karpiskova, R.; Rychlik, I. Biofilm formation in field strains of Salmonella enterica serovar Typhimurium: Identification of a new colony morphology type and the role of SGI1 in biofilm formation. Vet. Microbiol. 2008, 129, 360–366. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Scher, K.; Romling, U.; Yaron, S. Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 2005, 71, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- White, A.P.; Gibson, D.L.; Kim, W.; Kay, W.W.; Surette, M.G. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J. Bacteriol. 2006, 188, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Rouf, S.F.; Sun, L.; Cimdins, A.; Shafeeq, S.; Le Guyon, S.; Schottkowski, M.; Rhen, M.; Römling, U. BcsZ inhibits biofilm phenotypes and promotes virulence by blocking cellulose production in Salmonella enterica serovar Typhimurium. Microb. Cell Fact. 2016, 15, 177. [Google Scholar] [CrossRef]
- Kumar, G.D.; Micallef, S.A. Biofilms: A community based strategy for bacterial persistence and relevance to food safety. In Trends in Food Safety Protection; Raid, V.R., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 107–130. [Google Scholar]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Speranza, B.; Corbo, M.R.; Sinigaglia, M. Effects of Nutritional and Environmental Conditions on Salmonella sp. Biofilm Formation. J. Food Sci. 2011, 76, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Olmos, I. Evaluación de la Efectividad del Ácido Peracético y Ácido Láctico para la Remoción de Biopelículas de Salmonella spp. Provenientes de Productos Hortofructícolas. Bachelor’s Thesis, Centro Universitario de la Ciénega (CuCiénega), Universidad de Guadalajara, Ocotlán, México, 28 August 2020. [Google Scholar]
- Andrews, W.H.; Wang, H.; Jacobson, A.; Ge, B.; Zhang, G.; Hammack, T. Salmonella (BAM Chapter 5). In Bacteriological Analytical Manual (BAM); FDA: Rockville, MD, USA, 2021. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; CLSI Supplement M100S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Zogaj, X.; Bokranz, W.; Nimtz, M.; Römling, U. Production of Cellulose and Curli Fimbriae by Members of the Family. Infect. Immun. 2003, 71, 4151–4158. [Google Scholar] [CrossRef] [PubMed]
- Paz-Méndez, A.M.; Lamas, A.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Effect of Food Residues in Biofilm Formation on Stainless Steel and Polystyrene Surfaces by Salmonella enterica Strains Isolated from Poultry Houses. Foods 2017, 6, 106. [Google Scholar] [CrossRef]
- Turki, Y.; Mehri, I.; Ouzari, H.; Khessairi, A.; Hassen, A. Molecular typing, antibiotic resistance, virulence gene and biofilm formation of different Salmonella enterica serotypes. J. Gen. Appl. Microbiol. 2011, 60, 123–130. [Google Scholar] [CrossRef]
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; Rosas-García, M.L.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J. Microbiol. Immunol. Infect. 2020, in press. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z. Differential Staining of DNA and RNA in intact cells and isolated cell nucleic with acridine orange. Methods Cell Biol. 1999, 33, 285–298. [Google Scholar]
- Rossoni, E.M.; Gaylarde, C. Comparison of sodium hypochlorite and peracetic acid as sanitising agents for stainless steel food processing surfaces using epifluorescence microscopy. Int. J. Food Microbiol. 2000, 61, 81–85. [Google Scholar] [CrossRef]
- Borucki, M.K.; Peppin, J.D.; White, D.; Loge, F.; Call, D.R. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7336–7342. [Google Scholar] [CrossRef]
- Fratesi, S.E.; Lynch, F.L.; Kirkland, B.L.; Brown, L.R. Effects of SEM Preparation Techniques on the appearance of bacteria and biofilms in the Carter Sandstone. J. Sediment. Res. 2004, 74, 858–867. [Google Scholar] [CrossRef]
- Trmcic, A.; Chen, H.; Trząskowska, M.; Tamber, S.; Wang, S. Biofilm-Forming Capacity of Five Salmonella Strains and Their Fate on Postharvest Mini Cucumbers. J. Food Prot. 2018, 81, 1871–1879. [Google Scholar] [CrossRef]
- Apellanis-Borges, K.; Quedi-Furian, T.; Neves-de Souza, S.; Menezes, R.; Alves-de Lima, D.; Borges-Fortes, F.B.; Pippi-Salle, C.T.; Souza-Moraes, H.L.; Pinheiro-Nascimento, V. Biofilm formation by Salmonella Enteritidis and Salmonella Typhimurium isolated from avian sources is partially related with their in vivo pathogenicity. Microb. Pathog. 2018, 118, 238–241. [Google Scholar] [CrossRef]
- Medalla, F.; Gu, W.; Mahon, B.E.; Judd, M.; Folster, J.; Griffin, P.M.; Hoekstra, R.M. Estimated incidence of antimicrobial drug-resistant nontyphoidal Salmonella infections, United States, 2004–2012. Emerg. Infect. Dis. 2017, 23, 29–37. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Kaur, R. Prevalence and growth of pathogens on salad vegetables, fruits and sprouts. Int. J. Hygen Environ. Health 2001, 203, 205–213. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, X.; Zou, W.; Wang, Y.; Lei, C.; Xiang, R.; Zhou, L.; Liu, B.; Zhang, A.; Wang, H. Co-occurrence of biofilm formation and quinolone resistance in Salmonella enterica serotype typhimurium carrying an IncHI2-type oqxAB-positive plasmid. Microbial. Pathog. 2018, 123, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Haghshenas, B.; Abdullah, N.; Barzegari, A.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotics or antibiotics: Future challenges in medicine. J. Med. Microbiol. 2015, 64, 137–146. [Google Scholar] [CrossRef]
- Sabtu, N.; Enoch, D.A.; Brown, N.M. Antibiotic resistance: What, why, where, when and how? Br. Med. Bull. 2015, 116, 105–113. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Study on E. coli and Salmonella biofilms from fresh fruits and vegetables. J. Food Sci. Technol. 2017, 54, 1091–1097. [Google Scholar] [CrossRef]
- Dhakal, J.; Sharma, C.S.; Nannapaneni, R.; McDaniel, C.D.; Kim, T.; Kiess, A. Effect of chlorine-induced sublethal oxidative stress on the biofilm-forming ability of Salmonella at different temperatures, nutrient conditions, and substrates. J. Food Prot. 2019, 82, 78–92. [Google Scholar] [CrossRef]
- Tassinari, E.; Duffy, G.; Bawn, M.; Burgess, C.M.; McCabe, E.M.; Lawlor, P.G.; Gardiner, G.; Kingsley, R.A. Microevolution of antimicrobial resistance and biofilm formation of Salmonella Typhimurium during persistence on pig farms. Sci. Rep. 2019, 9, 8832. [Google Scholar] [CrossRef]
- Bashir, A.; Azeem, A.; Stedman, Y.; Hilton, A.C. Pet food factory isolates of Salmonella serotypes do not demonstrate enhanced biofilm formation compared to rerotype-matched clinical and veterinary isolates. BioMed Res. Int. 2019, 2019, 8569459. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Bokranz, W.; Rabsch, W.; Zogaj, X.; Nimtz, M.; Tschäpe, H. Occurrence and regulation of the multicellular morphotype in Salmonella serovars important in human disease. Int. J. Med. Microbiol. 2003, 293, 273–285. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Salmonella and Campylobacter biofilm formation: A comparative assessment from farm to fork. J. Sci. Food Agric. 2018, 98, 4014–4032. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Klu, Y.A.K.; Chen, J. Attachment and biofilm formation by selected strains of Salmonella enterica and entrohemorrhagic Escherichia coli of fresh produce origin. J. Food Sci. 2017, 82, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Luo, Y.; Nou, X.; Bauchan, G.; Zhou, B.; Wang, Q.; Millner, P. Enhanced inactivation of Salmonella and Pseudomonas biofilms on stainless steel by Use of T-128, a fresh-produce washing aid, in chlorinated wash solutions. Appl. Environ. Microbiol. 2012, 78, 6789–6798. [Google Scholar] [CrossRef]
- Singla, R.; Goel, H.; Ganguli, A. Novel synergistic approach to exploit the bactericidal efficacy of commercial disinfectants on the biofilms of Salmonella enterica serovar Typhimurium. J. Biosci. Bioeng. 2014, 118, 34–40. [Google Scholar] [CrossRef]
- Cook, K.L.; Givan, E.C.; Mayton, H.M.; Parekh, R.R.; Taylor, R.; Walker, S.L. Using the agricultural environment to select better surrogates for foodborne pathogens associated with fresh produce. Int. J. Food Microbiol. 2017, 262, 80–88. [Google Scholar] [CrossRef]
- Jain, S.; Chen, J. Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. J. Food Prot. 2007, 70, 2473–2479. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M. Differences in attachment of Salmonella enterica serovars to cabbage and lettuce leaves. Int. J. Food Microbiol. 2010, 139, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.O.; Cruz, E.A.; Souza, E.G.F.; Oliveira, T.C.M.; Alvarenga, V.O.; Peña, W.E.L.; Sant-Ana, A.S.; Magnani, M. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. Int. J. Food Microbiol. 2018, 281, 90–100. [Google Scholar] [CrossRef] [PubMed]
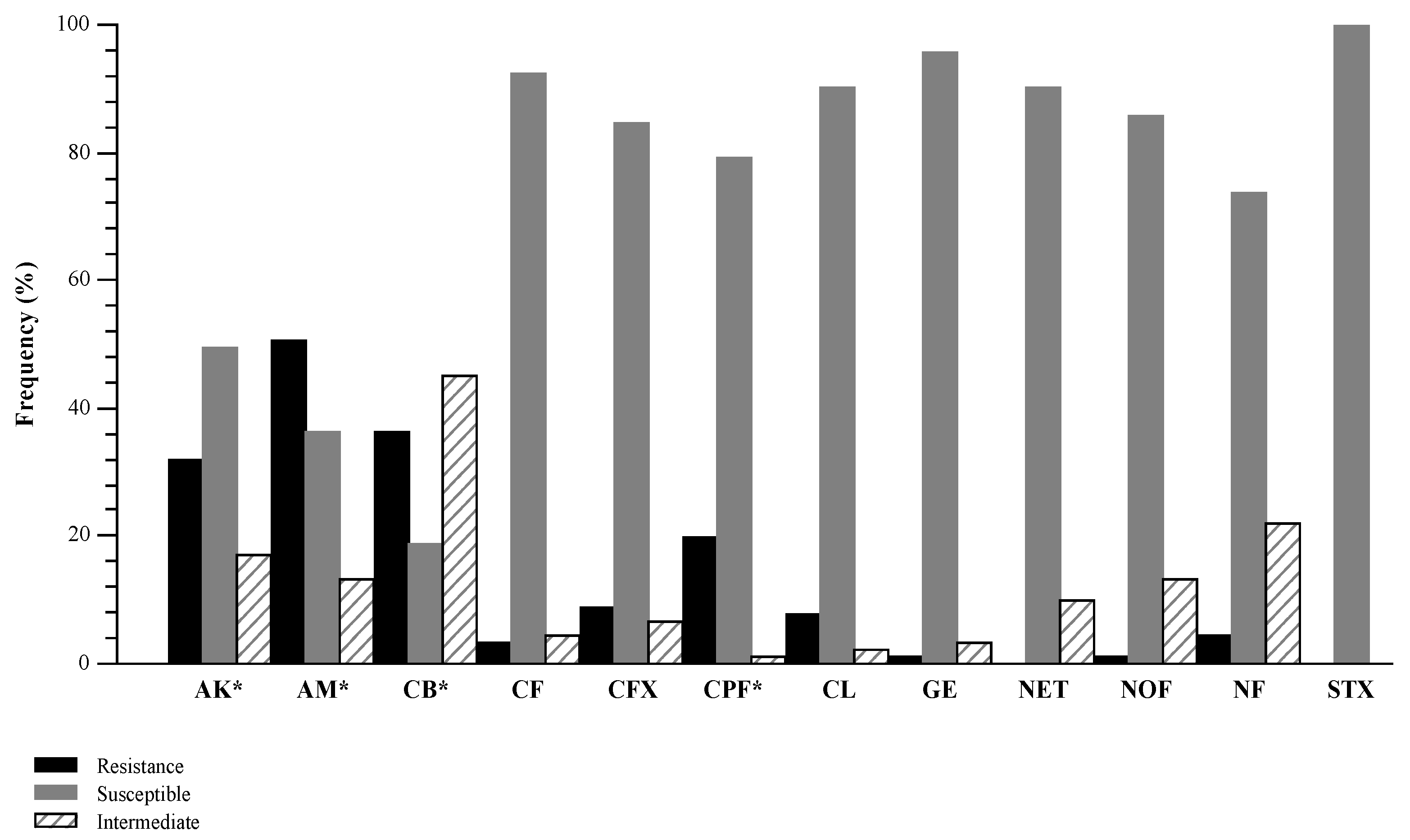
| Strains | No. (%) of Isolates with Resistance | Resistance Profile |
|---|---|---|
| Salmonella Rubislaw (n = 17) | 4 (4.3) | AM-CB |
| 1 (1) | AM-CB-GE-NF | |
| 1 (1) | AK-CF-CL-NF | |
| Salmonella Newport (n = 17) | 3 (3.2) | AM-CB |
| 1 (1) | AK-AM | |
| 1 (1) | AK-AM-CB | |
| 2 (2) | AK-CFX-CPF-CL | |
| 1 (1) | AK-CF-CFX-CPF-CL | |
| Salmonella Oranienburg (n = 3) | 1 (1) | AM-CB |
| 1 (1) | AK-AM-CB | |
| Salmonella Infantis (n = 40) | 7 (7) | AM-CB |
| 2 (2) | AK-CPF | |
| 1 (1) | AK-CFX | |
| 2 (2) | AK-AM-CPF | |
| 1 (1) | AK-CPF-CL | |
| 1 (1) | AK-CFX-CPF | |
| 1 (1) | AK-AM-CB | |
| 1 (1) | AM-CB-NF | |
| 1 (1) | AK-CB-CFX-CPF | |
| 1 (1) | AM-CB-CF-NF | |
| 1 (1) | AK-CFX-CPF-CL | |
| 1 (1) | AK-AM-CFX-CPF-CL | |
| Salmonella Thyphimurium (n = 14) | 4 (4.3) | AM-CB |
| 3 (3.2) | AK-AM-CPF | |
| 1 (1) | AK-CFX-CPF | |
| 1 (1) | AK-AM-CB |
| Morphotype CRA (n = 91) | Polystyrene Biofilm Formation (n = 91) | ||||||
|---|---|---|---|---|---|---|---|
| Strains | rdar | pdar | bdar | saw | Strong Biofilm Producers | Weak Biofilm Producers | OD570 * |
| Salmonella Rubislaw (n = 17) | 6 | 2 | 6 | 3 | 16 | 1 | Mean = 1.083 ± 0.25 |
| Salmonella Newport (n = 17) | 11 | - | 4 | 2 | 17 | - | Mean = 1.086 ± 0.01 |
| Salmonella Infantis (n = 40) | 24 | - | 14 | 2 | 40 | - | Mean = 1.085 ± 0.11 |
| Salmonella Typhimurium (n = 14) | 13 | - | 1 | - | 14 | - | Mean = 1.081 ± 0.00 |
| Salmonella Oranienburg (n = 3) | - | - | - | 3 | - | 3 | Mean = 0.014 ± 0.00 |
| Phenotypic Characteristics | |||||
|---|---|---|---|---|---|
| Serotype | Resistance Profile | Morphotype CRA | Polystyrene Biofilm Formation/OD570 * | ||
| 22 °C | 35 °C | ||||
| Salmonella Rubislaw-1 | AM-CB-GE-NF | rdar | rdar | SBP | Mean = 1.088 ± 0.002 |
| Salmonella Newport-6 | AMK-CF-CFX-CPF-CL | rdar | rdar | SBP | Mean = 1.094 ± 0.001 |
| Salmonella Typhimurium-8 | AK-CFX-CPF | rdar | rdar | SBP | Mean = 1.088 ± 0.001 |
| Salmonella Infantis-9 | AM-CB-CF-NF | rdar | rdar | SBP | Mean = 1.086 ± 0.001 |
| Serotype | Culture Medium | log10 de CFU/cm2 ± SD |
|---|---|---|
| Salmonella Rubislaw-1 | TSBS | 8.3 ± 0.51 |
| Salmonella Newport-6 | TSBS | 8.2 ± 0.35 |
| Salmonella Typhimurium-8 | TSBS | 8.3 ± 0.04 |
| Salmonella Infantis-9 | TSBS | 9.6 ± 0.13 |
| Salmonella Typhimurium ATCC 14028 | TSBS | 9.3 ± 0.10 |
| Salmonella Rubislaw-1 | TSBA | 7.8 ± 0.02 |
| Salmonella Newport-6 | TSBA | 8.5 ± 0.27 |
| Salmonella Typhimurium-8 | TSBA | 8.4 ± 0.06 |
| Salmonella Infantis-9 | TSBA | 8.9 ± 0.03 |
| Salmonella Typhimurium ATCC 14028 | TSBA | 8.3 ± 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Novoa, M.-G.; Guerrero-Medina, P.-J.; Navarrete-Sahagún, V.; Gómez-Olmos, I.; Velázquez-Suárez, N.-Y.; De la Cruz-Color, L.; Gutiérrez-Lomelí, M. Biofilm Formation by Multidrug-Resistant Serotypes of Salmonella Isolated from Fresh Products: Effects of Nutritional and Environmental Conditions. Appl. Sci. 2021, 11, 3581. https://doi.org/10.3390/app11083581
Avila-Novoa M-G, Guerrero-Medina P-J, Navarrete-Sahagún V, Gómez-Olmos I, Velázquez-Suárez N-Y, De la Cruz-Color L, Gutiérrez-Lomelí M. Biofilm Formation by Multidrug-Resistant Serotypes of Salmonella Isolated from Fresh Products: Effects of Nutritional and Environmental Conditions. Applied Sciences. 2021; 11(8):3581. https://doi.org/10.3390/app11083581
Chicago/Turabian StyleAvila-Novoa, María-Guadalupe, Pedro-Javier Guerrero-Medina, Velia Navarrete-Sahagún, Itzel Gómez-Olmos, Noemí-Yolanda Velázquez-Suárez, Lucia De la Cruz-Color, and Melesio Gutiérrez-Lomelí. 2021. "Biofilm Formation by Multidrug-Resistant Serotypes of Salmonella Isolated from Fresh Products: Effects of Nutritional and Environmental Conditions" Applied Sciences 11, no. 8: 3581. https://doi.org/10.3390/app11083581
APA StyleAvila-Novoa, M.-G., Guerrero-Medina, P.-J., Navarrete-Sahagún, V., Gómez-Olmos, I., Velázquez-Suárez, N.-Y., De la Cruz-Color, L., & Gutiérrez-Lomelí, M. (2021). Biofilm Formation by Multidrug-Resistant Serotypes of Salmonella Isolated from Fresh Products: Effects of Nutritional and Environmental Conditions. Applied Sciences, 11(8), 3581. https://doi.org/10.3390/app11083581







