Measurement of Temperature and H2O Concentration in Premixed CH4/Air Flame Using Two Partially Overlapped H2O Absorption Signals in the Near Infrared Region
Abstract
1. Introduction
2. Theory
2.1. Recovery of Curve Fitting for Partially Overlapped Absorption Signal
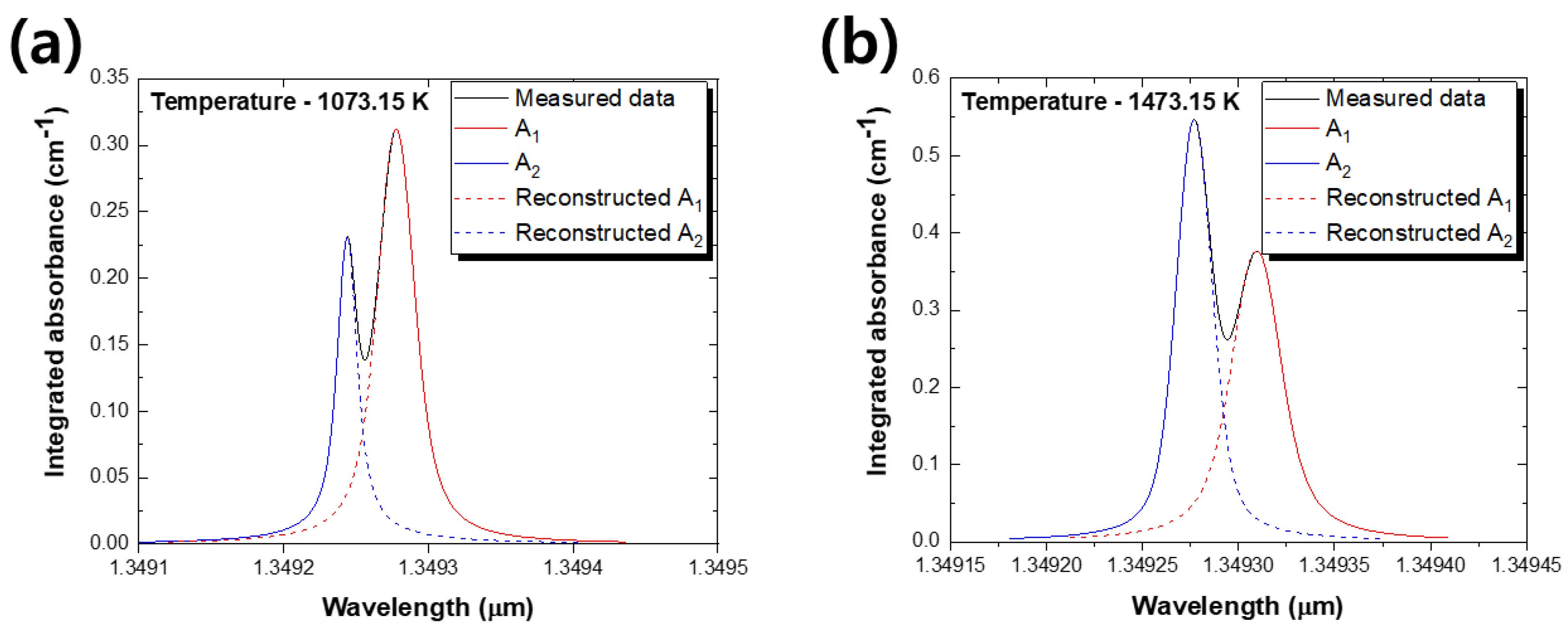
2.1.1. Reconstruction Method Using Half Absorbance
2.1.2. Multi-Peak Voigt Fitting
3. Line Selection
- (1)
- The two selected absorption lines should have sufficient absorption signals in the temperature range;
- (2)
- The two absorption lines are within a single laser scan range and do not overlap with each other in the atmosphere;
- (3)
- The R of the integrated absorbance area should be single-valued with temperature, and the line strengths of the two absorption lines ( = 0.2–5.0) should be similar;
- (4)
- The two absorption lines have sufficiently different lower-state energy E” to yield an absorption ratio that is sensitive to the probed temperature;
- (5)
- There should be no interference from other gas molecules near the two absorption signals.
4. Experimental Setup and Condition
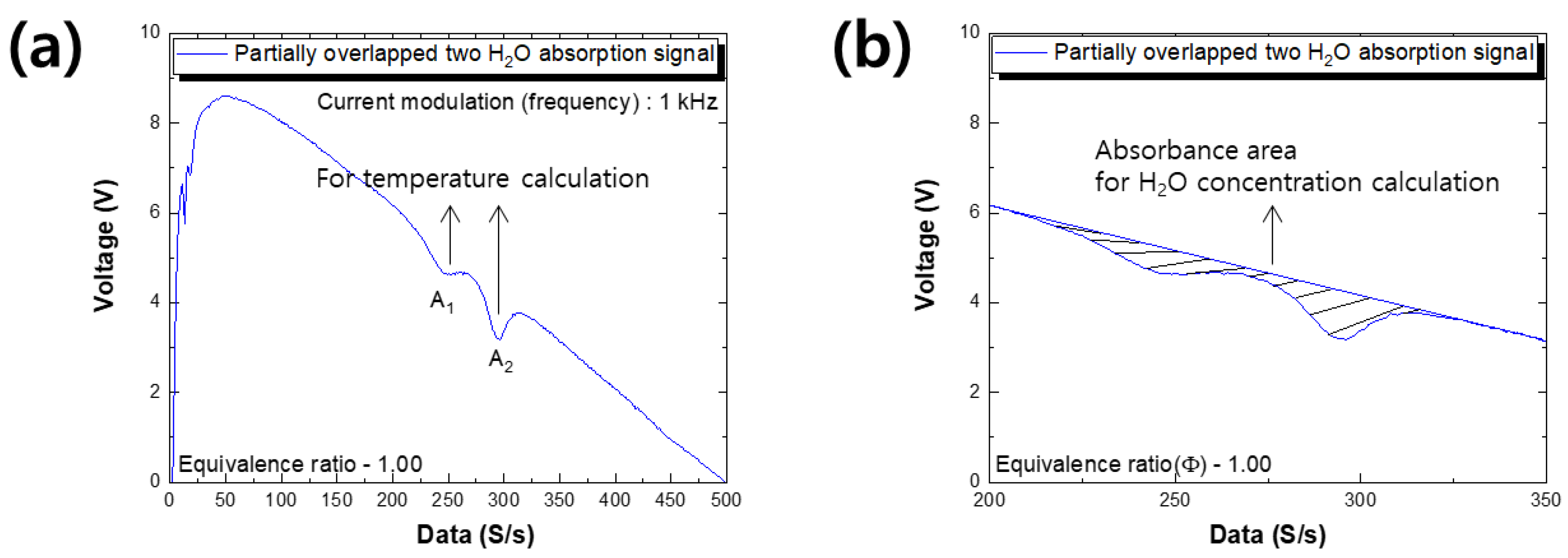
4.1. Preliminary Experimental Setup
4.2. Combustion Experimental Setup
5. Results and Discussion
5.1. Results of Preliminary Experiment
5.2. Results of Combustion Experiment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Uncertainty of energy level | |
| Lifetime | |
| Absorbance of multiple transitions | |
| Voigt function | |
| Wavelength | |
| Peak position | |
| Half-width at half maximum | |
| The number of peaks | |
| The serial number of each peak | |
| Doppler’s half-width at half maximum | |
| Lorentzian’s half-width at half maximum | |
| Molecular weight | |
| Lower-state energy | |
| Linestrength ratio |
References
- U.S. Energy Information Administration. Annual Energy Outlook; U.S. Energy Information Administration: Washington, DC, USA, 2020. [Google Scholar]
- Qu, Z.; Steinvall, E.; Ghorbani, R.; Schmidt, F.M. Tunable diode laser atomic absorption spectroscopy for detection of potassium under optically thick conditions. Anal. Chem. 2016, 88, 3754–3760. [Google Scholar] [CrossRef]
- Witzel, O.; Klein, A.; Meffert, C.; Schulz, C.; Kaiser, S.; Ebert, V. Calibration-free, high-speed, in-cylinder laser absorption sensor for cycle-resolved, absolute H2O measurements in a production IC engine. Proc. Combust. Inst. 2015, 35, 3653–3661. [Google Scholar] [CrossRef]
- Sepman, A.; Ögren, Y.; Qu, Z.; Wiinikka, H.; Schmidt, F.M. Real-time in situ multi-parameter TDLAS sensing in the reactor core of an entrained-flow biomass gasifier. Proc. Combust. Inst. 2017, 36, 4541–4548. [Google Scholar] [CrossRef]
- Spearrin, R.M.; Goldenstein, C.S.; Schultz, I.A.; Jeffries, J.B.; Hanson, R.K. Simultaneous sensing of temperature, CO, and CO2 in a scramjet combustor using quantum cascade laser absorption spectroscopy. Appl. Phys. A 2014, 117, 689–698. [Google Scholar] [CrossRef]
- Sur, R.; Sun, K.; Jeffries, J.B.; Socha, J.G.; Hanson, R.K. Scanned-wavelength-modulation-spectroscopy sensor for CO, CO2, CH4 and H2O in a high-pressure engineering-scale transport-reactor coal gasifier. Fuel 2015, 150, 102–111. [Google Scholar] [CrossRef]
- Witzel, O.; Klein, A.; Meffert, C.; Wagner, S.; Kaiser, S.; Schulz, C.; Ebert, V. VCSEL-based, high-speed, in situ TDLAS for in-cylinder water vapor measurements in IC engines. Opt. Express 2013, 21, 19951–19965. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.K.; Falcone, P.K. Temperature measurement technique for high-temperature gases using a tunable diode laser. Appl. Opt. 1978, 17, 2477–2480. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.E.; Wang, J.; Sanders, S.T.; Baer, D.S.; Hanson, R.K. In situ combustion measurements of CO, CO2, H2O and temperature using diode laser absorption sensors. Proc. Combust. Inst. 2000, 28, 407–413. [Google Scholar] [CrossRef]
- Torek, P.V.; Hall, D.L.; Miller, T.A.; Wooldridge, M.S. H2O absorption spectroscopy for determination of temperature and H2O mole fraction in high-temperature particle synthesis systems. Appl. Opt. 2002, 41, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Goldenstein, C.S.; Jeffries, J.B.; Hanson, R.K. Diode laser measurements of linestrength and temperature-dependent lineshape parameters of H2O-, CO2-, and N2-perturbed H2O transitions near 2474 and 2482nm. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 100–111. [Google Scholar] [CrossRef]
- Wu, K.; Li, F.; Cheng, X.; Yang, Y.; Lin, X.; Xia, Y. Sensitive detection of CO2 concentration and temperature for hot gases using quantum-cascade laser absorption spectroscopy near 4.2 μm. Appl. Phys. A 2014, 117, 659–666. [Google Scholar] [CrossRef]
- Ma, L.H.; Lau, L.Y.; Ren, W. Non-uniform temperature and species concentration measurements in a laminar flame using multi-band infrared absorption spectroscopy. Appl. Phys. A 2017, 123, 83. [Google Scholar] [CrossRef]
- Wei, W.; Peng, W.Y.; Wang, Y.; Shao, J.; Strand, C.L.; Hanson, R.K. Two-color frequency-multiplexed IMS technique for gas thermometry at elevated pressures. Appl. Phys. A 2020, 126, 1–16. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, R.; Enemali, G.; Cao, Z.; Zhou, B.; McCann, H.; Liu, C. Relative entropy regularized TDLAS tomography for robust temperature imaging. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Jeffries, J.B.; Hanson, R.K. Development of a sensor for temperature and water concentration in combustion gases using a single tunable diode laser. Meas. Sci. Technol. 2003, 14, 1459–1468. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, P.; Chao, X. Laser absorption sensing systems: Challenges, modeling, and design optimization. Appl. Sci. 2019, 9, 2723. [Google Scholar] [CrossRef]
- Penner, S.S. Quantitative Molecular Spectroscopy and Gas Emissivities, 1st ed.; Addison-Wesley: Boston, MA, USA, 1959. [Google Scholar]
- Hanson, R.K.; Spearrin, R.M.; Goldenstein, C.S. Spectroscopy and Optical Diagnostics for Gases; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zhou, S.; Yang, W.; Liu, C.; Zhang, L.; Yu, B.; Li, J. CO2-broadening coefficients for the NO2 transitions at 6.2 µm measured by mid-infrared absorption spectroscopy. J. Quant. Spectrosc. Radiat. Transf. 2020, 242, 106754. [Google Scholar] [CrossRef]
- Yang, W.; Li, B.; Zhou, J.; Han, Y.; Wang, Q. Continuous-wavelet-transform-based automatic curve fitting method for laser-induced breakdown spectroscopy. Appl. Opt. 2018, 57, 7526–7532. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN 2016 molecular spectroscopic database. J. Quant. Spec. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- So, S.; Park, J.; Song, A.; Hwang, J.; Yoo, M.; Lee, C. Detection limit of CO concentration measurement in LPG/air flame flue gas using tunable diode laser absorption spectroscopy. Energies 2020, 13, 4234. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Spearrin, R.M.; Jeffries, J.B.; Hanson, R.K. Infrared laser-absorption sensing for combustion gases. Prog. Energy Combust. Sci. 2017, 60, 132–176. [Google Scholar] [CrossRef]
- Zhou, X. Diode-Laser Absorption Sensors for Combustion Control. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2005. [Google Scholar]
- Bolshov, M.; Kuritsyn, Y.; Romanovskii, Y. Tunable diode laser spectroscopy as a technique for combustion diagnostics. Spectrochim. Acta Part B At. Spectrosc. 2015, 106, 45–66. [Google Scholar] [CrossRef]
- Liu, C.; Xu, L. Laser absorption spectroscopy for combustion diagnosis in reactive flows: A review. Appl. Spectrosc. Rev. 2019, 54, 1–44. [Google Scholar] [CrossRef]
- Hindasageri, V.; Vedula, R.P.; Prabhu, S.V. Thermocouple error correction for measuring the flame temperature with determination of emissivity and heat transfer coefficient. Rev. Sci. Instrum. 2013, 84, 024902. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.T. An Introduction to Combustion: Concepts and Applications, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2002. [Google Scholar]
- Lee, J.; Bong, C.; Yoo, J.; Bak, M.S. Combined use of TDLAS and LIBS for reconstruction of temperature and concentration fields. Opt. Express 2020, 28, 21121–21133. [Google Scholar] [CrossRef]
- So, S.; Park, D.G.; Jeong, N.; Kim, D.; Hwang, J.; Lee, C. Study on the simultaneous measurement of O2 and CO concentrations in the exhaust gas of a methane/air flame using tunable diode laser absorption spectroscopy. Energy Fuels 2020, 34, 3780–3787. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C.; et al. GRI-Mech 3.0. Available online: http://www.meberkeley.edu/gri_mech/ (accessed on 15 February 2021).
- Law, C.K. Combustion Physics, 1st ed.; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
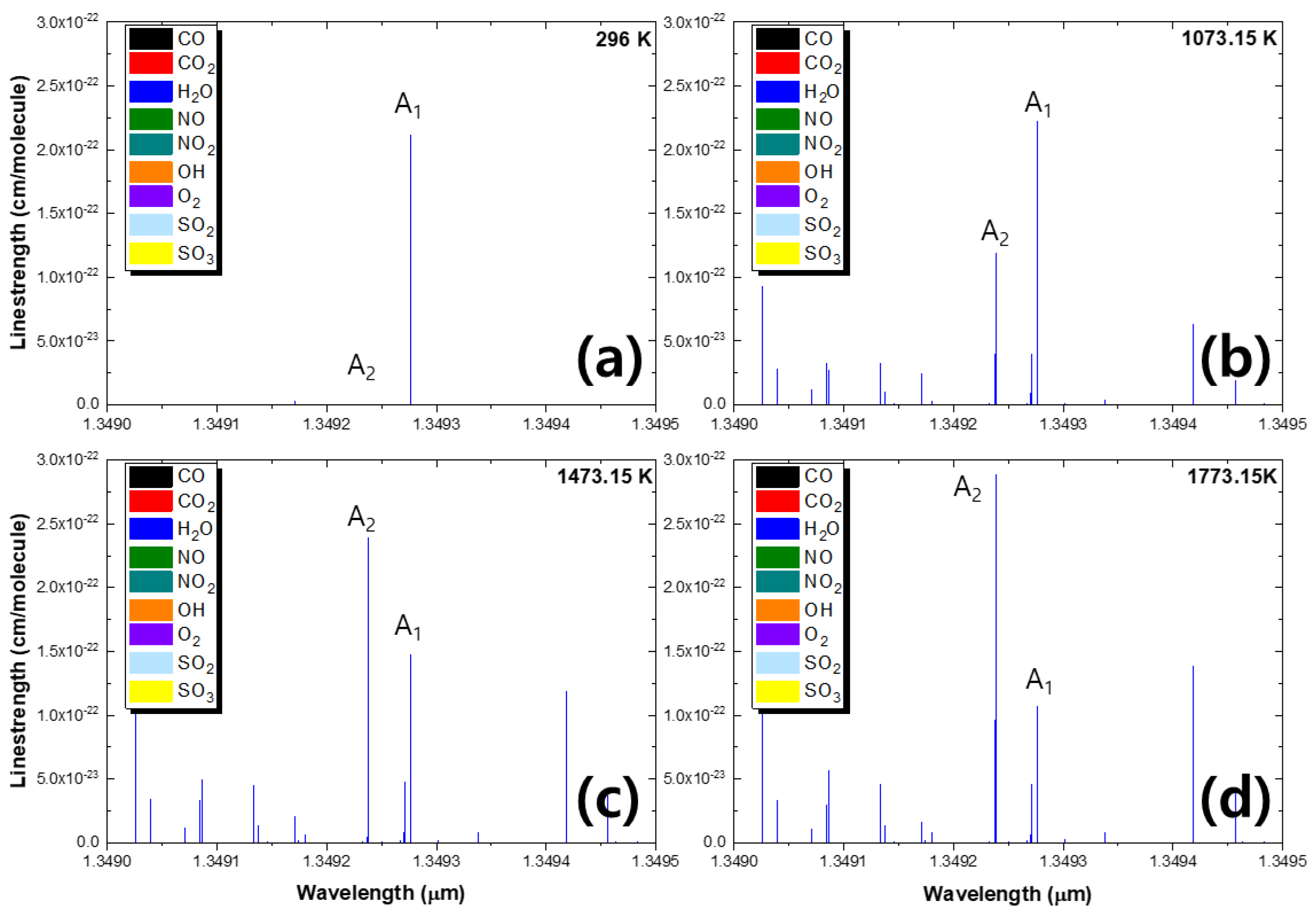








| 1.34923 | 7411.589 | 1.188 10−22 | 3655.4833 | 3052.709 | 0.2126 |
| 1.34927 | 7411.376 | 2.227 10−22 | 602.7735 |
| Equivalence Ratio (Φ) | Fuel (L/min) | Air (L/min) |
|---|---|---|
| 0.70 | 252.57 | |
| 0.85 | 208.68 | |
| 1.00 | 8.46 | 176.44 |
| 1.15 | 154.58 | |
| 1.30 | 137.03 |
| Set Temperature (K) | 1073.15 | 1173.15 | 1273.15 | 1373.15 | 1473.15 | 1573.15 |
| Measured temperature (K) | 797.30 | 924.08 | 1069.39 | 1165.48 | 1273.34 | 1360.28 |
| Equivalence Ratio | Measured Temp. Using TDLAS (K) | Standard Deviation (±K) | Measured Conc. Using TDLAS (%) | Standard Deviation (±%) |
|---|---|---|---|---|
| 0.70 | 1342.3 | 13.1 | 13.89 | 0.32 |
| 0.85 | 1374.9 | 16.2 | 15.39 | 0.33 |
| 1.00 | 1385.8 | 11.2 | 18.05 | 0.31 |
| 1.15 | 1384.3 | 15.6 | 18.92 | 0.32 |
| 1.30 | 1337.0 | 13.8 | 18.62 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, S.; Jeong, N.; Song, A.; Hwang, J.; Kim, D.; Lee, C. Measurement of Temperature and H2O Concentration in Premixed CH4/Air Flame Using Two Partially Overlapped H2O Absorption Signals in the Near Infrared Region. Appl. Sci. 2021, 11, 3701. https://doi.org/10.3390/app11083701
So S, Jeong N, Song A, Hwang J, Kim D, Lee C. Measurement of Temperature and H2O Concentration in Premixed CH4/Air Flame Using Two Partially Overlapped H2O Absorption Signals in the Near Infrared Region. Applied Sciences. 2021; 11(8):3701. https://doi.org/10.3390/app11083701
Chicago/Turabian StyleSo, Sunghyun, Nakwon Jeong, Aran Song, Jungho Hwang, Daehae Kim, and Changyeop Lee. 2021. "Measurement of Temperature and H2O Concentration in Premixed CH4/Air Flame Using Two Partially Overlapped H2O Absorption Signals in the Near Infrared Region" Applied Sciences 11, no. 8: 3701. https://doi.org/10.3390/app11083701
APA StyleSo, S., Jeong, N., Song, A., Hwang, J., Kim, D., & Lee, C. (2021). Measurement of Temperature and H2O Concentration in Premixed CH4/Air Flame Using Two Partially Overlapped H2O Absorption Signals in the Near Infrared Region. Applied Sciences, 11(8), 3701. https://doi.org/10.3390/app11083701





