The Biocompatibility of Wireless Power Charging System on Human Neural Cells
Abstract
:1. Introduction
2. Material and Methods
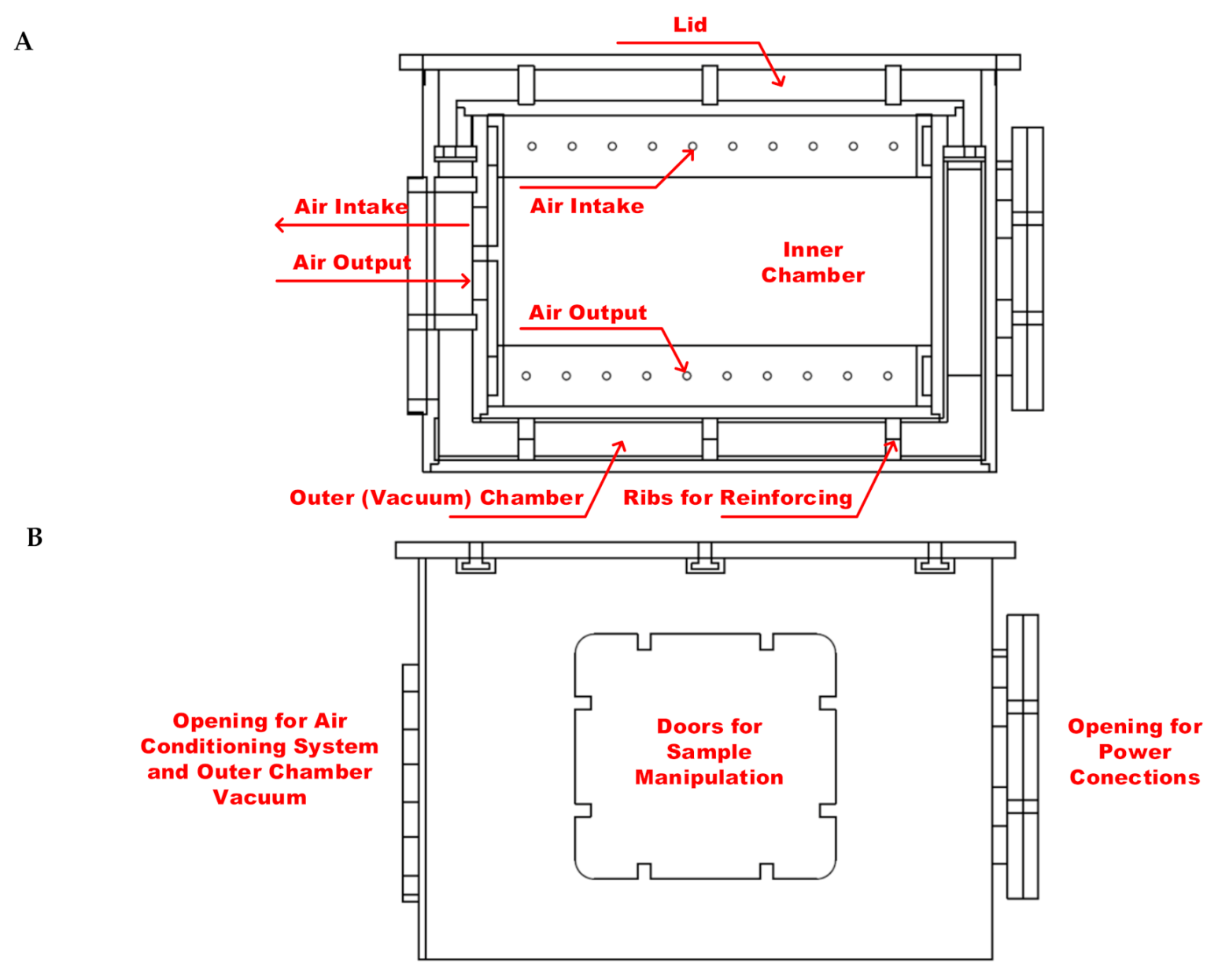
2.1. Design of Experimental Thermo Incubator
2.2. WPT System Operational Settings and Scaling of the EMF for Exposure
2.3. Simulation Analysis of the EMF Intensity and Distribution within Proposed WPT System
2.4. Cell Cultures and Cultivation Conditions
2.5. Exposure of Cell Cultures
2.6. MTT Cell Viability Assay
2.7. Annexin V Assay and Flow Cytometry Analysis
2.8. Immunocytochemistry
3. Results
3.1. The Effect of EMF on Cell Morphology and Viability
3.2. The Effect of EMF on Cell Death and Inner Cellular Organization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saliev, T.; Begimbetova, D.; Masoud, A.R.; Matkarimov, B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog. Biophys. Mol. Biol. 2019, 141, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.E.; de Llobet, P.; Dalmau, A.; Wiart, J.; Goedhart, G.; Hours, M.; Benke, G.P.; Bouka, E.; Bruchim, R.; Choi, K.H.; et al. Patterns of cellular phone use among young people in 12 countries: Implications for RF exposure. Environ. Int. 2017, 107, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, L.L.; Miller, A.B.; Sasco, A.; Davis, D.L. Mobile phone radiation causes brain tumors and should be classified as a probable human carcinogen (2A). Int. J. Oncol. 2015, 46, 1865–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.P.; Li, J.H.; Zhang, J.; Xu, S.L.; Kuang, F.; Lang, H.Y.; Wang, Y.F.; An, G.Z.; Li, J.; Guo, G.Z. Long-term electromagnetic pulse exposure induces Abeta deposition and cognitive dysfunction through oxidative stress and overexpression of APP and BACE1. Brain Res. 2016, 1642, 10–19. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, D.H.; Huh, Y.H.; Lee, E.H.; Kim, H.G.; Kim, H.R. Long-term exposure to 835 MHz RF-EMF induces hyperactivity, autophagy and demyelination in the cortical neurons of mice. Sci. Rep. 2017, 7, 41129. [Google Scholar] [CrossRef]
- Falzone, N.; Huyser, C.; Becker, P.; Leszczynski, D.; Franken, D.R. The effect of pulsed 900-MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int. J. Androl. 2011, 34, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Altun, G.; Deniz, Ö.G.; Yurt, K.K.; Davis, D.; Kaplan, S. Effects of mobile phone exposure on metabolomics in the male and female reproductive systems. Environ. Res. 2018, 167, 700–707. [Google Scholar] [CrossRef]
- Koniar, D.; Hargas, L.; Stofan, S. high speed video system for tissue measurement based on pwm regulated dimming and virtual instrumentation. Elektron. Elektrotechn. 2010, 10, 169–172. [Google Scholar]
- Ohtani, S.; Ushiyama, A.; Maeda, M.; Ogasawara, Y.; Wang, J.; Kunugita, N.; Ishii, K. The effects of radio-frequency electromagnetic fields on T cell function during development. J. Radiat. Res. 2015, 56, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, E.; Mortazavi, S.M.; Ali-Ghanbari, A.; Sharifzadeh, S.; Ranjbaran, R.; Mostafavi-Pour, Z.; Zal, F.; Haghani, M. Effect of 900 MHz electromagnetic radiation on the induction of ROS in human peripheral blood mononuclear cells. J. Biomed. Phys. Eng. 2015, 5, 105–114. [Google Scholar]
- Ruediger, H.W. Genotoxic effects of radiofrequency electromagnetic fields. Pathophysiology 2009, 16, 89–102. [Google Scholar] [CrossRef]
- Son, Y.; Kim, J.S.; Jeong, Y.J.; Jeong, Y.K.; Kwon, J.H.; Choi, H.D.; Pack, J.K.; Kim, N.; Lee, Y.S.; Lee, H.J. Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neurosci. Lett. 2018, 666, 64–69. [Google Scholar] [CrossRef]
- Gruber, M.J.; Palmquist, E.; Nordin, S. Characteristics of perceived electromagnetic hypersensitivity in the general population. Scand. J. Psychol. 2018, 59, 422–427. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kang, G.Y.; Kwon, J.H.; Choi, H.D.; Pack, J.K.; Kim, N.; Lee, Y.S.; Lee, H.J. 1950 MHz electromagnetic fields ameliorate Aβ pathology in Alzheimer’s disease mice. Curr. Alzheimer Res. 2015, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.K.; Kim, H.G.; Kim, K.B.; Kim, H.R. Possible effects of radiofrequency electromagnetic field exposure on central nerve system. Biomol. Ther. 2019, 27, 265–275. [Google Scholar] [CrossRef]
- Koniar, D.; Hargas, L.; Loncova, Z. Visual system-based object tracking using image segmentation for biomedical applications. Electr. Eng. 2017, 99, 1349–1366. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Hargas, L.; Koniar, D.; Hrianka, M. Adjusting and conditioning of high speed videosequences for diagnostic purposes in medicine. In Proceedings of the 10th International Conference ELEKTRO, Rajecké Teplice, Slovakia, 19–20 May 2014; pp. 548–552. [Google Scholar]
- IEEE Standards Coordinating Committee. IEEE standard for safety levels with respect to human exposure to radio frequency electromagnetic fields, 3 kHz to 300 GHz amendment 1: Specifies ceiling limits for induced and contact current, clarifies distinctions between localized exposure and spatial. IEEE Stand. Coordinat. Comm. 2010, 95, 1–9. [Google Scholar]
- Yoshie, S.; Ogasawara, Y.; Ikehata, M.; Ishii, K.; Suzuki, Y.; Wada, K.; Wake, K.; Nakasono, S.; Taki, M.; Ohkubo, C. Evaluation of biological effects of intermediate frequency magnetic field on differentiation of embryonic stem cell. Toxicol. Rep. 2016, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Barabáš, J.; Radil, R.; Malíková, I. Modification of S. cerevisiae growth dynamics using low frequency electromagnetic fields in the 1–2 kHz range. Biomed. Res. Int. 2015, 2015, 694713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Zhu, C.; Lu, R.; Mao, S.; Qi, Y. Intermediate frequency magnetic field generated by a wireless power transmission device does not cause genotoxicity in vitro. Bioelectromagnetics 2014, 35, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Frivaldsky, M.; Pavelek, M.; Donic, T. Modeling and experimental verification of induction heating of thin molybdenum sheets. Appl. Sci. 2021, 11, 647. [Google Scholar] [CrossRef]
- Frivaldsky, M.; Pavelek, M. In loop design of the coils and the electromagnetic shielding elements for the wireless charging systems. Energies 2020, 13, 6661. [Google Scholar] [CrossRef]
- Kindl, V.; Frivaldsky, M.; Zavrel, M.; Pavelek, M. Generalized design approach on industrial wireless chargers. Energies 2020, 13, 2697. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Baan, R.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Islami, F.; Galichet, L.; Straif, K.; et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011, 12, 624–626. [Google Scholar] [CrossRef]
- Wagner, P.; Röschke, J.; Mann, K.; Hiller, W.; Frank, C. Human sleep under the influence of pulsed radiofrequency electromagnetic fields: A polysomnographic study using standardized conditions. Bioelectromagnetics 1998, 19, 199–202. [Google Scholar] [CrossRef]
- Frey, A.H. Headaches from cellular telephones: Are they real and what are the implications? Environ. Health Perspect. 1998, 106, 101–103. [Google Scholar] [CrossRef]
- Braune, S.; Wrocklage, C.; Raczek, J.; Gailus, T.; Lücking, C.H. Resting blood pressure increase during exposure to a radio-frequency electromagnetic field. Lancet 1998, 351, 1857–1858. [Google Scholar] [CrossRef]
- Mann, K.; Wagner, P.; Brunn, G.; Hassan, F.; Hiemke, C.; Röschke, J. Effects of pulsed high-frequency electromagnetic fields on the neuroendocrine system. Neuroendocrinology 1998, 67, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.J.; Benke, G.P.; Dimitriadis, C.; Inyang, I.O.; Sim, M.R.; Wolfe, R.S.; Croft, R.J. Mobile telephone use is associated with changes in cognitive function in young adolescents. Bioelectromagnetics 2009, 30, 678–686. [Google Scholar] [CrossRef]
- Fragopoulou, A.F.; Samara, A.; Antonelou, M.H.; Xanthopoulou, A.; Papadopoulou, A.; Vougas, K.; Koutsogiannopoulou, E.; Anastasiadou, E.; Stravopodis, D.J.; Tsangaris, G.T.; et al. Brain proteome response following whole body exposure of mice to mobile phone or wireless DECT base radiation. Electromagn. Biol. Med. 2012, 31, 250–274. [Google Scholar] [CrossRef]
- Misek, J.; Veterník, M.; Tonhajzerova, I.; Jakusova, V.; Janousek, L.; Jakus, J. Radiofrequency electromagnetic field affects heart rate variability in rabbits. Physiol. Res. 2020, 69, 633–643. [Google Scholar] [CrossRef]
- Nittby, H.; Brun, A.; Eberhardt, J.; Malmgren, L.; Persson, B.R.; Salford, L.G. Increased blood-brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology 2009, 16, 103–112. [Google Scholar] [CrossRef]
- Sudan, M.; Olsen, J.; Arah, O.A.; Obel, C.; Kheifets, L. Prospective cohort analysis of cellphone use and emotional and behavioural difficulties in children. J. Epidemiol. Commun. Health 2016, 70, 1207–1213. [Google Scholar] [CrossRef]
- Zastko, L.; Petrovičová, P.; Račková, A.; Jakl, L.; Jakušová, V.; Marková, E.; Belyaev, I. DNA damage response and apoptosis induced by hyperthermia in human umbilical cord blood lymphocytes. Toxicol. In Vitro 2021, 73, 105127. [Google Scholar] [CrossRef]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 2012, 7, e45069. [Google Scholar] [CrossRef]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Reale, M.; Kamal, M.A.; Patruno, A.; Costantini, E.; D’Angelo, C.; Pesce, M.; Greig, N.H. Neuronal cellular responses to extremely low frequency electromagnetic field exposure: Implications regarding oxidative stress and neurodegeneration. PLoS ONE 2014, 9, e104973. [Google Scholar] [CrossRef]
- Kiseleva, L.N.; Kartashev, A.V.; Vartanyan, N.L.; Pinevich, A.A.; Samoilovich, M.P. A172 and T98G cell lines characteristics. Cell Tissue Biol. 2016, 10, 341–348. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Dibdiakova, K.; Saksonova, S.; Stefanikova, A.; Vidomanova, E.; Lichardusova, L.; Hatok, J.; Racay, P. Proteasome stress triggers death of SH-SY5Y and T98G cells via different cellular mechanisms. Neurochem. Res. 2017, 42, 3170–3185. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Electromagnetic Fields & Public Health: Intermediate Frequencies (IF). 2005. Available online: https://www.who.int/peh-emf/publications/facts/intermediatefrequencies_infosheet.pdf (accessed on February 2005).
- Bodewein, L.; Schmiedchen, K.; Dechent, D.; Stunder, D.; Graefrath, D.; Winter, L.; Kraus, T.; Driessen, S. Systematic review on the biological effects of electric, magnetic and electromagnetic fields in the intermediate frequency range (300 Hz to 1 MHz). Environ. Res. 2019, 171, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Taki, M.; Ohkubo, C. Safety assessment of human exposure to intermediate frequency electromagnetic fields. Electr. Eng. Jpn. 2016, 197, 3–11. [Google Scholar] [CrossRef]
- Zastko, L.; Makinistian, L.; Moravčíková, A.; Jakuš, J.; Belyaev, I. Effect of intermittent ELF MF on umbilical cord blood lymphocytes. Bioelectromagnetics 2020, 41, 649–655. [Google Scholar] [CrossRef]
- Laakso, I.; Hirata, A. Evaluation of the induced electric field and compliance procedure for a wireless power transfer system in an electrical vehicle. Phys. Med. Biol. 2013, 58, 7583–7593. [Google Scholar] [CrossRef] [Green Version]
- Bereta, M.; Janoušek, L.; Cifra, M.; Červinková, K. Low frequency electromagnetic field effects on ultra-weak photon emission from yeast cells. In Proceedings of the ELEKTRO 2016—11th International Conference, Vysoké Tatry, Slovakia, 16–18 May 2016; pp. 478–481. [Google Scholar] [CrossRef]
- Blumenthal, N.C.; Ricci, J.; Breger, L.; Zychlinsky, A.; Solomon, H.; Chen, G.G.; Kuznetsov, D.; Dorfman, R. Effects of low-intensity AC and/or DC electromagnetic fields on cell attachment and induction of apoptosis. Bioelectromagnetics 1997, 18, 264–272. [Google Scholar] [CrossRef]
- Gottwald, E.; Wobus, A.M.; Guan, K.; Sontag, W.; Weibezahn, K.F.; Dertinger, H. Interferential electric field treatment revealed a low increase of spontaneous cardiac differentiation but no cyclic AMP changes nor induction of cardiac-specific gene expression in pluripotent embryonal carcinoma P19 cells. Electromagn. Biol. Med. 2002, 21, 105–118. [Google Scholar] [CrossRef]
- Wang, J.; Tang, N.; Xiao, Q.; Zhang, L.; Li, Y.; Li, J.; Wang, J.; Zhao, Z.; Tan, L. Pulsed electromagnetic field may accelerate in vitro endochondral ossification. Bioelectromagnetics 2015, 36, 35–44. [Google Scholar] [CrossRef]
- Choi, Y.K.; Urnukhsaikhan, E.; Yoon, H.H.; Seo, Y.K.; Cho, H.; Jeong, J.S.; Kim, S.C.; Park, J.K. Combined effect of pulsed electromagnetic field and sound wave on In vitro and In vivo neural differentiation of human mesenchymal stem cells. Biotechnol. Prog. 2017, 33, 201–211. [Google Scholar] [CrossRef]
- Koyama, S.; Narita, E.; Shinohara, N.; Miyakoshi, J. Effect of an intermediate-frequency magnetic field of 23 kHz at 2 mT on chemotaxis and phagocytosis in neutrophil-like differentiated human HL-60 cells. Int. J. Environ. Res. Public Health 2014, 11, 9649–9659. [Google Scholar] [CrossRef] [Green Version]
- Tituschkin, I.; Cho, M. Regulation of cell cytoskeleton and membrane mechanism by electric field: Role of linker protein. Biophys. J. 2009, 96, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Markov, M.S. Expanding use of pulse electromagnetic field therapies. Electromag. Biol. Med. 2007, 26, 257–274. [Google Scholar] [CrossRef]
- Muñoz-Lasso, D.C.; Romá-Mateo, C.; Pallardó, F.V.; Gonzalez-Cabo, P. Much more than a scaffold: Cytoskeletal proteins in neurological disorders. Cells 2020, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Sul, A.R.; Park, S.N.; Suh, H. Effects of sinusoidal electromagnetic field on structure and function of different kinds of cell lines. Yonsei Med. J. 2006, 47, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Čermak, A.M.M.; Ilić, K.; Pavičić, I. Microtubular structure impairment after GSM-modulated RF radiation exposur. Arch. Indust. Hyg. Tox. 2020, 71, 205–210. [Google Scholar] [CrossRef]
- Tuszynski, J.A.; Wenger, C.; Friesen, D.E.; Preto, J. An overview of sub-cellular mechanisms involved in the action of TTFields. Int. J. Environ. Res. Public Health 2016, 13, 1128. [Google Scholar] [CrossRef] [Green Version]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]



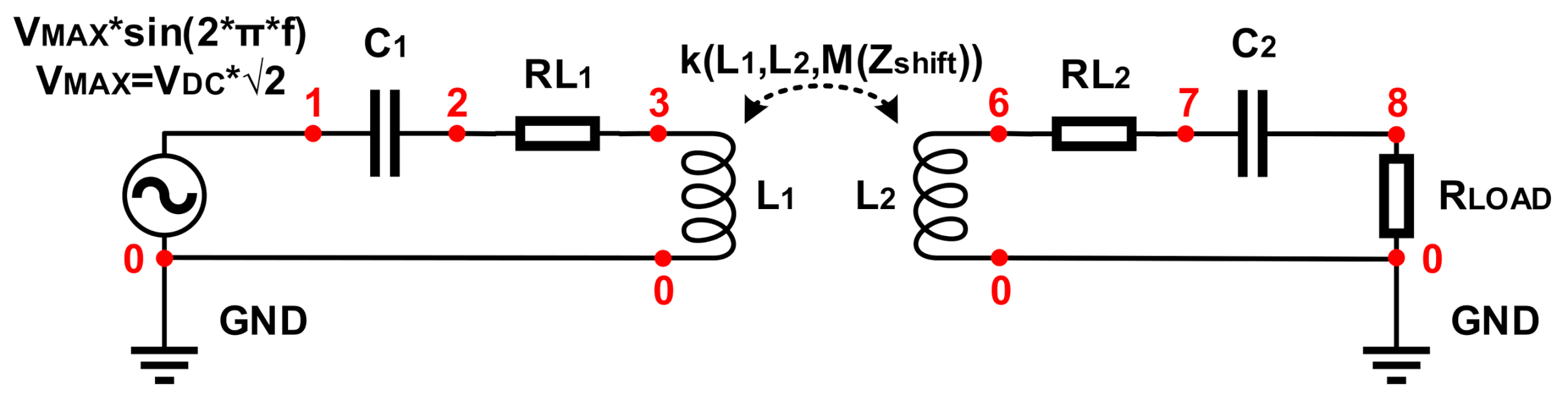







| Circuit Element | Value | Point (+) | Point (−) |
|---|---|---|---|
| Ground | GND | 0 | 0 |
| Voltage source | VMAX*sin(ωt) | 0 | 1 |
| Capacitor 1 | 1/(ω^2*comp1.mf.LCoil_1) [F] | 1 | 2 |
| Resistor 1 | RCoil1 [Ω] | 2 | 3 |
| External I vs. V1 | Coil voltage (mf3/coil1) | 3 | 0 |
| External I vs. V2 | Coil voltage (mf3/coil2) | 0 | 6 |
| Resistor 2 | RCoil2 [Ω] | 6 | 7 |
| Capacitor 2 | 1/(ω^2*comp2.mf2.LCoil_1) [F] | 7 | 8 |
| Load | RLOAD [Ω] | 8 | 0 |
| Location | Living Cells | Early Apoptotic | Late Apoptotic | Dead Cells | |
|---|---|---|---|---|---|
| HA | Control | 87.3% | 4.7% | 6.2% | 1.8% |
| Coil | 88.5% | 4.6% | 4.5% | 2.3% | |
| Shielded | 89.9% | 3.5% | 3.5% | 3.0% | |
| T98G | Control | 86.2% | 4.1% | 6.5% | 3.2% |
| Coil | 86.1% | 4.0% | 6.5% | 3.3% | |
| Shielded | 87.3% | 4.0% | 5.7% | 3.0% | |
| SH-SY5Y | Control | 84.9% | 4.3% | 7.1% | 3.7% |
| Coil | 83.7% | 4.8% | 8.5% | 3.0% | |
| Shielded | 87.8% | 3.0% | 5.1% | 4.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skovierova, H.; Pavelek, M.; Okajcekova, T.; Palesova, J.; Strnadel, J.; Spanik, P.; Halašová, E.; Frivaldsky, M. The Biocompatibility of Wireless Power Charging System on Human Neural Cells. Appl. Sci. 2021, 11, 3611. https://doi.org/10.3390/app11083611
Skovierova H, Pavelek M, Okajcekova T, Palesova J, Strnadel J, Spanik P, Halašová E, Frivaldsky M. The Biocompatibility of Wireless Power Charging System on Human Neural Cells. Applied Sciences. 2021; 11(8):3611. https://doi.org/10.3390/app11083611
Chicago/Turabian StyleSkovierova, Henrieta, Miroslav Pavelek, Terezia Okajcekova, Janka Palesova, Jan Strnadel, Pavol Spanik, Erika Halašová, and Michal Frivaldsky. 2021. "The Biocompatibility of Wireless Power Charging System on Human Neural Cells" Applied Sciences 11, no. 8: 3611. https://doi.org/10.3390/app11083611
APA StyleSkovierova, H., Pavelek, M., Okajcekova, T., Palesova, J., Strnadel, J., Spanik, P., Halašová, E., & Frivaldsky, M. (2021). The Biocompatibility of Wireless Power Charging System on Human Neural Cells. Applied Sciences, 11(8), 3611. https://doi.org/10.3390/app11083611







